Assessment of Efficacy and Safety of Lipid-Lowering Treatment and Its Importance in Risk Assessment and Prevention in a Hungarian Myositis Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Biochemical Measurements
2.3. Cardiovascular Disease Risk Assessment
2.4. IIM Disease Activity and Muscular Side Effects of Lipid-Lowering Treatment
2.5. Statistical Analysis
3. Results
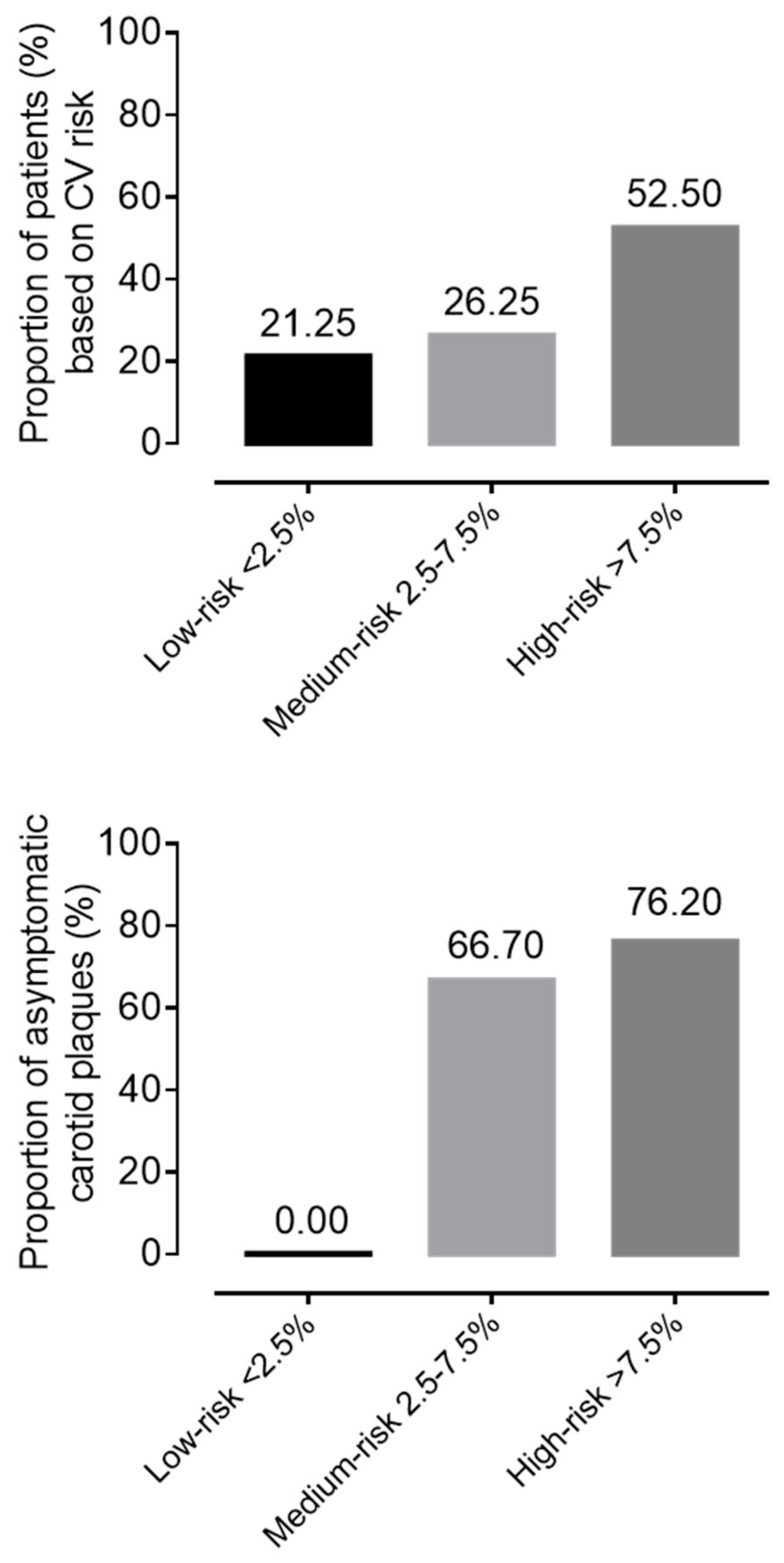
3.1. General Characteristics of the IIM Cohort
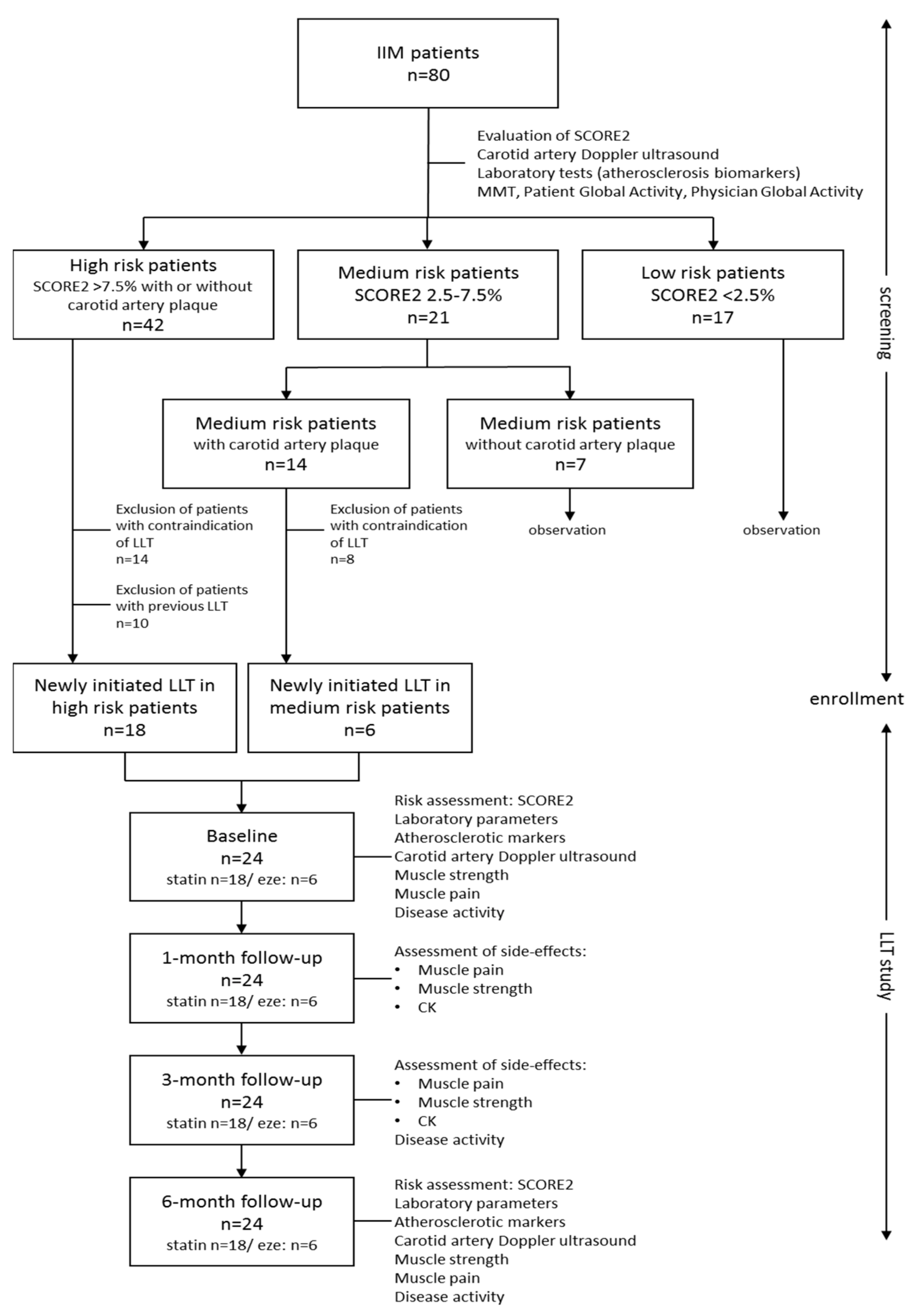
3.2. Patient Selection for Lipid Lowering Therapy (LLT)
3.3. Efficacy of Lipid-Lowering Therapy
3.4. Safety and Tolerability of Lipid-Lowering Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| anti–HMGCR | anti- 3-hydroxy-3-methylglutaryl coenzyme-A reductase antibody |
| ALT | alanine aminotransferase |
| ApoA1 | apolipoprotein A1 |
| ApoB100 | apolipoprotein B100 |
| ASS | antisynthetase syndrome |
| AST | aspartate aminotransferase |
| CV | cardiovascular |
| CVD | cardiovascular diseases |
| CK | creatine kinase |
| DM | dermatomyositis |
| ECG | electrocardiography |
| ELISA | enzyme linked immunoassay |
| ESC | European Society of Cardiology |
| EULAR | European Alliance of Associations for Reumatology |
| EZE | ezetimibe |
| GGT | glutamyl transpeptidase |
| HDL-C | high-density lipoprotein cholesterol |
| HgbA1c | hemoglobin A1C |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme-A reductase |
| hs CRP | high-sensitivity C-reactive protein |
| IBM | inclusion body myositis |
| IIM | Idiopathic inflammatory myopathies (IIM) |
| IMNM | immune-mediated necrotizing myopathy |
| IMT | carotid artery intima media thickness |
| LDH | lactate dehydrogenase |
| LDL-C | low-density lipoprotein-cholesterol |
| LLT | lipid-lowering therapy |
| MAA | myositis associated autoantibody |
| MMT | manual muscle test |
| MSA | myositis specific autoantibody |
| OM | overlap myositis |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| PM | polymyositis |
| RMD | rheumatic and musculoskeletal diseases |
| sICAM-1 | soluble intercellular adhesion molecule-1 |
| sVCAM-1 | soluble vascular cell adhesion molecule 1 |
| oxLDL | oxidized LDL |
| VAS | visual analogue scales |
References
