Oral Findings Linked to Chronic Kidney Disease: A Comprehensive Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Analysis
3. Results
3.1. Study Selection and Flow Diagram
3.2. Data Extraction
Types of Studies
3.3. Quality Analysis
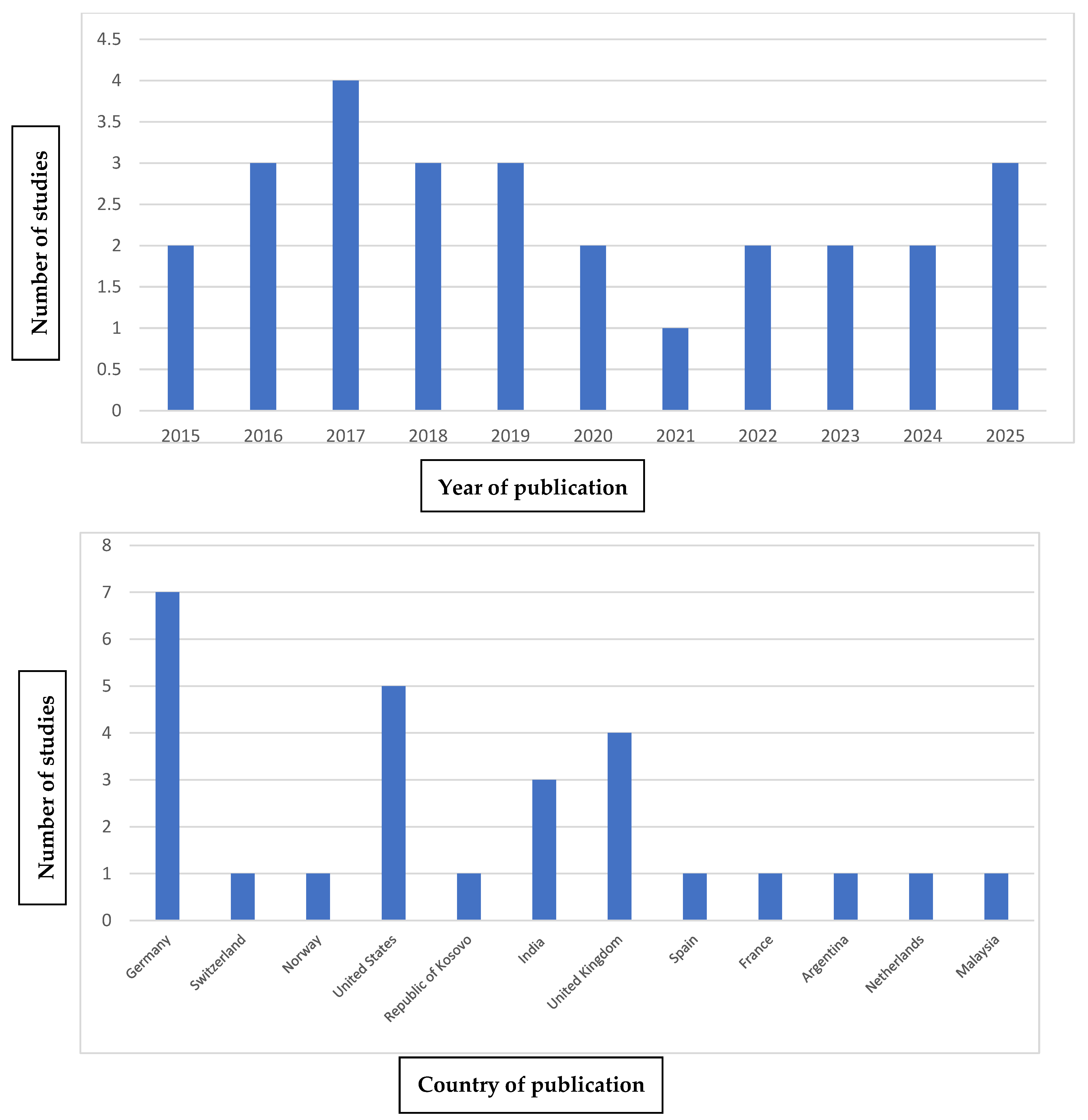
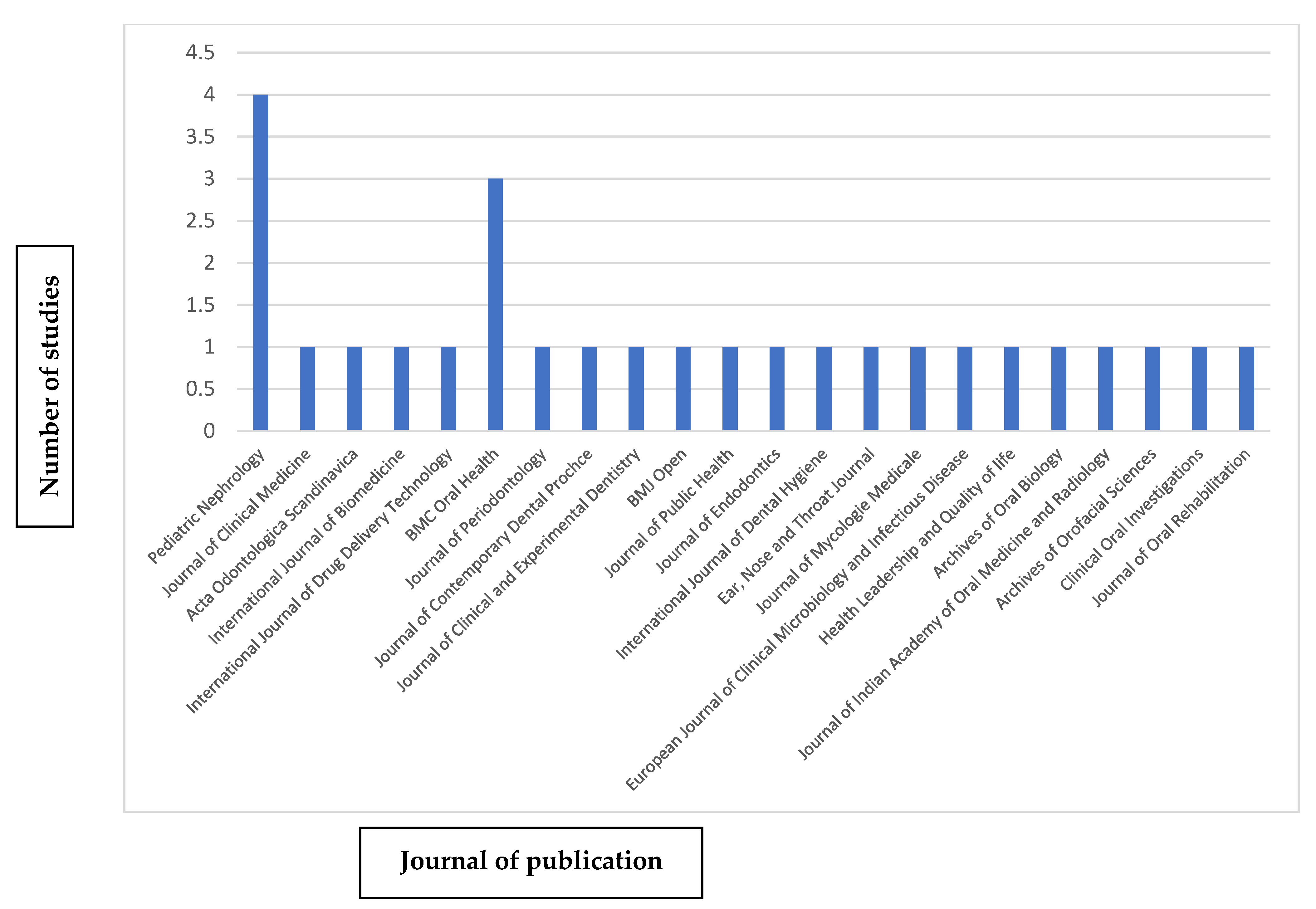
3.4. Bibliometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ammirati, A.L. Chronic Kidney Disease. Rev. Assoc. Méd. Bras. 2020, 66 (Suppl. 1), s03–s09. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Hamasaki, Y.; Okuda, Y.; Hamada, R.; Ishikura, K. Epidemiology of pediatric chronic kidney disease/kidney failure: Learning from registries and cohort studies. Pediatr. Nephrol. 2022, 37, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care Clin. Off. Pract. 2020, 47, 585–595. [Google Scholar] [CrossRef]
- VanSickle, J.S.; Warady, B.A. Chronic Kidney Disease in Children. Pediatr. Clin. N. Am. 2022, 69, 1239–1254. [Google Scholar] [CrossRef]
- Rao, I.R.; Bangera, A.; Nagaraju, S.P.; Shenoy, S.V.; Prabhu, R.A.; Rangaswamy, D.; Bhojaraja, M.V. Chronic kidney disease of unknown aetiology: A comprehensive review of a global public health problem. Trop. Med. Int. Health 2023, 28, 588–600. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Database Syst. Rev. Implement. Rep. 2019; publish ahead of print. [Google Scholar] [CrossRef]
- Almeida, P.A.; Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; Almeida, F.C.L.; Souza, I.P.R.; Valente, A.P. Salivary metabolic profile of children and adolescents after hemodialysis. Metabolomics 2017, 13, 141. [Google Scholar] [CrossRef]
- Kodaman Dokumacıgil, N.; Sezer, B.; Oktay, Ş.; Alpay, H.; Kargül, B. Dental caries, oral hygiene and salivary characteristics in children with chronic kidney disease: A case–control study. Pediatr. Nephrol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Taranta-Janusz, K.; Wasilewska, A.; Zalewska, A. Salivary gland dysfunction, protein glycooxidation and nitrosative stress in children with chronic kidney disease. J. Clin. Med. 2020, 9, 1285. [Google Scholar] [CrossRef]
- Beyer, A.; Ebach, F.; Reutter, H.; Sauerstein, K.; Hilger, A.C.; Krickau, T.; Tzschoppe, A.; Woelfe, J.; Galiano, M.; Schaefer, J.T. Oral health status in children with chronic kidney disease, kidney transplantation, and nephrotic syndrome: A cross-sectional study. Pediatr. Nephrol. 2025, 40, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Caliento, R.; Sarmento, D.J.D.S.; Silva, É.M.P.; Tozetto-Mendoza, T.R.; Tobouti, P.L.; Benini, V.; Braz-Silva, P.H.; Gallottini, M. Oral shedding of HSV-1 and EBV and oral manifestations in paediatric chronic kidney disease patients and renal transplant recipients. Acta Odontol. Scand. 2018, 76, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.; Laing, D.G.; Hutchinson, I.; Jinks, A.L.; Armstrong, J.E.; Kainer, G. Reduced taste function and taste papillae density in children with chronic kidney disease. Pediatr. Nephrol. 2015, 30, 2003–2010. [Google Scholar] [CrossRef]
