Adverse Drug Reactions Associated with Concomitant Use of Calcium Channel Blockers and Cocaine: An Analysis of FDA Adverse Events Reporting System Data
Abstract
1. Introduction
Aim of the Study
2. Methods
2.1. Study Design and Data Collection
2.2. Descriptive and Exploratory Analyses
2.3. Correlation and Predictive Modeling
2.4. Dimensionality Reduction and Clustering
2.5. Ethical Considerations
3. Results
- One cluster was characterized by high rates of opioid and alcohol co-use.
- The second cluster was dominated by suicide-related ADRs.
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ANSA.it. Con la Droga Sequestrati Anti-Ipertensivi, Allarme a Cuneo. 2 March 2025. Available online: https://www.ansa.it/piemonte/notizie/2025/03/02/con-la-droga-sequestrati-anti-ipertensivi-allarme-a-cuneo_29d72eb9-3a22-45b0-a340-f1d6133724ba.html (accessed on 4 April 2025).
- Davis, S.; Zhu, J. Substance abuse and neurotransmission. Adv. Pharmacol. 2022, 93, 403–441. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 6583–6588. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. The neurobiology of cocaine addiction. Sci. Pract. Perspect. 2005, 3, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.R.; Hollander, J.E.; Ramoska, E.A.; Fareed, F.N.; Sand, I.C.; Izquierdo Gómez, M.M.; Lange, R.A. β-Blockers, Cocaine, and the Unopposed α-Stimulation Phenomenon. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 239–249. [Google Scholar] [CrossRef]
- Kudlacek, O.; Hofmaier, T.; Luf, A.; Mayer, F.P.; Stockner, T.; Nagy, C.; Holy, M.; Freissmuth, M.; Schmid, R.; Sitte, H.H. Cocaine adulteration. J. Chem. Neuroanat. 2017, 83–84, 75–81. [Google Scholar] [CrossRef]
- Mumba, M.N.; Tice, J.; Brown, W. Xylazine: The Drug Taking the World By Storm: What You Need to Know. J. Psychosoc. Nurs. Ment. Health Serv. 2023, 61, 7–10. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Sall, S.; Upshaw, W.C.; Spillers, N.J.; Vincik, L.Y.; De Witt, A.S.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Xylazine: A Drug Adulterant of Clinical Concern. Curr. Pain Headache Rep. 2024, 28, 417–426. [Google Scholar] [CrossRef]
- Yuan, T.H.; Kerns, W.P.; Tomaszewski, C.A.; Ford, M.D.; Kline, J.A.; Kline, J. Insulin-Glucose as Adjunctive Therapy for Severe Calcium Channel Antagonist Poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 463–474. [Google Scholar] [CrossRef]
- Bronstein, A.C.; Spyker, D.A.; Cantilena, L.R.; Green, J.L.; Rumack, B.H.; Giffin, S.L. 2009 Annual Report of the American Association Of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin. Toxicol. 2010, 48, 979–1178. [Google Scholar] [CrossRef]
- Bronstein, A. 2011 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th Annual Report. Clin. Toxicol. 2012, 50, 911–1164. [Google Scholar] [CrossRef]
- Mowry, J. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin. Toxicol. 2013, 51, 949–1229. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Spyker, D.A.; Brooks, D.E.; Beuhler, M.C.; Rivers, L.J.; Hashem, H.A.; Ryan, M.L. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin. Toxicol. 2019, 57, 1220–1413. [Google Scholar] [CrossRef] [PubMed]
