Association Between Oral Dysbiosis and Alzheimer’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Article Selection: Inclusion and Exclusion Criteria
- Population: Patients with Alzheimer’s disease.
- Exposure: Altered oral microbiota (dysbiosis);
- Comparison/Control: Healthy individuals.
- Outcome: Relationship between altered oral dysbiosis and Alzheimer’s disease in patients.
2.2. Search Strategy
2.2.1. Information Sources
2.2.2. Search Terms
2.2.3. Study Selection
2.2.4. Quality Assessment
- 1.
- Were the groups comparable other than the presence of disease in cases or the absence of disease in controls?
- 2.
- Were cases and controls matched appropriately?
- 3.
- Were the same criteria used for identification of cases and controls?
- 4.
- Was exposure measured in a standard, valid and reliable way?
- 5.
- Was exposure measured in the same way for cases and controls?
- 6.
- Were confounding factors identified?
- 7.
- Were strategies to deal with confounding factors stated?
- 8.
- Were outcomes assessed in a standard, valid and reliable way for cases and controls?
- 9.
- Was the exposure period of interest long enough to be meaningful?
- 10.
- Was appropriate statistical analysis used?
- Low risk: 9–10 items
- Moderate risk: 6–8 items
- High risk: 0–5 items
- 1.
- Were the criteria for inclusion in the sample clearly defined?
- 2.
- Were the study subjects and the setting described in detail?
- 3.
- Was the exposure measured in a valid and reliable way?
- 4.
- Were objective, standard criteria used for measurement of the condition?
- 5.
- Were confounding factors identified?
- 6.
- Were strategies to deal with confounding factors stated?
- 7.
- Were the outcomes measured in a valid and reliable way?
- 8.
- Was appropriate statistical analysis used?
- 1–4 High
- 5–6 Moderate
- 7–8 Low
2.2.5. Data Extraction
3. Results
3.1. Article Selection and Flow Diagram
3.2. Quality Assessment Results of the Included Studies
3.3. Data Extraction Results
3.4. Bibliometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BBB | Blood–brain barrier |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| PG | Porphyromonas gingivalis |
References
- Bello-Corral, L.; Alves-Gomes, L.; Fernández-Fernández, J.A.; Fernández-García, D.; Casado-Verdejo, I.; Sánchez-Valdeón, L. Implications of gut and oral microbiota in neuroinflammatory responses in Alzheimer’s disease. Life Sci. 2023, 333, 122132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mi, N.; Ying, Z.; Lin, X.; Jin, Y. Advances in the prevention and treatment of Alzheimer’s disease based on oral bacteria. Front. Psychiatry 2023, 14, 1291455. [Google Scholar] [CrossRef]
- Fernandes, G.V.O.; Mosley, G.A.; Ross, W.; Dagher, A.; Martins, B.; Fernandes, J.C.H. Revisiting Socransky’s Complexes: A Review Suggesting Updated New Bacterial Clusters (GF-MoR Complexes) for Periodontal and Peri-Implant Diseases and Conditions. Microorganisms 2024, 12, 2214. [Google Scholar] [CrossRef]
- Giordano-Kelhoffer, B.; Lorca, C.; March Llanes, J.; Rábano, A.; Del Ser, T.; Serra, A.; Gallart-Palau, X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines 2022, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Maurotto, M.; Costa, L.G.; Manso, M.C.; Mosley, G.A.; Fernandes, J.C.H.; Fernandes, G.V.O.; Castro, F. Correlation between Periodontitis and Gastritis Induced by Helicobacter pylori: A Comprehensive Review. Microorganisms 2024, 12, 1579. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, D.K.; Allen, S.J. The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 725–738. [Google Scholar] [CrossRef]