- Muhaxheri, G.; Kelmendi, J.; Cakolli, V.H.; Kolgeci, D. Oral Changes in Chronic Renal Failure Patients in One of the Regional Hospitals in Kosovo. Int. J. Biomed. 2023, 13, 306–311. [Google Scholar] [CrossRef]
- Al-Zaidi, R.R. Oral Health Status among a Group of Population with Chronic Kidney Disease in Iraq. Int. J. Drug Deliv. Technol. 2022, 12, 51–54. [Google Scholar] [CrossRef]
- Abou-Bakr, A.; Hussein, R.R.; Khalil, E.; Ahmed, E. The frequency of periodontitis in end-stage renal disease on hemodialysis in a sample of Egyptian population: Multi-center clinical cross-sectional study. BMC Oral Health 2022, 22, 1. [Google Scholar] [CrossRef]
- Han, K.; Park, J.-B. Tooth loss and risk of end-stage renal disease: A nationwide cohort study. J. Periodontol. 2021, 92, 371–377. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, U.; Mallapragada, S.; Agarwal, P. Comparative Evaluation of Periodontal Status of Chronic Renal Failure Patients and Systemically Healthy Individuals. J. Contemp. Dent. Pract. 2018, 19, 324–330. [Google Scholar] [CrossRef]
- Ausavarungnirun, R.; Wisetsin, S.; Rongkiettechakorn, N.; Chaichalermsak, S.; Udompol, U.; Rattanasompattikul, M. Association of dental and periodontal disease with chronic kidney disease in patients of a single, tertiary care centre in Thailand. BMJ Open 2016, 6, e011836. [Google Scholar] [CrossRef] [PubMed]
- Parente, I.T.; Lima, Z.R.; Teixeira, L.H.; Lisboa, M.R.; de Melo, I.M.; Santos, P.R.; Goes, P. Gingivitis, increased probing depth, clinical attachment loss and tooth loss among patients with end-stage chronic kidney disease: A case-control study. J. Public Health 2018, 26, 75–80. [Google Scholar] [CrossRef]
- Khalighinejad, N.; Aminoshariae, A.; Kulild, J.C.; Sahly, K.; Mickel, A. Association of End-stage Renal Disease with Radiographically and Clinically Diagnosed Apical Periodontitis: A Hospital-based Study. J. Endod. 2017, 43, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.V.; Le, D.D. Dental condition and salivary characteristics in Vietnamese patients with chronic kidney disease. Int. J. Dent. Hyg. 2019, 17, 253–260. [Google Scholar] [CrossRef]
- Honarmand, M.; Farhad-Mollashahi, L.; Nakhaee, A.; Sargolzaie, F. Oral manifestation and salivary changes in renal patients undergoing hemodialysis. J. Clin. Exp. Dent. 2017, 9, e207–e210. [Google Scholar] [CrossRef] [PubMed]
- Oyetola, E.O.; Owotade, F.J.; Agbelusi, G.A.; Fatusi, O.A.; Sanusi, A.A. Oral findings in chronic kidney disease: Implications for management in developing countries. BMC Oral Health 2015, 15, 24. [Google Scholar] [CrossRef]
- Yusuf, T.; Raji, Y.R.; Lasisi, T.J.; Daniel, A.; Bamidele, O.T.; Fasunla, A.J.; Lasisi, A.O. Predictors of Taste Dysfunction and Its Severity Among Patients With Chronic Kidney Disease. Ear Nose Throat J. 2023, 102, 787–793. [Google Scholar] [CrossRef]
- de la Rosa-García, E.; Olalde-Hernández, M.J.; Irigoyen-Camacho, M.E.; Mondragón-Padilla, A.; Mendoza-Juache, A.; Sánchez-Vargas, L.O. Antifungal susceptibility of oral isolates of Candida species from chronic kidney disease patients on chronic dialysis. J. Mycol. Med. 2020, 30, 101009. [Google Scholar] [CrossRef]