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, L.P. Cardiovascular involvement in myositis. Curr. Opin. Rheumatol. 2017, 29, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Shinjo, S.K.; Day, J.; Gupta, L. Cardiovascular manifestations in idiopathic inflammatory myopathies. Clin. Rheumatol. 2023, 42, 2557–2575. [Google Scholar] [CrossRef] [PubMed]
- Khoo, T.; Stokes, M.B.; Teo, K.; Proudman, S.; Basnayake, S.; Sanders, P.; Limaye, V. Cardiac involvement in idiopathic inflammatory myopathies detected by cardiac magnetic resonance imaging. Clin. Rheumatol. 2019, 38, 3471–3476. [Google Scholar] [CrossRef]
- Lundberg, I.E. The heart in dermatomyositis and polymyositis. Rheumatology 2006, 45 (Suppl. S4), iv18–iv21. [Google Scholar] [CrossRef]
- Péter, A.; Balogh, Á.; Csanádi, Z.; Dankó, K.; Griger, Z. Subclinical systolic and diastolic myocardial dysfunction in polyphasic polymyositis/dermatomyositis: A 2-year longitudinal study. Arthritis Res. Ther. 2022, 24, 219. [Google Scholar] [CrossRef]
- Myhr, K.A.; Pecini, R. Management of Myocarditis in Myositis: Diagnosis and Treatment. Curr. Rheumatol. Rep. 2020, 22, 49. [Google Scholar] [CrossRef]
- Limaye, V.; Hakendorf, P.; Woodman, R.J.; Blumbergs, P.; Roberts-Thomson, P. Mortality and its predominant causes in a large cohort of patients with biopsy-determined inflammatory myositis. Intern. Med. J. 2012, 42, 191–198. [Google Scholar] [CrossRef]
- Mc Namara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Guimarães, F.; Yildirim, R.; Isenberg, D.A. Long-term survival of patients with idiopathic inflammatory myopathies: Anatomy of a single-centre cohort. Clin. Exp. Rheumatol. 2023, 41, 322–329. [Google Scholar] [CrossRef]
- Oreska, S.; Storkanova, H.; Pekacova, A.; Kudlicka, J.; Tuka, V.; Mikes, O.; Krupickova, Z.; Satny, M.; Chytilova, E.; Kvasnicka, J.; et al. Cardiovascular risk in myositis patients compared with the general population. Rheumatology 2024, 63, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Prieto, C.P.; Ibrahim, F.; Campbell, R.; Chinoy, H.; Galloway, J.; Gordon, P. Similar risk of cardiovascular events in idiopathic inflammatory myopathy and rheumatoid arthritis in the first 5 years after diagnosis. Clin. Rheumatol. 