- Sureda, A.; Daglia, M.; Arguelles Castilla, S.; Sanadgol, N.; Fazel Nabavi, S.; Khan, H.; Belwal, T.; Jeandet, P.; Marchese, A.; Pistollato, F.; et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef] [PubMed]
- Maitre, Y.; Micheneau, P.; Delpierre, A.; Mahalli, R.; Guerin, M.; Amador, G.; Denis, F. Did the Brain and Oral Microbiota Talk to Each Other? A Review of the Literature. J. Clin. Med. 2020, 9, 3876. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Xiong, W.; Luo, Y.; Feng, H.; Zhou, H.; Peng, Y.; He, Y.; Ye, Q. The oral-brain axis: Can periodontal pathogens trigger the onset and progression of Alzheimer’s disease? Front. Microbiol. 2024, 15, 1358179. [Google Scholar] [CrossRef]
- Narengaowa; Kong, W.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Weber, C.; Dilthey, A.; Finzer, P. The role of microbiome-host interactions in the development of Alzheimer’s disease. Front. Cell Infect. Microbiol. 2023, 13, 1151021. [Google Scholar] [CrossRef]
- Plachokova, A.S.; Gjaltema, J.; Hagens, E.R.C.; Hashemi, Z.; Knüppe, T.B.A.; Kootstra, T.J.M.; Visser, A.; Bloem, B.R. Periodontitis: A Plausible Modifiable Risk Factor for Neurodegenerative Diseases? A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 4504. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Asadinejad, S.M.; Kakavand, D.; Jafari Doudaran, P.; Fathi, A.H. Association of Periodontitis and Aging-Related Diseases: A Review of Mechanistic Studies. J. Res. Dent. Maxillofac. Sci. 2023, 8, 62–70. [Google Scholar] [CrossRef]
- Orr, M.E.; Reveles, K.R.; Yeh, C.K.; Young, E.H.; Han, X. Can oral health and oral-derived biospecimens predict progression of dementia? Oral. Dis. 2020, 26, 249–258. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, S.; Zhang, W.; Qu, J.; Liu, G.H. Unraveling brain aging through the lens of oral microbiota. Neural Regen. Res. 2025, 20, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef]
- Sritana, N.; Phungpinij, A. Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2024, 21, 1242. [Google Scholar] [CrossRef]
- Holmer, J.; Aho, V.; Eriksdotter, M.; Paulin, L.; Pietiäinen, M.; Auvinen, P.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Subgingival microbiota in a population with and without cognitive dysfunction. J. Oral. Microbiol. 2021, 13, 1854552. [Google Scholar] [CrossRef]
- Taati Moghadam, M.; Amirmozafari, N.; Mojtahedi, A.; Bakhshayesh, B.; Shariati, A.; Masjedian Jazi, F. Association of perturbation of oral bacterial with incident of Alzheimer’s disease: A pilot study. J. Clin. Lab. Anal. 2022, 36, e24483. [Google Scholar] [CrossRef]
- Panzarella, V.; Mauceri, R.; Baschi, R.; Maniscalco, L.; Campisi, G.; Monastero, R. Oral Health Status in Subjects with Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Data from the Zabút Aging Project. J. Alzheimers Dis. 2022, 87, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sansores-España, L.D.; Díaz-Zúñiga, J.; Martínez-Aguilar, V.; Melgar-Rodríguez, S.; Carrillo-Ávila, A.; Paula-Lima, A.; Astorga, J.; Arriola-Pacheco, F.; Morales, F. Gingival Crevicular Fluid as Biomarker’s Source for Alzheimer’s Disease. Odovtos-Int. J. Dent. Sci. 2022, 24, 156–176. [Google Scholar] [CrossRef]