- Pieralisi, N.; de Souza Bonfim-Mendonça, P.; Negri, M.; Jarros, I.C.; Svidzinski, T. Tongue coating frequency and its colonization by yeasts in chronic kidney disease patients. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1455–1462. [Google Scholar] [CrossRef]
- Gonzales, C.B.; Brusca, M.I.; Garzón, M.L.; Vela Ferreira, A.; Jewtuchowicz, V. Frequency and factors associated with oral Candida growth in patients with chronic kidney disease. Health Leadersh. Qual. Life 2024, 3, 540. [Google Scholar] [CrossRef]
- Luo, Q.; Chu, S.; Wu, Y.; Jin, L.; Liu, R.; Xu, Y.; Yu, Y.; Jin, Y.; Houndekon, L.O.E.P.; Hu, H.; et al. Characteristics of tongue coating microbiota in diabetic and non-diabetic kidney patients receiving hemodialysis. BMC Oral Health 2025, 25, 104. [Google Scholar] [CrossRef] [PubMed]
- Marinoski, J.; Bokor-Bratic, M.; Mitic, I.; Cankovic, M. Oral mucosa and salivary findings in non-diabetic patients with chronic kidney disease. Arch. Oral Biol. 2019, 102, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mahay, P.; Singh, M.P.; Nahar, P.; Bhuvaneshwari, S.; Goel, S.; Masih, U. Oral manifestations of patients with chronic kidney diseases: A cross-sectional study. J. Indian Acad. Oral Med. Radiol. 2024, 36, 63–67. [Google Scholar] [CrossRef]
- Kassim, N.K.; Feun, L.W.; Zainuddin, S.L.A.; Adnan, A.S.; Ibrahim, H.A. Oral manifestation and caries experience in pre-dialysis chronic kidney disease patients. Arch. Orofac. Sci. 2019, 14, 157–168. [Google Scholar] [CrossRef]
- Nylund, K.M.; Meurman, J.H.; Heikkinen, A.M.; Furuholm, J.O.; Ortiz, F.; Ruokonen, H.M. Oral health in patients with renal disease: A longitudinal study from predialysis to kidney transplantation. Clin. Oral Investig. 2018, 22, 339–347. [Google Scholar] [CrossRef]
- Limeres, J.; Garcez, J.F.; Marinho, J.S.; Loureiro, A.; Diniz, M.; Diz, P. Early tooth loss in end-stage renal disease patients on haemodialysis. Oral Dis. 2016, 22, 530–535. [Google Scholar] [CrossRef]
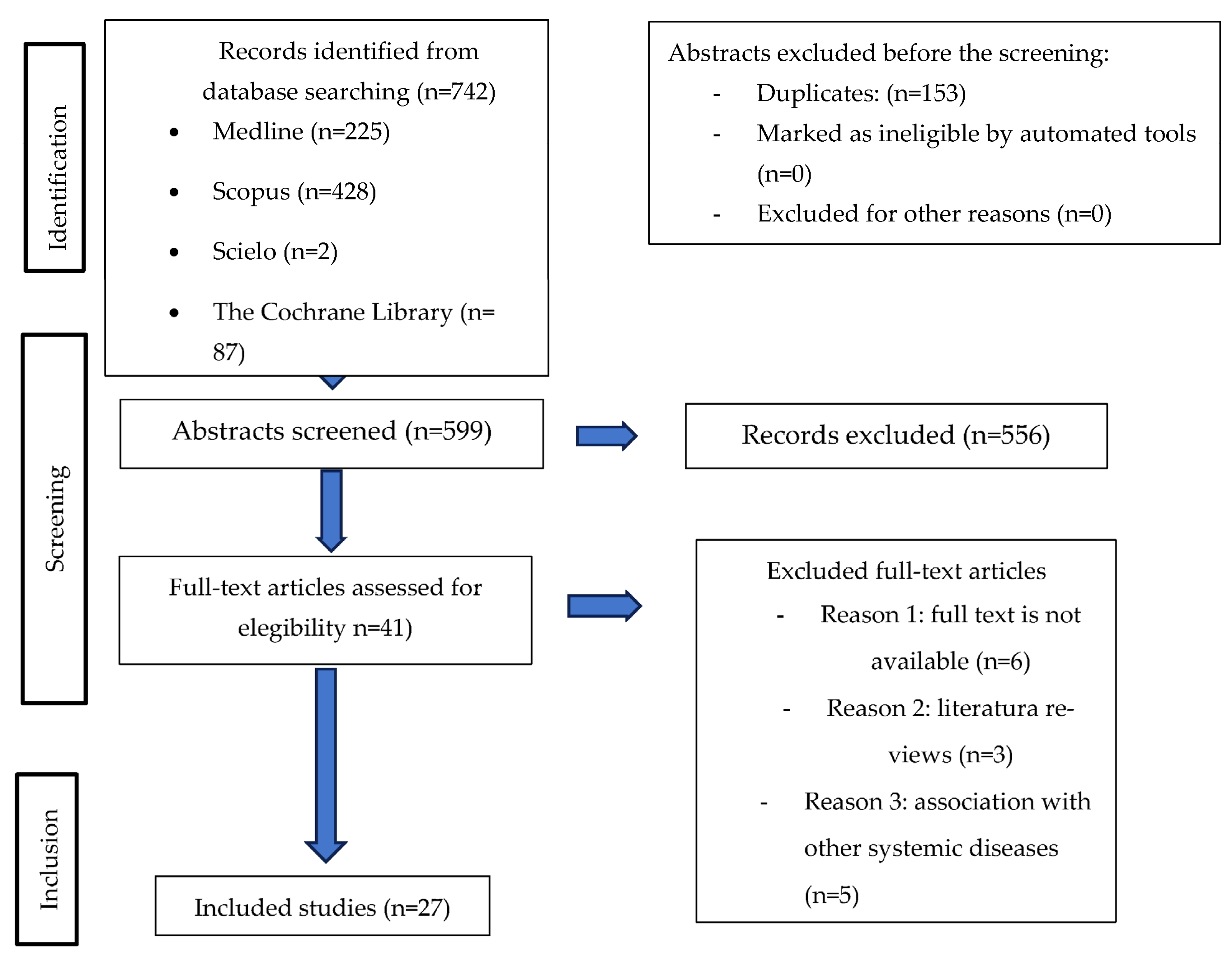

| Databases | Search Field | Results |
|---|---|---|
| Medline (Pubmed) | 1# “chronic kidney disease”, “chronic renal insufficiency”, “renal insufficiency, chronic”, “kidney failure, chronic” | 53,296 |
| 2# “Oral Manifestations”, “Oral Diseases”, “Oral Health”, “Mouth”, “Dental Care”, “Salivation”, “Gingival Diseases”, “Periodontal Diseases”, “Dry Mouth”, “Candidiasis, Oral” | 107,559 | |
| 1# AND 2# | 225 | |
| SCOPUS | 1# “chronic kidney disease”, “chronic renal insufficiency”, “renal insufficiency, chronic”, “kidney failure, chronic” | 74,795 |
| 2# “Oral Manifestations”, “Oral Diseases”, “Oral Health”, “Mouth”, “Dental Care”, “Salivation”, “Gingival Diseases”, “Periodontal Diseases”, “Dry Mouth”, “Candidiasis, Oral” | 159,019 | |
| 1# AND 2# | 428 | |
| Scielo | 1# “chronic kidney disease”, “chronic renal insufficiency”, “renal insufficiency, chronic”, “kidney failure, chronic” | 2077 |
