Advanced Wound Care Strategies in Patients with NSTIs
Abstract
1. Introduction
2. Process of Wound Healing
2.1. Inflammation Stage
2.2. Proliferative Stage
2.3. Remodeling Stage
3. Factors Associated with Poor Wound Healing
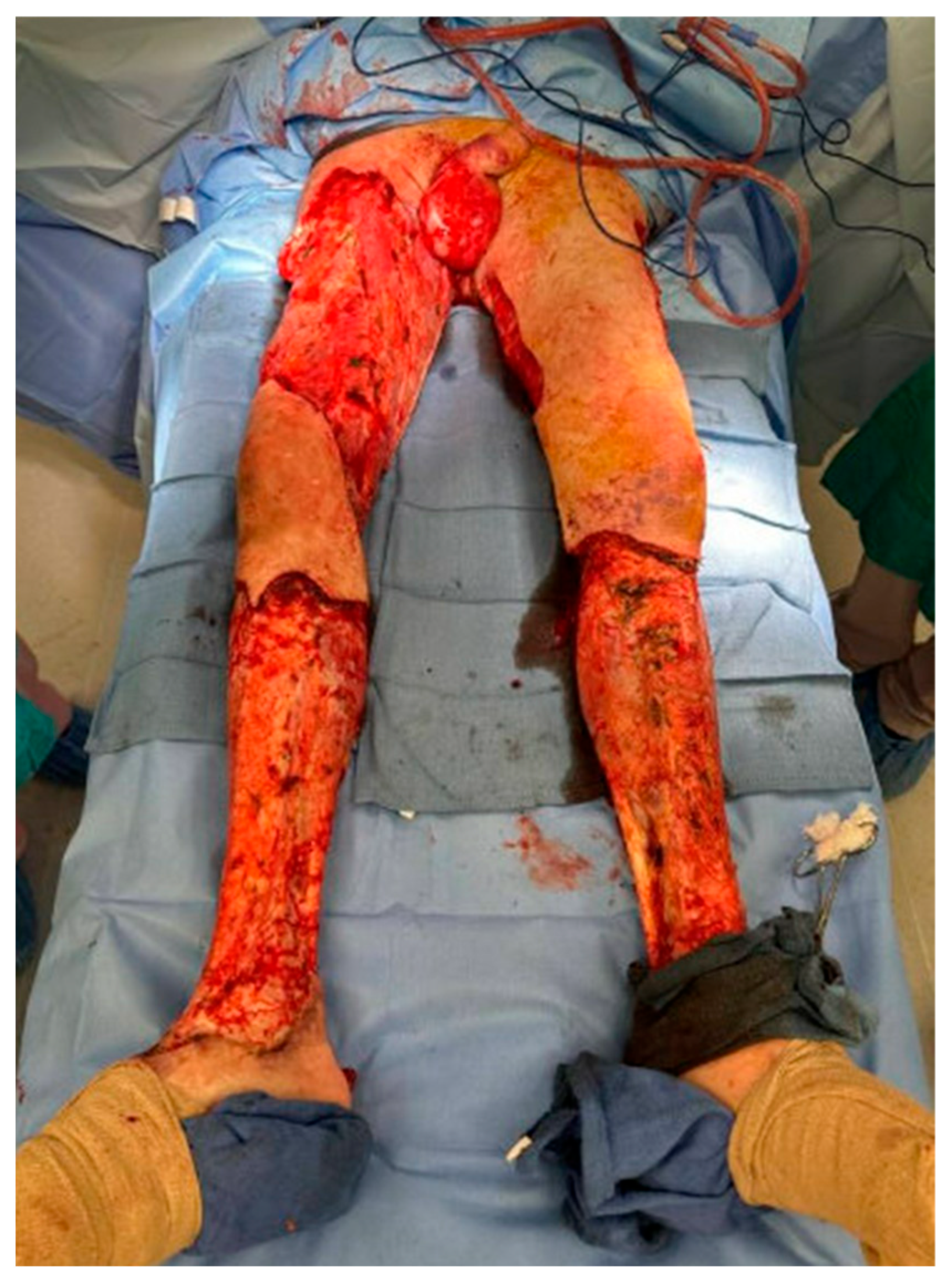
3.1. Infection and Necrotic Tissue
3.2. Perfusion and Ischemia
3.3. Edema
3.4. Diabetes
3.5. Age
3.6. Smoking
3.7. Sepsis and Multiorgan Failure