- Issilbayeva, A.; Kaiyrlykyzy, A.; Vinogradova, E.; Jarmukhanov, Z.; Kozhakhmetov, S.; Kassenova, A.; Nurgaziyev, M.; Mukhanbetzhanov, N.; Alzhanova, D.; Zholdasbekova, G.; et al. Oral Microbiome Stamp in Alzheimer’s Disease. Pathogens 2024, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Lee, W.F.; Salamanca, E.; Yao, W.L.; Su, J.N.; Wang, S.Y.; Hu, C.J.; Chang, W.J. Oral Microbiota Changes in Elderly Patients, an Indicator of Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 4211. [Google Scholar] [CrossRef]
- Qiu, C.; Zhou, W.; Shen, H.; Wang, J.; Tang, R.; Wang, T.; Xie, X.; Hong, B.; Ren, R.; Wang, G.; et al. Profiles of subgingival microbiomes and gingival crevicular metabolic signatures in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 41. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Franciotti, R.; Pignatelli, P.; Carrarini, C.; Romei, F.M.; Mastrippolito, M.; Gentile, A.; Mancinelli, R.; Fulle, S.; Piattelli, A.; Onofrj, M.; et al. Exploring the Connection between Porphyromonas gingivalis and Neurodegenerative Diseases: A Pilot Quantitative Study on the Bacterium Abundance in Oral Cavity and the Amount of Antibodies in Serum. Biomolecules 2021, 11, 845. [Google Scholar] [CrossRef]
- Nicholson, J.S.; Landry, K.S. Oral Dysbiosis and Neurodegenerative Diseases: Correlations and Potential Causations. Microorganisms 2022, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Larvin, H.; Gao, C.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. The impact of study factors in the association of periodontal disease and cognitive disorders: Systematic review and meta-analysis. Age Ageing 2023, 52, afad015. [Google Scholar] [CrossRef]
- Tavares, L.T.R.; Saavedra-Silva, M.; López-Marcos, J.F.; Veiga, N.J.; Castilho, R.M.; Fernandes, G.V.O. Blood and Salivary Inflammatory Biomarkers Profile in Patients with Chronic Kidney Disease and Periodontal Disease: A Systematic Review. Diseases 2022, 10, 12. [Google Scholar] [CrossRef]
- Yadav, P.; Lee, Y.H.; Panday, H.; Kant, S.; Bajwa, N.; Parashar, R.; Jha, S.K.; Jha, N.K.; Nand, P.; Lee, S.S.; et al. Implications of Microorganisms in Alzheimer’s Disease. Curr. Issues Mol. Biol. 2022, 44, 4584–4615. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Pushalkar, S.; Gulivindala, D.; Butler, T.; Li, Y.; Annam, K.R.C.; Glodzik, L.; Ballman, K.V.; Corby, P.M.; Blennow, K.; et al. Periodontal dysbiosis associates with reduced CSF Aβ42 in cognitively normal elderly. Alzheimers Dement. 2021, 13, e12172. [Google Scholar] [CrossRef] [PubMed]

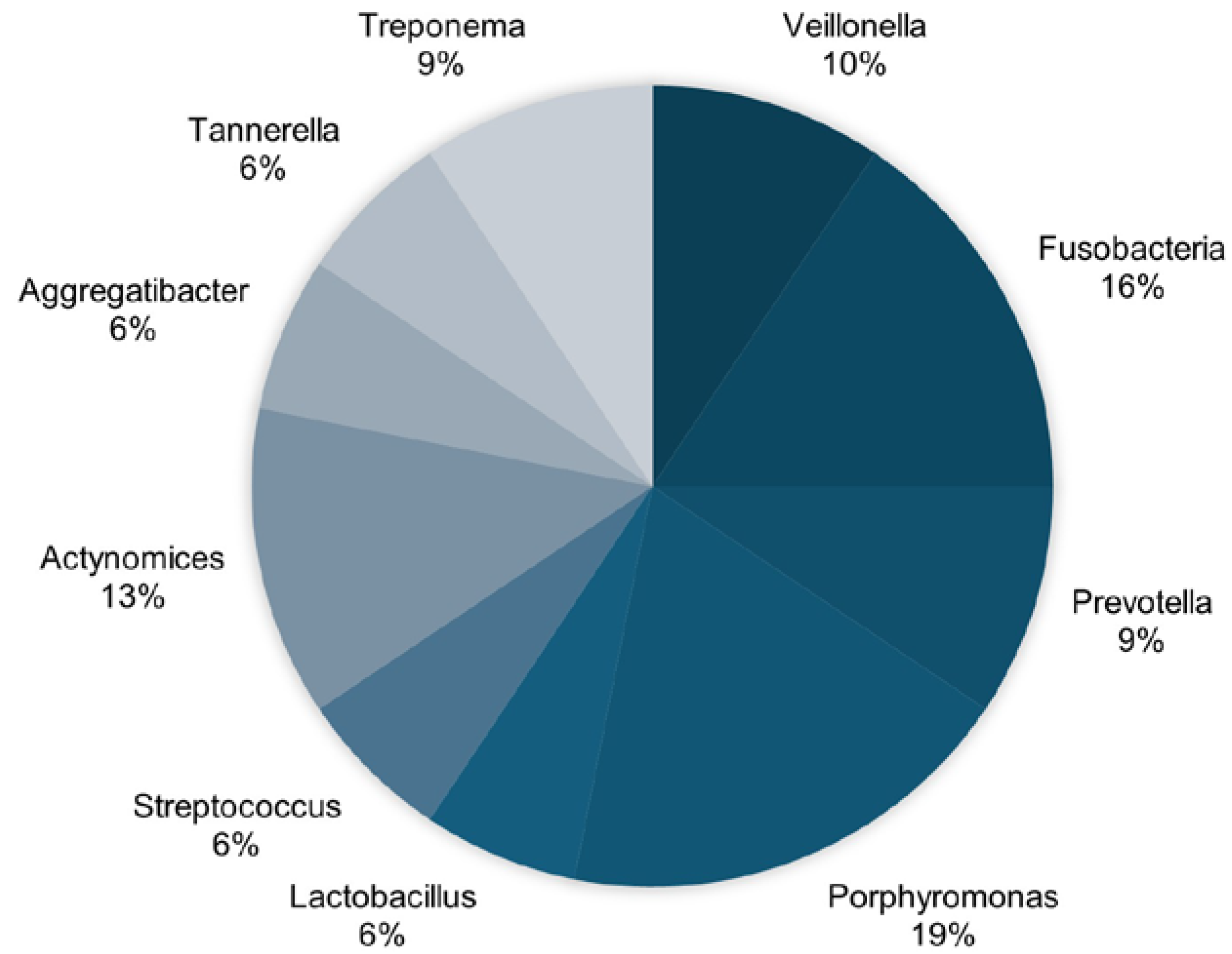


| Key Items | Sritana, N. et al. (2024) [18] | Holmer et al. (2021) [19] | Taati Moghadam et al. (2022) [20] | Panzarella et al. (2022) [21] | Sonsores-España et al. (2022) [22] | Issilbayeva et al. (2024) [23] | Wu et al. (2021) [24] |
|---|---|---|---|---|---|---|---|
| 1 | Y | Y | Y | Y | Y | Y | Y |
| 2 | N | Y | N | N | N | Y | N |
| 3 | Y | Y | Y | Y | Y | Y | Y |
| 4 | Y | Y | Y | Y | Y | Y | Y |
| 5 | Y | Y | Y | Y | Y | Y | Y |
| 6 | N | Y | Y | Y | N | Y | N |
| 7 | N | Y | N | Y | N | N | N |
| 8 | Y | Y | Y | Y | Y | Y | Y |
| 9 | U | U | U | U | U | U | U |