| 2# “Oral Manifestations”, “Oral Diseases”, “Oral Health”, “Mouth”, “Dental Care”, “Salivation”, “Gingival Diseases”, “Periodontal Diseases”, “Dry Mouth”, “Candidiasis, Oral” | 169 | |
| 1# AND 2# | 2 | |
| The Cochrane Library | 1# “chronic kidney disease”, “chronic renal insufficiency”, “renal insufficiency, chronic”, “kidney failure, chronic” | 17,899 |
| 2# “Oral Manifestations”, “Oral Diseases”, “Oral Health”, “Mouth”, “Dental Care”, “Salivation”, “Gingival Diseases”, “Periodontal Diseases”, “Dry Mouth”, “Candidiasis, Oral” | 37,524 | |
| 1# AND 2# | 87 |
| Author and Year | Type of Study | Number of Participants and Comparison | Age | Manifestations Studied | Conclusions |
|---|---|---|---|---|---|
| Almeida et al. (2017) [10] | Transversal | 30 patients with CKD (chronic kidney disease) before and after hemodialysis. 40 healthy patients. There was comparison. | 10–23 | Caries Dental calculus Saliva characteristics | There are significant differences in the salivary composition of patients with CKD treated with dialysis, those without previous dialysis, and healthy patients. Patients with CKD showed a significantly higher amount of dental calculus compared to healthy patients. |
| Dokumacigil et al. (2025) [11] | Case-control | 43 patients with CKD divided into stages (stage 1–3 (n = 14) and stage 4–5 (n = 29)). 40 healthy patients. There was comparison. | 8–17 | Caries Dental calculus | The plaque and calculus indices were significantly higher in healthy patients. Salivary flow was lower in patients with CKD. No significant differences were found regarding caries. |
| Maciejczyk et al. (2020) [12] | Transversal | 30 patients with CKD divided into stages (stage 1–3 and stage 4–5). 30 healthy patients. There was comparison. | 9–17 | Reduction of salivary flow, Xerostomia, Fungal infections, Caries | Salivary flow was significantly lower in patients with CKD, but their salivary pH was significantly higher. |
| Beyer et al. (2025) [13] | Transversal | 86 patients with CKD divided into stage 1–3, stage 4–5, kidney transplant recipient, and nephrotic syndrome. 81 healthy patients. There was comparison. | 4–17 | Caries, Dental plaque, Enamel development defects | CKD and kidney transplant significantly influence plaque and calculus accumulation, as well as the prevalence of enamel development defects. No significant differences were found regarding caries. |
| Caliento et al. (2018) [14] | Transversal | 25 patients with CKD. 25 kidney transplant recipients, 50 healthy patients. There was comparison. | 0–15 | Enamel development defects, Xerostomia, Candidiasis, Pale oral mucosa | Transplant recipients showed more oral manifestations than patients with CKD and healthy patients. Patients with CKD had a higher prevalence of pale oral mucosa and enamel hypoplasia than kidney transplant recipients and healthy patients. |
| Correa et al. (2015) [15] | Transversal | 12 patients with CKD. 12 healthy patients. There was comparison. | 5–24 | Loss of taste | The group with CKD had a significantly lower papillary density and poorer taste sensitivity compared to healthy patients. |