- Mowry, J.B.; Spyker, D.A.; Brooks, D.E.; Zimmerman, A.; Schauben, J.L. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin. Toxicol. 2016, 54, 924–1109. [Google Scholar] [CrossRef]
- UNODC. World Drug Report 2024 (United Nations Publication, 2024); UNODC: Vienna, Austria, 2024. [Google Scholar]
- Chiappini, S.; Schifano, F. What about “Pharming”? Issues Regarding the Misuse of Prescription and Over-the-Counter Drugs. Brain Sci. 2020, 10, 736. [Google Scholar] [CrossRef]
- Chiappini, S.; Vickers-Smith, R.; Guirguis, A.; Corkery, J.M.; Martinotti, G.; Schifano, F. A Focus on Abuse/Misuse and Withdrawal Issues with Selective Serotonin Reuptake Inhibitors (SSRIs): Analysis of Both the European EMA and the US FAERS Pharmacovigilance Databases. Pharmaceuticals 2022, 15, 565. [Google Scholar] [CrossRef]
- Vitcheva, V.; Simeonova, R.; Karova, D.; Mitcheva, M. Nifedipine lowers cocaine-induced brain and liver enzyme activity and cocaine urinary excretion in rats. Arh. Za Hig. Rada I Toksikol. 2011, 62, 131–137. [Google Scholar] [CrossRef]
- Schindler, C.W.; Tella, S.R.; Prada, J.; Goldberg, S.R. Calcium channel blockers antagonize some of cocaine’s cardiovascular effects, but fail to alter cocaine’s behavioral effects. J. Pharmacol. Exp. Ther. 1995, 272, 791–798. [Google Scholar] [CrossRef]
- Ansah, T.A.; Wade, L.H.; Kopsombut, P.; Shockley, D.C. Nifedipine potentiates the toxic effects of cocaine in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 357–362. [Google Scholar] [CrossRef]
- Derlet, R.W.; Albertson, T.E. Potentiation of cocaine toxicity with calcium channel blockers. Am. J. Emerg. Med. 1989, 7, 464–468. [Google Scholar] [CrossRef]
- Leenen, F.H.; Ruzicka, M.; Huang, B.S. Central sympathoinhibitory effects of calcium channel blockers. Curr. Hypertens. Rep. 2001, 3, 314–321. [Google Scholar] [CrossRef]
- Yeo, K.R.; Yeo, W.W. Inhibitory effects of verapamil and diltiazem on simvastatin metabolism in human liver microsomes. Br. J. Clin. Pharmacol. 2001, 51, 461–470. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.; Jneid, H.; Hollander, J.E.; de Lemos, J.A.; Cercek, B.; Hsue, P.; Gibler, W.B.; Ohman, E.M.; Drew, B.; Philippides, G.; et al. Management of cocaine-associated chest pain and myocardial infarction: A scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 2008, 117, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Alawoè, C.; Chapet, N.; Roubille, F.; Peyrière, H.; Eiden, C. Narrative Review of Heart Failure Related to Cocaine Consumption and Its Therapeutic Management. J. Clin. Med. 2024, 13, 7275. [Google Scholar] [CrossRef]
- Darke, S.; Duflou, J.; Peacock, A.; Chrzanowska, A.; Farrell, M.; Lappin, J. Clinical characteristics of fatal cocaine toxicity in Australia, 2000–2021. Drug Alcohol Rev. 2023, 42, 582–591. [Google Scholar] [CrossRef]
- Hsue, P.Y.; McManus, D.; Selby, V.; Ren, X.; Pillutla, P.; Younes, N.; Goldschlager, N.; Waters, D.D. Cardiac arrest in patients who smoke crack cocaine. Am. J. Cardiol. 2007, 99, 822–824. [Google Scholar] [CrossRef]
- Verapamil. Available online: https://www.medicines.org.uk/emc/product/10613/smpc (accessed on 7 May 2025).
- Diltiazem. Available online: https://www.medicines.org.uk/emc/product/7583/smpc (accessed on 7 May 2025).