| 10 | Y | Y | Y | Y | Y | Y | Y |
| Total risk of bias | 6 Yes 3 No 1 Unclear Moderate | 9 Yes 1 Unclear Low | 7 Yes 2 No 1 Unclear Moderate | 8 Yes 1 No 1 Unclear Low | 6 Yes 3 No 1 Unclear Moderate | 8 Yes 1 No 1 Unclear Low | 6 Yes 3 No 1 Unclear Moderate |
| Key Item | Qiu et al. (2024) [25] |
|---|---|
| 1 | Y |
| 2 | Y |
| 3 | Y |
| 4 | Y |
| 5 | Y |
| 6 | N |
| 7 | Y |
| 8 | Y |
| Overall | 7 Yes 1 No Low |
| Author | Study Type | Sample and Group | Oral Microbiota | Microbiota Collection Method | Association Between OD and AD |
| Sritana, N. and Phungpinii, A. 2024 [18] | Case-control study | n = 100 AD patients (n = 10); Mean age 66.9 y Patients with mild cognitive impairment (MCI) (n = 46); Mean age 68.5 y Healthy patients (n = 44); Mean age 64.73 y | Cyanobacteria, Pseudomonadales, Fusobacteriota, Peptostreptococcaceae, Veillonella | OMNIgene® ORAL collection kit (DNA Genotek, Ottawa, ON, Canada) was used to collect saliva samples according to the manufacturer’s instructions. | YES |
| Taati Moghadam M et al. 2022 [20] | Case-control study | n = 30 AD patients (n = 15) Healthy patients (n = 15) | Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Streptococcus mutans | Oral bacterial microbiome composition was analyzed by quantitative real-time PCR (qPCR) using the 16S rDNA bacterial gene. Systemic inflammatory cytokine levels in both groups were assessed by ELISA. | YES |
| Panzarella V et al. 2022 [21] | Case-control study | n = 60 AD subjects (n = 20) Subjects with amnestic mild cognitive impairment (aMCI) (n = 20) Controls (n = 20) | Aggregatibacter actinomycetemcomitans (A.a), Fusobacterium nucleatum (F.n.), Porphyromonas gingivalis (P.g.), Prevotella intermedia (P.i.), Treponema denticola (T.d.), Tannerella forsythia (T.f.) | Samples were collected from subgingival plaque bacterial load (using the Carpegen® Perio Diagnostics kit with paper points on gingival crevicular fluid) for RT-PCR quantitative analysis of six periodontitis marker organisms. | YES |
| Sansores-España LD et al. 2022 [22] | Case-control study | n = 30 AD patients (n = 10) Healthy patients (n = 20) | Porphyromonas gingivalis | Subgingival microbiota and GCF samples were collected from the deepest sites. Total DNA was isolated to quantify the 16S ribosomal subunit. Pro-inflammatory mediators and ApoE were quantified from gingival crevicular fluid (GCF). | YES |
| Issilbayeya A et al. 2024 [23] | Case-control study | n = 135 AD patients (n = 64) Healthy patients (n = 71) | Firmicutes, Bacteroidota, Haemophilus parainfluenzae, Prevotella melaninogenica, Prevotella histicola, Actinomyces oris, Limosilactobacillus, Lactobacillus, Lacticaseibacillus, Bacteroides, Catenibacterium, Parabacteroides, Eubacterium_eligens_group, Fusobacterium, Turicibacter, Anaerostipes genera | Saliva was collected using a calibrated pipette from the floor of the mouth. Soft tissue samples were obtained from the dorsal tongue, hard palate, buccal mucosa, keratinized (attached) gingiva, palatine tonsils, and throat using a DNA/RNA shield collection tube with a swab. Supragingival and subgingival plaque were collected using a Gracey curette. DNA extraction was performed using the ZymoBIOMICS DNA miniprep kit. | YES |