| Muhaxheri et al. (2023) [16] | Transversal | 48 male patients with CKD. 42 female patients with CKD. There was comparison. | >60 | Xerostomia, Caries, Halitosis, Gingival bleeding | Men with CKD had a higher prevalence of halitosis, while in women, the presence of stains was more common. There were no significant differences regarding xerostomia or gingival bleeding. |
| Al-Zaidi et al. (2022) [17] | Transversal | 30 patients with CKD. 30 healthy patients. There was comparison. | 24–72 | Caries, Lost teeth, Filled teeth, Pale oral mucosa, Xerostomia, Candidiasis | The presence of cavities, calculus index, and lost teeth was significantly higher in patients with CKD. No significant differences were observed in the plaque index and gingival index between the CKD group and healthy patients. |
| Abou-Bakr et al. (2022) [18] | Transversal | 263 patients with end-stage renal disease with different stages of periodontitis. There was comparison. | 20–70 | Periodontitis | There was a significant association between the patient’s age and the duration of dialysis with respect to the stage of periodontitis. No significant association was found between sex and the stage of periodontitis. |
| Han et al. (2019) [19] | Cohorts | 4,544,610 patients with CKD divided according to the number of teeth. There was comparison. | >20 | Periodontitis, Lost teeth | The number of lost teeth showed a strong association with end-stage renal disease. |
| Gupta et al. (2018) [20] | Transversal | 30 patients with CKD on dialysis. 30 patients with CKD without prior dialysis 30 healthy patients. There was comparison. | 18–70 | Periodontitis, Gingivitis, Dental Plaque, Dental Calculus, Xerostomia, Enamel development defects | The periodontal status of patients with CKD was worse compared to healthy patients. For example, significant differences were found in the clinical attachment level parameter, but not in the probing depth. |
| Ausavarungnirum et al. (2016) [21] | Transversal | 46 patients with mild CKD, 48 patients with moderate CKD, 35 patients with severe CKD. There was comparison. | 30–86 | Caries, Lost teeth, Filled teeth, Periodontitis | Patients with more severe CKD had more severe periodontal disease than those with less severe CKD. The DMFT index (decayed, missing, and filled teeth) and the number of lost teeth were higher in the moderate CKD group than in healthy patients. However, no significant differences were found regarding sex. |
| Parente et al. (2023) [22] | Case-control | 45 patients with end-stage renal disease. 26 healthy patients. There was comparison. | 30–50 | Gingival bleeding, Dental plaque, Periodontal status | Patients with end-stage renal disease had a significantly higher prevalence of periodontal disease and plaque accumulation. |