3.8. Nutrition
4. Wound Management
4.1. Surgical Debridement
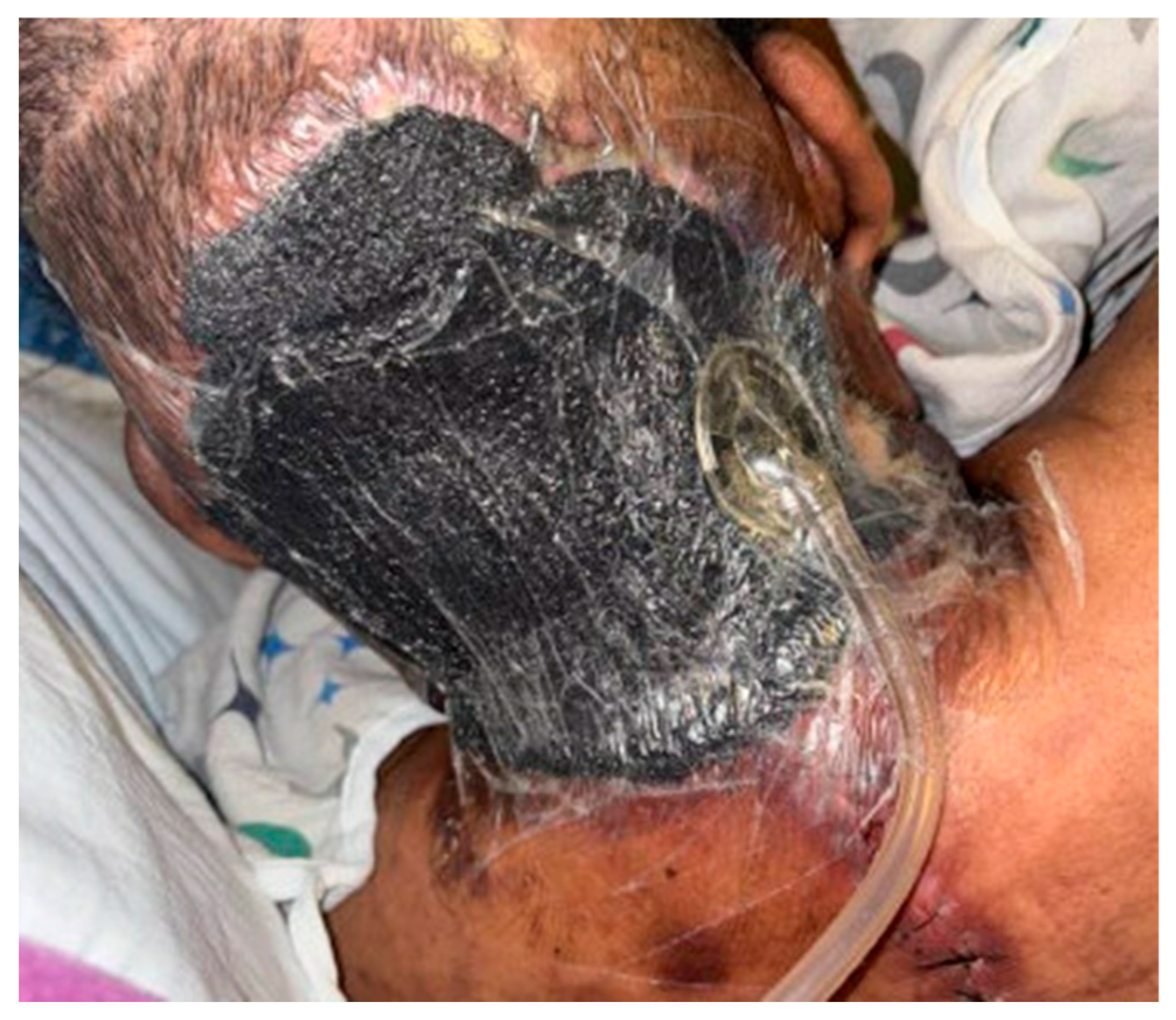
4.2. Wound Dressings
- Protecting wounds from mechanical damage;
- Protecting wounds from infection;
- Addressing dead space;
- Providing a moist wound bed;
- Absorbing excess exudate;
- Minimizing trauma from dressing changes;
- Allowing frequent re-evaluation of the wound;
- Protection of surrounding skin.