2021, 40, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Mizus, M.C.; Tiniakou, E. Lipid-lowering Therapies in Myositis. Curr. Rheumatol. Rep. 2020, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Drosos, G.C.; Vedder, D.; Houben, E.; Boekel, L.; Atzeni, F.; Badreh, S.; Boumpas, D.T.; Brodin, N.; Bruce, I.N.; González-Gay, M.Á.; et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann. Rheum. Dis. 2022, 81, 768–779. [Google Scholar] [CrossRef]
- Linos, E.; Fiorentino, D.; Lingala, B.; Krishnan, E.; Chung, L. Atherosclerotic cardiovascular disease and dermatomyositis: An analysis of the Nationwide Inpatient Sample survey. Arthritis Res. Ther. 2013, 15, R7. [Google Scholar] [CrossRef]
- Weng, M.-Y.; Lai, E.C.-C.; Yang, Y.-H.K. Increased risk of coronary heart disease among patients with idiopathic infammatory myositis: A nationwide population study in Taiwan. Rheumatology 2019, 58, 1935–1941. [Google Scholar] [CrossRef]
- Leclair, V.; Svensson, J.; Lundberg, I.E.; Holmqvist, M. Acute coronary syndrome in idiopathic infammatory myopathies: A population-based study. J. Rheumatol. 2019, 46, 1509–1514. [Google Scholar] [CrossRef]
- Rai, S.K.; Choi, H.K.; Sayre, E.C.; Aviña-Zubieta, J.A. Risk of myocardial infarction and ischaemic stroke in adults with polymyositis and dermatomyositis: A general population-based study. Rheumatology 2016, 55, 461–469. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Lin, C.-L.; Chang, K.-C.; Kao, C.-H. Increased subsequent risk of acute coronary syndrome for patients with dermatomyositis/polymyositis: A nationwide population-based retrospective cohort study. Scand. J. Rheumatol. 2015, 44, 42–47. [Google Scholar] [CrossRef]
- Diederichsen, L.P.; Diederichsen, A.C.P.; Simonsen, J.A.; Junker, P.; Søndergaard, K.; Lundberg, I.E.; Tvede, N.; Gerke, O.; Christensen, A.F.; Dreyer, L.; et al. Traditional cardiovascular risk factors and coronary artery calcifcation in adults with polymyositis and dermatomyositis: A danish multicenter study. Arthritis Care Res. 2015, 67, 848–854. [Google Scholar] [CrossRef]