| Khalighinejad et al. (2017) [23] | Transversal | 40 patients with end-stage renal disease. 40 healthy patients. There was comparison. | 49–64 | Apical periodontitis | Apical periodontitis was significantly more prevalent in patients with end-stage renal disease. There were no significant differences regarding age, sex, or smoking habit. |
| Pham et al. (2018) [24] | Transversal | 111 patients with CKD, 109 healthy patients. There was comparison. | 30–63 | Caries, Xerostomia | Patients with CKD had a significantly reduced salivary flow rate but a higher level of xerostomia. They also had a significantly higher DMFT index. |
| Honarmand et al. (2017) [25] | Transversal | 30 patients with CKD on dialysis. 30 healthy patients. There was comparison. | 22–58 | Halitosis, Xerostomia, Pale oral mucosa, Gingival bleeding | Halitosis, xerostomia, presence of calculus, and gingival bleeding were significantly more prevalent in patients with CKD than in healthy patients. No significant differences were found regarding the presence of salivary calcium. |
| Oyetola et al. (2015) [26] | Transversal | 90 patients with CKD. 90 healthy patients. There was comparison. | 31–65 | Candidiasis, Xerostomia, Gingival Bleeding, Periodontitis, Halitosis, Lip pigmentation | The prevalence of oral lesions was significantly higher in subjects with CKD than in healthy patients, including abnormal lip pigmentation, halitosis, periodontitis, and candidiasis. Age and sex were not significantly related to the likelihood of developing oral lesions. |
| Yusuf et al. (2021) [27] | Case-control | 100 patients with CKD. 100 healthy patients. There was comparison. | 31–59 | Taste dysfunction | The taste dysfunction was significantly correlated with the increased duration of CKD. Neither age nor the stage of CKD were significantly related to the presence of taste dysfunction. |
| Rosa-García et al. (2020) [28] | Transversal | 119 patients with CKD divided into patients with CKD without the presence of candida, with the presence of candida, and with candidiasis. There was comparison. | 22–87 | Candidiasis, Xerostomia, Pale oral mucosa | The oral cavity of patients with CKD on dialysis is frequently colonized by Candida spp. In addition, its presence is more frequent in woman, smokers, and older individuals. |
| Pieralisi et al. (2016) [29] | Transversal | 33 patients with CKD. There was no comparison. | 18–75 | Candidiasis | Patients with CKD exhibited a high frequency of tongue coating which was frequently colonized by yeasts, especially C. albicans and C. parapsilosis. There were no significant differences regarding age. |
| Gonzales et al. (2024) [30] | Transversal | 21 patients with CKD. There was no comparison. | 29–43 | Candidiasis | A significant relationship was found between oral Candida and CKD. Its presence is significantly associated with woman, smokers, oral hygiene, and age. |
| Luo et al. (2025) [31] | Transversal | 31 non-diabetic patients on hemodialysis. 29 diabetic patients on hemodialysis. 33 healthy patients. There was comparison. | 44–70 | Changes in the lingual microbiome | The lingual coating microbiome was different in patients with CKD compared to healthy patients. |