4.3. Other Advanced Wound Care Options
5. Reconstructive Surgical Techniques
6. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117 (Suppl. S7), 1e-S–32e-S. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [CrossRef]
- Attinger, C.E.; Janis, J.E.; Steinberg, J.; Schwartz, J.; Al-Attar, A.; Couch, K. Clinical approach to wounds: Débridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg. 2006, 117 (Suppl. S7), 72S–109S. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, A.; LaMuraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J. Vasc. Surg. 2016, 63, 3S–21S. [Google Scholar] [CrossRef] [PubMed]
- Wounds International. Best Practice Recommendations for Prevention and Management of Periwound Skin Complications. Available online: https://woundsinternational.com/best-practice-statements/best-practice-recommendations-prevention-and-management-periwound-skin-complications/ (accessed on 23 April 2025).
- Wounds International. Use of Wound Antiseptics in Practice. Available online: https://woundsinternational.com/consensus-documents/use-of-wound-antiseptics-in-practice/ (accessed on 23 April 2025).
- Wounds International. White Paper–Wound Balance: Achieving Wound Healing with Confidence. Available online: https://woundsinternational.com/best-practice-statements/white-paper-wound-balance-achieving-wound-healing-with-confidence/ (accessed on 23 April 2025).
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection. 2016. Available online: https://iris.who.int/handle/10665/250680 (accessed on 23 April 2025).
- Duane, T.M.; Huston, J.M.; Collom, M.; Beyer, A.; Parli, S.; Buckman, S.; Shapiro, M.; McDonald, A.; Diaz, J.; Tessier, J.M.; et al. Surgical Infection Society 2020 Updated Guidelines on the Management of Complicated Skin and Soft Tissue Infections. Surg. Infect. 2021, 22, 383–399. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Nutrition in Wound Care Management: A Comprehensive Overview. HMP Global Learning Network. December 10, 2015. Available online: https://www.hmpgloballearningnetwork.com/site/wounds/reviews/nutrition-wound-care-management-comprehensive-overview (accessed on 23 February 2025).
- Timing of Operative Debridement for Necrotizing Soft Tissue-Practice Management Guideline. Available online: https://www.east.org/education-resources/practice-management-guidelines/details/timing-of-operative-debridement-for-necrotizing-soft-tissue (accessed on 10 April 2025).
- Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America | Clinical Infectious Diseases|Oxford Academic. Available online: https://academic.oup.com/cid/article/59/2/e10/2895845?login=false (accessed on 8 April 2025).
- Sartelli, M.; Malangoni, M.A.; May, A.K.; Viale, P.; Kao, L.S.; Catena, F.; Ansaloni, L.; Moore, E.E.; Moore, F.A.; Peitzman, A.B.; et al. World Society of Emergency Surgery (WSES) guidelines for management of skin and soft tissue infections. World J. Emerg. Surg. 2014, 9, 57. [Google Scholar] [CrossRef]
- World Health Organization. Summary of a Systematic Review on Advanced Dressings. In Global Guidelines for the Prevention of Surgical Site Infection; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536417/ (accessed on 21 April 2025).