- Xiong, A.; Hu, Z.; Zhou, S.; Qiang, Y.; Song, Z.; Chen, H.; Xiang, Q.; Zhang, Y.; Cao, Y.; Cui, H.; et al. Cardiovascular events in adult polymyositis and dermatomyositis: A meta-analysis of observational studies. Rheumatology 2022, 61, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Suksaranjit, P.; Spanuchart, I.; Leeaphorn, N.; Permpalung, N. Risk of coronary artery disease in patients with idiopathic infammatory myopathies: A systematic review and meta-analysis of observational studies. Semin. Arthritis Rheum. 2014, 44, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Dobloug, G.C.; Svensson, J.; Lundberg, I.E.; Holmqvist, M. Mortality in idiopathic inflammatory myopathy: Results from a Swedish nationwide population-based cohort study. Ann. Rheum. Dis. 2018, 77, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Dankó, K.; Ponyi, A.; Constantin, T.; Borgulya, G.; Szegedi, G. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: A longitudinal study of 162 cases. Medicine 2004, 83, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Kim, H.; Park, W.; Lim, M.; Kang, T.; Kang, M.; Kim, K.-B.; Ahn, H. Incidence, survival, and risk of cardiovascular events in adult inflammatory myopathies in South Korea: A nationwide population-based study. Scand. J. Rheumatol. 2020, 49, 323–331. [Google Scholar] [CrossRef]
- Shah, M.; Shrestha, K.; Tseng, C.W.; Goyal, A.; Liewluck, T.; Gupta, L. Statin-associated muscle symptoms: A comprehensive exploration of epidemiology, pathophysiology, diagnosis, and clinical management strategies. Int. J. Rheum. Dis. 2024, 27, e15337. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clin. 2018, 150, 398–402. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Muñoz-Braceras, S.; Casal-Dominguez, M.; Pak, K.; Torres-Ruiz, J.; Musai, J.; Dell, S.; Naz, F.; Islam, S.; Mammen, A.L.; et al. Pathogenic autoantibody internalization in myositis. Ann. Rheum. Dis. 2024, 83, 1549–1560. [Google Scholar] [CrossRef]
- Bae, S.S.; Oganesian, B.; Golub, I.; Charles-Schoeman, C. Statin use in patients with non-HMGCR idiopathic inflammatory myopathies: A retrospective study. Clin. Cardiol. 2020, 43, 732–742. [Google Scholar] [CrossRef]
- Rastegar, T.F.; Khan, I.A.; Christopher-Stine, L. Decoding the Intricacies of Statin-Associated Muscle Symptoms. Curr. Rheumatol. Rep. 2024, 26, 260–268. [Google Scholar] [CrossRef]
- Blake, G.J.; Ridker, P.M. Are statins anti-inflammatory? Curr. Control Trials Cardiovasc. Med. 2000, 1, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.escardio.org/Education/Practice-Tools/CVD-prevention-toolbox/HeartScore (accessed on 1 March 2023).