| Marinoski et al. (2019) [32] | Transversal | 50 patients with CKD on dialysis. 25 patients with CKD without prior dialysis. 25 healthy patients. There was comparison. | 18–82 | Pale oral mucosa, Burning mouth, Xerostomia, Coated tongue, Defects in the oral mucosa | Pre-dialysis patients showed a significantly higher prevalence of oral lesions such as pale mucosa and coated tongue. No significant differences were found related to age or regarding hyposalivation between the pre-dialysis and dialysis groups. |
| Mahay et al. (2024) [33] | Transversal | 150 patients with CKD on dialysis. 150 healthy patients. There was comparison. | 55–57 | Xerostomia, Halitosis, Pale oral mucosa, Coated tongue, Candidiasis, Defects in the oral mucosa, Gingivitis, Periodontitis | Pre-dialysis patients exhibit a high prevalence of various oral lesions compared to healthy patients. An example would be the presence of coated tongue, candidiasis, or angular cheilitis. There are no significant differences regarding the presence of herpes simplex. |
| Kassim et al. (2019) [34] | Transversal | 29 patients with CKD without prior dialysis. 29 healthy patients. There was comparison. | >18 | Candidiasis, Lip pigmentation, Tongue coating, Pale oral mucosa, Xerostomia, Oral ulcers | Pale mucosa is the most common oral manifestation in pre-dialysis patients. Xerostomia, halitosis, and lip pigmentation are also commonly present in these patients. |
| Nylund et al. (2017) [35] | Transversal | 53 patients with CKD. There was no comparison with the other group. | 31–86 | Dental calculus, Lost teeth, Caries, Filled teeth, Candidiasis, salivary flow | A significant decrease was observed in the total dental index (TDI) and the periodontal inflammatory load index from pre-dialysis to follow-up. |
| Limeres et al. (2016) [36] | Transversal | 44 patients with CKD. 44 healthy patients. There was comparison. | 60–70 | Lost teeth | The average number of lost teeth was higher in patients with end-stage renal disease (ESRD) on hemodialysis than in the controls. |
| Cohorts Studies (NOS) | Selection | Comparability | Outcome | Total Score |
|---|---|---|---|---|
| Han et al. [19] |  |  |  | 9 |
| Case-Control Studies (NOS) | Selection | Comparability | Exposure | Total Score |
|---|---|---|---|---|
| Dokumacigl et al. [11] |  |  |  | 6 |
| Parente et al. [22] |  |  |  | 6 |
| Yusuf et al. [27] |  |  |  | 8 |
| Article Title | Clear Inclusion Criteria | Subjects and Setting Described | Exposure Measured Validly | Standard Criteria for Condition | Confounding Factors Identified | Strategies to Deal with Confounding | Outcomes Measured Validly | Appropriate Statistical Analysis | Overall Appraisal | % |
|---|---|---|---|---|---|---|---|---|---|---|
| Almeida et al. (2017) [10] | Partial | Yes | Yes | Yes | Partial | No | Yes | Yes | Include | 62.5 |
| Maciejczyk et al. (2020) [12] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Beyer et al. (2025) [13] | Yes | Yes | Yes | Yes | Yes | No | Partial | Yes | Include | 75 |