| Wu YF et al. 2021 [24] | Case-control study | n = 35 AD patients (n = 17) Healthy patients (n = 18) | Firmicutes, Bacteroidetes, Fusobacteria, Fusobacterium, Cardiobacterium, Porphyromonas, Lactobacillus, Streptococcaceae, Actinomycetaceae, Veillonella | Plaque was collected by a trained dentist using Gracey periodontal curettes. Genomic DNA was extracted using a bacterial genomic DNA kit. | YES |
| Franciotti R et al. 2021 [27] | Case-control study | n = 78 Patients with neurodegenerative disease (n = 21) Patients with non-neurodegenerative disease (n = 28) Healthy patients (n = 29) | Porphyromonas gingivalis | Tongue biofilm was collected from each patient and control subject by the same dentist (P.P.) under identical conditions, 8 h after the last tooth brushing. The swab was obtained by brushing five times from the middle third of the tongue dorsum. | YES |
| Qiu C et al. 2024 [25] | Cross-sectional study | AD patients (n = 32) Amnestic MCI patients (n = 32) Healthy patients (n = 32) | Veillonella parvula, Lancefieldella parvula, Prevotella melaninogenica, Anaeroglobus geminatus, Streptococcus anginosus, Campylobacter gracilis, Dialister pneumosintes, [Eubacterium] yurii, Pseudoleptotrichia goodfellowii, Campylobacter rectus, Leptotrichia buccalis, Streptococcus sanguinis, Actinomyces massiliensis, Haemophilus parainfluenzae, Campylobacter concisus | Subgingival plaque was obtained using Gracey curettes and placed in a sterile Eppendorf tube containing 0.5 mL of 1× phosphate-buffered solution (pH 7.2) and stored at −80 °C. Subgingival microbiota composition was determined by high-throughput sequencing of the 16S rRNA amplicon. | YES |
| Holmer J et al., 2021. [19] | Case-control study | n = 154 AD-diagnosed patients (50–80 y) (n = 52) Patients with mild cognitive impairment (n = 51) Patients with subjective cognitive decline (n = 51) Healthy patients (n = 76) | Fusobacterium, Porphyromonas (P. gingivalis), Capnocytophaga, Treponema, Prevotella (P. intermedia), Campylobacter, Streptococcus, Slackia exigua, Lachnospiraceae, Actinomyces, Rothia | Samples were collected using curettes; DNA extraction, PCR amplification, and sequencing of V3-V4 regions of the 16S rRNA gene were conducted by the DNA Sequencing and Genomics Laboratory, Institute of Biotechnology, University of Helsinki. | YES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Martínez, V.; Rodríguez-Lozano, F.J.; Pecci-Lloret, M.P.; Pérez-Guzmán, N. Association Between Oral Dysbiosis and Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2025, 14, 3415. https://doi.org/10.3390/jcm14103415
Martínez-Martínez V, Rodríguez-Lozano FJ, Pecci-Lloret MP, Pérez-Guzmán N. Association Between Oral Dysbiosis and Alzheimer’s Disease: A Systematic Review. Journal of Clinical Medicine. 2025; 14(10):3415. https://doi.org/10.3390/jcm14103415
Chicago/Turabian StyleMartínez-Martínez, Valeria, Francisco Javier Rodríguez-Lozano, María Pilar Pecci-Lloret, and Nuria Pérez-Guzmán. 2025. "Association Between Oral Dysbiosis and Alzheimer’s Disease: A Systematic Review" Journal of Clinical Medicine 14, no. 10: 3415. https://doi.org/10.3390/jcm14103415
APA StyleMartínez-Martínez, V., Rodríguez-Lozano, F. J., Pecci-Lloret, M. P., & Pérez-Guzmán, N. (2025). Association Between Oral Dysbiosis and Alzheimer’s Disease: A Systematic Review. Journal of Clinical Medicine, 14(10), 3415. https://doi.org/10.3390/jcm14103415