- Ihle-Hansen, H.; Vigen, T.; Berge, T.; Walle-Hansen, M.M.; Hagberg, G.; Thommessen, B.; Ariansen, I.; Røsjø, H.; Rønning, O.M.; Tveit, A.; et al. Carotid Plaque Score for Stroke and Cardiovascular Risk Prediction in a Middle-Aged Cohort from the General Population. J. Am. Heart Assoc. 2023, 12, e030739. [Google Scholar] [CrossRef]
- Miller, F.W.; Rider, L.G.; Chung, Y.L.; Cooper, R.; Danko, K.; Farewell, V.; Lundberg, I.; Morrison, C.; Oakley, L.; Isenberg, D.A.; et al. Proposed preliminary core set measures for disease outcome assessment in adult and juvenile idiopathic inflammatory myopathies. Rheumatology 2001, 40, 1262–1273. [Google Scholar] [CrossRef]
- Rider, L.G.; Werth, V.P.; Huber, A.M.; Alexanderson, H.; Rao, A.P.; Ruperto, N.; Herbelin, L.; Barohn, R.; Isenberg, D.; Miller, F.W. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res. 2011, 63 (Suppl. S11), S118–S157. [Google Scholar]
- Michaeli, D.T.; Michaeli, J.C.; Albers, S.; Boch, T.; Michaeli, T. Established and Emerging Lipid-Lowering Drugs for Primary and Secondary Cardiovascular Prevention. Am. J. Cardiovasc. Drugs 2023, 23, 477–495. [Google Scholar] [CrossRef]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-wide cross-sec-tional observational study of lipid-modifying therapy use in secondary and primary care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Glavinovic, T.; Thanassoulis, G.; de Graaf, J.; Couture, P.; Hegele, R.A.; Sniderman, A.D. Physiological Bases for the Superiority of Apolipoprotein B Over Low-Density Lipoprotein Cholesterol and Non–High-Density Lipoprotein Cholesterol as a Marker of Cardiovascular Risk. J. Am. Heart Assoc. 2022, 11, 20. [Google Scholar] [CrossRef]
- Brinjikji, W.; Cloft, H.; Cekirge, S.; Fiorella, D.; Hanel, R.A.; Jabbour, P.; Lylyk, P.; McDougall, C.; Moran, C.; Siddiqui, A.; et al. Lack of Association between Statin Use and Angiographic and Clinical Outcomes after Pipeline Embolization for Intracranial Aneurysms AJNR. Am. J. Neuroradiol. 2017, 38, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: An individual participant data meta-analysis. Lancet Diabetes Endocrinol. 2024, 12, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Soomro, A.; Ferreira, D.; Elashri, I.; McGee, M. The Use of PCSK9 Inhibitors in Treating Statin Induced Necrotising Myositis. Heart Lung Circ. 2021, 30 (Suppl. S3), S273–S274. [Google Scholar] [CrossRef]