- Chiappini, S.; Miuli, A.; Mosca, A.; Pettorruso, M.; Guirguis, A.; John, M.C.; Martinotti, G.; Di Giannantonio, M.; Schifano, F. The Benzydamine Experience: A Systematic Review of Benzydamine Abuse. Curr. Neuropharmacol. 2021, 19, 1728–1737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

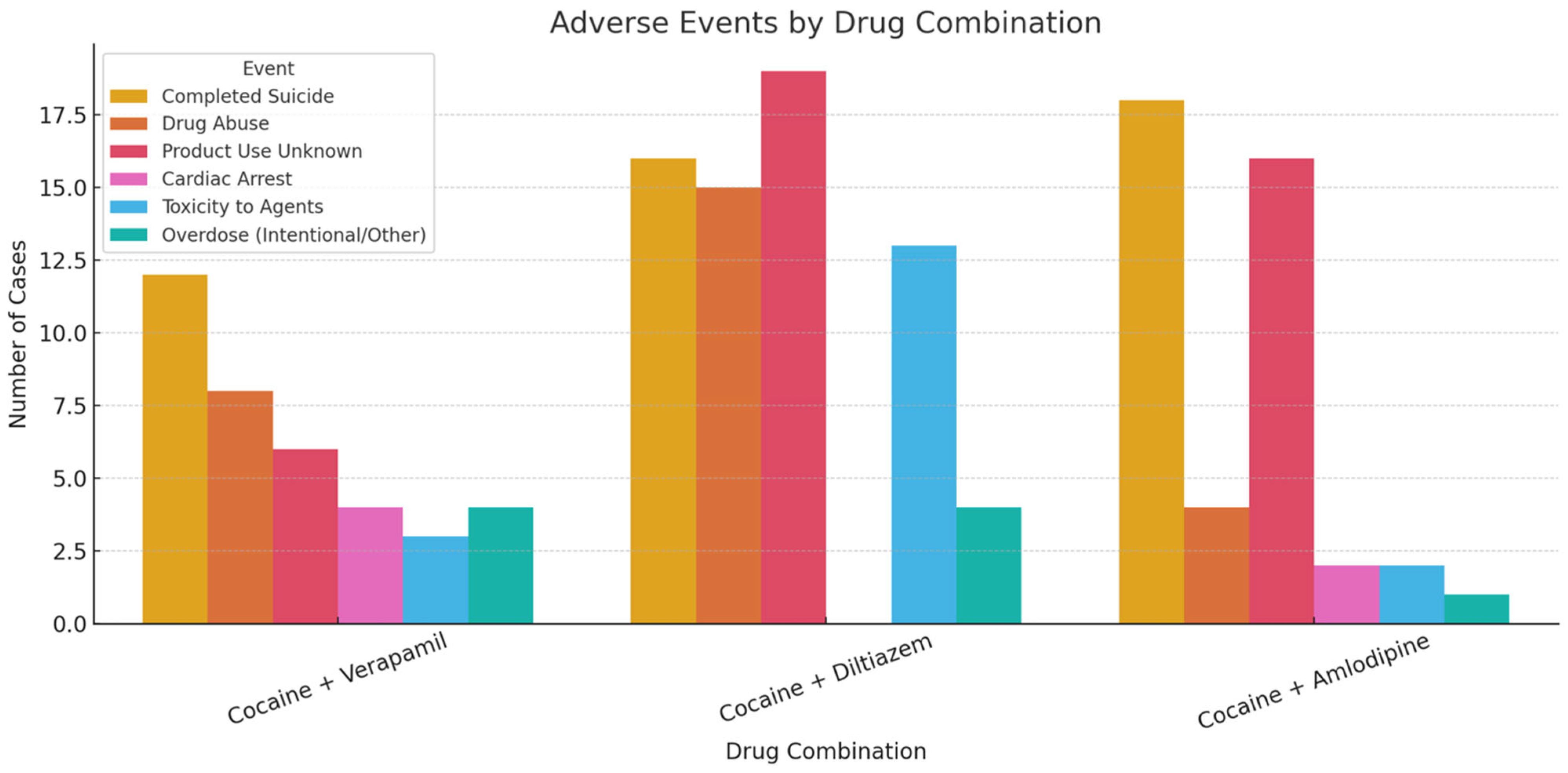
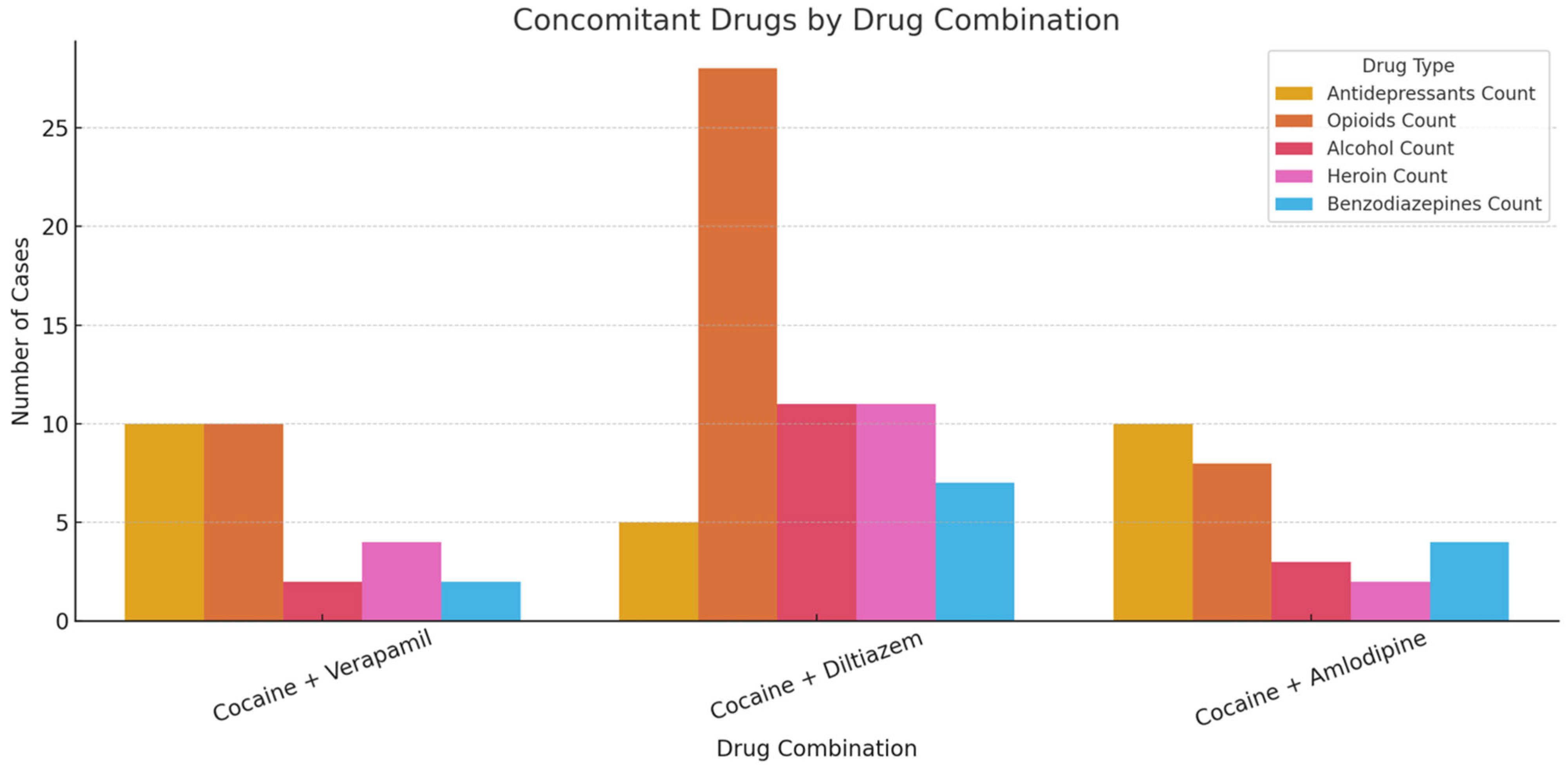
| Cocaine + Verapamil (Tot. Cases: 19) | Cocaine + Diltiazem (Tot. Cases: 30) | Cocaine + Amlodipine (Tot. Cases: 18) | |
|---|---|---|---|
| AGE (years) | 0–25: 0 26–40: 1 41–65: 11 >65: 1 Not Specified: 5 | 0–25: 2 26–40: 4 41–65: 10 >65: 2 Not Specified: 12 | 0–25: 0 26–40: 3 41–65: 7 >65: 2 Not Specified: 6 |
| SEX (F/M) | 5/10 Not Specified 4 | 4/21 Not Specified 5 | 8/5 Not Specified 5 |
| OUTCOME | Fatal 18 Life Threatening 1 | Fatal 29 Other 1 | Fatal 18 |
| ADVERSE DRUG REACTIONS | Completed suicide 12 Drug abuse 8 Product use for unknown indication 6 Cardiac arrest 4 Toxicity to various agents 3 Suicide attempt 2 Overdose/Intentional overdose 4 Respiratory arrest 2 | Product use for unknown indication 19 Completed suicide 16 Drug abuse 15 Toxicity to various agents 13 Overdose 4 Intentional drug misuse 3 Homicide 1 Suspected suicide 1 Suicidal ideation 1 Accidental death 1 Poisoning 1 | Completed suicide 18 Product use for unknown indication 16 Drug abuse 4 Product use issue 1 Cardiac arrest 2 Homicide 1 Toxicity to various agents 2 Overdose 1 Substance abuse 1 |
| COUNTRY | United States 13 Germany 1 Not Specified 5 | United States 15 Germany 3 France 1 South Africa 1 Not Specified 16 | United States 15 South Africa 1 Germany 1 Not Specified 1 |