- Bhoyar, S.D.; Malhotra, K.; Madke, B. Dressing Materials: A Comprehensive Review. J. Cutan. Aesthet. Surg. 2023, 16, 81–89. [Google Scholar] [CrossRef]
- World Union of Wound Healing Societies (WUWHS) Consensus Document. Wound Exudate: Effective Assessment and Management; Wounds International: London, UK, 2019. [Google Scholar]
- Dumville, J.C.; O’Meara, S.; Deshpande, S.; Speak, K. Hydrogel dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev. 2013, 2013, CD009101. [Google Scholar] [CrossRef]
- Dumville, J.C.; Stubbs, N.; Keogh, S.J.; Walker, R.M.; Liu, Z. Hydrogel Dressings for Treating Pressure Ulcers-Dumville, JC-2015|Cochrane Library. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011226.pub2/full (accessed on 22 April 2025).
- Dumville, J.C.; Deshpande, S.; O’Meara, S.; Speak, K. Foam dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev. 2013, CD009111. [Google Scholar] [CrossRef]
- Trial, C.; Darbas, H.; Lavigne, J.P.; Sotto, A.; Simoneau, G.; Tillet, Y.; Toét, L. Assessment of the antimicrobial effectiveness of a new silver alginate wound dressing: A RCT. J. Wound Care 2010, 19, 20–26. [Google Scholar] [CrossRef]
- Labib, A.; Winters, R. Complex Wound Management. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK576385/ (accessed on 22 April 2025).
- Rosin, N.R.; Tabibi, R.S.; Trimbath, J.D.; Henzel, M.K. A Primary Care Provider’s Guide to Prevention and Management of Pressure Injury and Skin Breakdown in People with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2020, 26, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Münter, K.C.; Lázaro-Martínez, J.L.; Kanya, S.; Sawade, L.; Schwenke, C.; Pegalajar-Jurado, A.; Swanson, T.; Leaper, D. Clinical efficacy and safety of a silver ion-releasing foam dressing on hard-to-heal wounds: A meta-analysis. J. Wound Care 2024, 33, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Antony, J.; Vafaei, A.; Khan, P.A.; Harrington, A.; Cogo, E.; Wilson, C.; Perrier, L.; Hui, W.; Straus, S.E. Seeking effective interventions to treat complex wounds: An overview of systematic reviews. BMC Med. 2015, 13, 89. [Google Scholar] [CrossRef]
- Bergin, S.M.; Wraight, P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2006, CD005082. [Google Scholar] [CrossRef] [PubMed]
- Storm-Versloot, M.N.; Vos, C.G.; Ubbink, D.T.; Vermeulen, H. Topical silver for preventing wound infection. Cochrane Database Syst Rev. 2010, CD006478. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Y.; Jiang, F. Analysis of therapeutic effect of silver-based dressings on chronic wound healing. Int. Wound J. 2024, 21, e70006. [Google Scholar] [CrossRef]
- Luo, Y.; Li, L.; Zhao, P.; Yang, C.; Zhang, J. Effectiveness of silver dressings in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. J. Wound Care 2022, 31, 979–986. [Google Scholar] [CrossRef]
- Capobianco, C.M.; Zgonis, T. An overview of negative pressure wound therapy for the lower extremity. Clin. Podiatr. Med. Surg. 2009, 26, 619–631. [Google Scholar] [CrossRef]
- Morykwas, M.J.; Argenta, L.C.; Shelton-Brown, E.I.; McGuirt, W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann. Plast. Surg. 1997, 38, 553–562. [Google Scholar] [CrossRef]
- Afzal, H.; Dawson, E.; Fonseca, R.; Canas, M.; Diaz, L.; Filippis, A.D.; Mazuski, J.; Bochicchio, K.M.; Bochicchio, G.V. Negative Pressure Wound Therapy with and Without Instillation in Necrotizing Soft Tissue Infections. Surg. Infect. 2024, 25, 199–205. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.M.; Barnes, S.; Nagel, M.; Fudge, M.; Lucius, A.; Garcia, B. Cost-effectiveness of negative pressure wound therapy for postsurgical patients in long-term acute care. Adv. Skin Wound Care 2009, 22, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2017, 377, 2253–2265. Available online: https://www.nejm.org/doi/full/10.1056/NEJMra1600673 (accessed on 15 February 2025). [CrossRef] [PubMed]