| Caliento et al. (2018) [14] | Partial | Yes | Yes | Partial | No | Yes | Yes | Yes | Include | 62.5 |
| Correa et al. (2015) [15] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Muhaxheri et al. (2023) [16] | Yes | Yes | Yes | No | Partial | No | Yes | Yes | Include | 62.5 |
| Al-Zaidi et al. (2022) [17] | Yes | Yes | Yes | Yes | Partial | No | Yes | Yes | Include | 75 |
| Abou-Bakr et al. (2022) [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Gupta et al. (2018) [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Ausavarungnirum et al. (2016) [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Khalighinejad et al. (2017) [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Pham et al. (2018) [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Honarmand et al. (2017) [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Oyetola et al. (2015) [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Rosa-García et al. (2020) [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Pieralisi et al. (2016) [29] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Include | 87.5 |
| Gonzales et al. (2024) [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Luo et al. (2025) [31] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Marinoski et al. (2019) [32] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Mahay et al. (2024) [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Kassim et al. (2019) [34] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Nylund et al. (2017) [35] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Include | 87.5 |
| Limeres et al. (2016) [36] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include | 100 |
| Oral Manifestation | Number of Studies Reporting | % of Total (n = 27) |
|---|---|---|
| Periodontitis | 12 | 44% |
| Xerostomia/Hyposalivation | 11 | 41% |
| Candidiasis | 9 | 33% |
| Enamel defects | 5 | 18% |
| Halitosis | 4 | 15% |
| Pale oral mucosa | 5 | 18% |
| Taste alteration | 3 | 11% |
| Caries | 10 | 37% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rios, P.; Rodríguez-Lozano, F.J.; Pérez-Guzmán, N. Oral Findings Linked to Chronic Kidney Disease: A Comprehensive Systematic Review. J. Clin. Med. 2025, 14, 4380. https://doi.org/10.3390/jcm14124380
García-Rios P, Rodríguez-Lozano FJ, Pérez-Guzmán N. Oral Findings Linked to Chronic Kidney Disease: A Comprehensive Systematic Review. Journal of Clinical Medicine. 2025; 14(12):4380. https://doi.org/10.3390/jcm14124380
Chicago/Turabian StyleGarcía-Rios, Paula, Francisco Javier Rodríguez-Lozano, and Nuria Pérez-Guzmán. 2025. "Oral Findings Linked to Chronic Kidney Disease: A Comprehensive Systematic Review" Journal of Clinical Medicine 14, no. 12: 4380. https://doi.org/10.3390/jcm14124380
APA StyleGarcía-Rios, P., Rodríguez-Lozano, F. J., & Pérez-Guzmán, N. (2025). Oral Findings Linked to Chronic Kidney Disease: A Comprehensive Systematic Review. Journal of Clinical Medicine, 14(12), 4380. https://doi.org/10.3390/jcm14124380