| REPORTER | Healthcare professional 14 Consumer 1 Not Specified 2 | Healthcare professional 26 Not Specified 4 | Healthcare professional 18 |
| CASES WITHOUT CONCOMITANT DRUGS | 3 | 1 | 5 |
| CONCOMITANT DRUGS | Antidepressant drugs (Bupropion, Citalopram, Venlafaxine, Trazodone, Amitriptyline, Mirtazapine) 10 Opioids (Fentanyl, Hydrocodone, Codeine, Methadone, Oxycodone, Tramadol) 10 Acetaminophen/Paracetamol 6 Heroin 4 Antipsychotic medications (Risperidone, Quetiapine, Doxepin): 3 Antihistamine drugs (Diphenhydramine, Promethazine) 2 Alcohol 2 Z-drugs (Zolpidem): 2 Mood stabilizers (Lamotrigine) 1 Benzodiazepines (Alprazolam) 2 Ketamine 1 | Opioids (Codeine, Fentanyl, Tramadol, Methadone, Hydrocodone, Oxycodone) 28 Alcohol 11 Heroin 11 Acetaminophen/Paracetamol 8 Antihistamine drugs (Diphenhydramine, Cetirizine, Promethazine) 7 Benzodiazepines (Alprazolam, Clonazepam, Diazepam, Midazolam) 7 Antidepressant drugs (Clomipramine, Fluoxetine, Nortriptyline, Trazodone) 5 Amphetamine/Methamphetamine 4 Dextromethorphan 3 THC 3 Mood stabilizers (Gabapentin, Topiramate) 1 Pseudoephedrine 1 Antipsychotic medications (Risperidone) 1 | Antidepressant drugs (Amitriptyline, Citalopram, Duloxetine, Clomipramine, Fluoxetine, Nortriptyline, Paroxetine, Trazodone, Venlafaxine) 10 Antihistamine drugs (Diphenhydramine, Promethazine) 4 Mood stabilizers (Gabapentin, Topiramate) 3 Opioids (Codeine, Fentanyl, Tramadol) 8 Benzodiazepines (Alprazolam, Clonazepam, Lorazepam) 4 Acetaminophen/Paracetamol 4 THC 3 Alcohol 3 Heroin 2 Antipsychotic medications (Quetiapine, Risperidone) 2 Pseudoephedrine 1 Amphetamine/Methamphetamine 2 Zolpidem 1 |
| Cocaine + Verapamil (1/19 Cases) | Cocaine + Diltiazem (1/30) | |
|---|---|---|
| OUTCOME | Life-threatening | Other |