- Huang, C.; Zhong, Y.; Yue, C.; He, B.; Li, Y.; Li, J. The effect of hyperbaric oxygen therapy on the clinical outcomes of necrotizing soft tissue infections: A systematic review and meta-analysis. World J. Emerg. Surg. 2023, 18, 23. [Google Scholar] [CrossRef]
- Wilkinson, D.; Doolette, D. Hyperbaric Oxygen Treatment and Survival from Necrotizing Soft Tissue Infection. Arch. Surg. 2004, 139, 1339–1345. [Google Scholar] [CrossRef]
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb. Med. 2017, 47, 24–32. [Google Scholar] [CrossRef]
- Hedetoft, M.; Bennett, M.H.; Hyldegaard, O. Adjunctive hyperbaric oxygen treatment for necrotising soft-tissue infections: A systematic review and meta-analysis. Diving Hyperb. Med. 2021, 51, 34–43. [Google Scholar] [CrossRef]
- Levett, D.; Bennett, M.H.; Millar, I. Adjunctive hyperbaric oxygen for necrotizing fasciitis. Cochrane Database Syst. Rev. 2015, 1, CD007937. [Google Scholar] [CrossRef]
- Bentaleb, M.; Abdulrahman, M.; Ribeiro, M.A.F., Jr. Nanomedicine and Its Role in Surgical Wound Infections: A Practical Approach. Bioengineering 2025, 12, 137. [Google Scholar] [CrossRef]
- Talbot, S.G.; Pribaz, J.J. Sophisticated surgical solutions for complex wound problems. Clin. Plast. Surg. 2012, 39, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Simman, R. Wound Closure and the Reconstructive Ladder in Plastic Surgery. J. Am. Col. Certif. Wound Spec. 2009, 1, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bay, C.; Chizmar, Z.; Reece, E.M.; Yu, J.Z.; Winocour, J.; Vorstenbosch, J.; Winocour, S. Comparison of Skin Substitutes for Acute and Chronic Wound Management. Semin. Plast. Surg. 2021, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Struble, S.L.; Patel, N.K.; Graham, E.M.; Tipps, J.A.; Vaile, J.R.; Leeflang, E.J.; Goodwin, I.; Mendenhall, S.D. Outcomes of Biodegradable Temporizing Matrix for Soft Tissue Reconstruction of the Hand and Extremities. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5956. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering. 2018, 5, 51. [Google Scholar] [CrossRef]
- Taupin, P.; Gandhi, A.; Saini, S. Integra® Dermal Regeneration Template: From Design to Clinical Use. Cureus 2023, 15, e38608. [Google Scholar] [CrossRef]
- Dardari, D.; Piaggesi, A.; Potier, L.; Sultan, A.; Diener, H.; Francois, M.; Dorweiler, B.; Bouillet, B.; M’bemba, J.; Chaillous, L.; et al. Intact Fish Skin Graft to Treat Deep Diabetic Foot Ulcers. NEJM Evid. 2024, 3, EVIDoa2400171. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, T.; Clark, J. Advanced Wound Care Strategies in Patients with NSTIs. J. Clin. Med. 2025, 14, 3514. https://doi.org/10.3390/jcm14103514
Miller T, Clark J. Advanced Wound Care Strategies in Patients with NSTIs. Journal of Clinical Medicine. 2025; 14(10):3514. https://doi.org/10.3390/jcm14103514
Chicago/Turabian StyleMiller, Taylor, and Jaclyn Clark. 2025. "Advanced Wound Care Strategies in Patients with NSTIs" Journal of Clinical Medicine 14, no. 10: 3514. https://doi.org/10.3390/jcm14103514
APA StyleMiller, T., & Clark, J. (2025). Advanced Wound Care Strategies in Patients with NSTIs. Journal of Clinical Medicine, 14(10), 3514. https://doi.org/10.3390/jcm14103514