| ACTIVE INGREDIENTS | Cocaine; Verapamil Hydrochloride; Alcohol | Fentanyl; Hydroxyzine Hydrochloride; Tramadol; Codeine; Prazepam; Tadalafil; Morphine; Benzoylecgonine; Diamorphine; Levamisole; Methadone Hydrochloride; Amphetamine; Ketamine; Cocaine; Sildenafil; Diltiazem; Lidocaine; Amitriptyline; Etifoxine; Nortriptyline; Cetirizine Hydrochloride; Methamphetamine; Dextromethorphan |
| REACTIONS | Suicide Attempt; Toxicity To Various Agents; Alcohol Poisoning; Hypomagnesaemia; Hypophosphataemia; Hypotension; Drug Interaction; Lethargy; Vomiting; Opiates Positive; Hypokalaemia | Drug Abuse; Toxicity to Various Agents; Product Use for Unknown Indication |
| SEX | Female | Male |
| AGE (years) | 36 | 42 |
| SENDER | Recro Pharma | Johnson And Johnson |
| REPORTER | Healthcare Professional | Healthcare professional |
| COUNTRY | United States | France |
| Ref. | Yuan et al., 1999 [9] | - |
| Cocaine + Verapamil (3/19 Cases) | Cocaine + Diltiazem (1/30) | Cocaine + Amlodipine (5/18) | |
|---|---|---|---|
| OUTCOME | Died (3) | Died | Died (5) |
| REACTIONS | Completed Suicide (3) | Completed Suicide | Completed Suicide (5) Other Outcomes (1) Toxicity to Various Agents (3) Product Used for Unknown Indication (1) Cardiac Arrest (1) Respiratory Arrest (1) Ill-Defined Disorder (1) Drug Abuser (1) |
| SEX | Female (1) Male (2) | Male | Male (2) Not Specified (3) |
| AGE (years) | 50 (1), 53 (1), 65 (1) | 47 | 29 (1), 38 (1), 52 (2), 59 (1) |
| SENDER | Pfizer (2) Novartis (1) | Apotex | Apotex (1) Aurobindo (1) Pfizer (2) Roxane (1) |
| REPORTER | Healthcare Professional (3) | Healthcare Professional | Healthcare Professional (5) |
| COUNTRY | United States (3) | Not Specified | United States (3) Not Specified (2) |
| Ref. | Bronstein et al., 2010 [10] Bronstein 2012 [11] Mowry 2013 [12] | - | Gummin et al., 2019 [13] Mowry et al., 2016 [14] -(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, S.; Mosca, A.; Papanti Pelletier, D.G.; Corkery, J.M.; Guirguis, A.; Arillotta, D.; Martinotti, G.; Schifano, F. Adverse Drug Reactions Associated with Concomitant Use of Calcium Channel Blockers and Cocaine: An Analysis of FDA Adverse Events Reporting System Data. J. Clin. Med. 2025, 14, 3461. https://doi.org/10.3390/jcm14103461
Chiappini S, Mosca A, Papanti Pelletier DG, Corkery JM, Guirguis A, Arillotta D, Martinotti G, Schifano F. Adverse Drug Reactions Associated with Concomitant Use of Calcium Channel Blockers and Cocaine: An Analysis of FDA Adverse Events Reporting System Data. Journal of Clinical Medicine. 2025; 14(10):3461. https://doi.org/10.3390/jcm14103461
Chicago/Turabian StyleChiappini, Stefania, Alessio Mosca, Duccio G. Papanti Pelletier, John M. Corkery, Amira Guirguis, Davide Arillotta, Giovanni Martinotti, and Fabrizio Schifano. 2025. "Adverse Drug Reactions Associated with Concomitant Use of Calcium Channel Blockers and Cocaine: An Analysis of FDA Adverse Events Reporting System Data" Journal of Clinical Medicine 14, no. 10: 3461. https://doi.org/10.3390/jcm14103461
APA StyleChiappini, S., Mosca, A., Papanti Pelletier, D. G., Corkery, J. M., Guirguis, A., Arillotta, D., Martinotti, G., & Schifano, F. (2025). Adverse Drug Reactions Associated with Concomitant Use of Calcium Channel Blockers and Cocaine: An Analysis of FDA Adverse Events Reporting System Data. Journal of Clinical Medicine, 14(10), 3461. https://doi.org/10.3390/jcm14103461