- Zeman, M.; Vecka, M.; Perlík, F.; Hromádka, R.; Staňková, B.; Tvrzická, E.; Žák, A. Niacin in the Treatment of Hyperlipidemias in Light of New Clinical Trials: Has Niacin Lost its Place? Med. Sci. Monit. 2015, 21, 2156–2162. [Google Scholar]
- Poli, A.; Marangoni, F.; Corsini, A.; Manzato, E.; Marrocco, W.; Martini, D.; Medea, G.; Visioli, F. Phytosterols, Cholesterol Control, and Cardiovascular Disease. Nutrients 2021, 13, 2810. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of Study Population (n = 80) | |
|---|---|
| Mean age (years ± SD) | 56.2 ± 13.4 |
| Duration of disease (years) | 9 (5–15) |
| Gender (female/male) | 67.5% (n = 54)/32.5% (n = 26) |
| Smoking status (n) | 17.5% (n = 14) |
| Polymyositis (including antisynthetase syndrome and necrotizing myopathy) | 49% (n = 39) |
| Dermatomyositis | 51% (n = 41) |
| Myositis-specific antibody (MSA) positivity | 60% (n = 48) |
| Anti-Jo1 Antisynthetase | n = 18 |
| Non-anti-Jo1 antisynthetase | n = 3 |
| DM-specific antibodies (SAE, TIF1γ, NXP2, MDA5, Mi2) | n = 22 |
| Anti-HMGCR antibody positivity | 2.5% (n = 2) |
| Anti-SRP positivity | 3.75% (n = 3) |
| Myositis-associated autoantibody (MAA) positivity (anti-Ro52/SSA, anti-Ku, anti-Pm/scl) | 43.7% (n = 32) |
| Glucocorticoid treatment at screening | 70% (n = 56) |
| Clinical symptoms | |
| Muscle weakness | 96.25% (n = 77) |
| Skin rashes | 70% (n = 56) |
| Interstitial lung disease | 37.50% (n = 30) |
| Dysphagia | 36.25% (n = 29) |
| Myocarditis | 16.25% (n = 13) |
| Arthralgia | 81.25% (n = 65) |
| Fever | 17.50% (n = 14) |
| Raynaud’s phenomenon | 38.75% (n = 31) |
| Cardiovascular comorbidities | |
| Hypertension | 71.25% (n = 57) |
| Diabetes mellitus | 25% (n = 20) |
| Heart failure | 6.25% (n = 5) |
| Arrythmias | 13.75% (n = 11) |
| Valvular problems | 8.75% (n = 7) |
| Thromboembolic events | 7.50% (n = 6) |
| Peripheral arterial diseases | 17.50% (n = 14) |
| Stroke | 0% (n = 0) |
| Acute myocardial infarction | 3.75% (n = 3) |
| Observed Parameters at Screening | Mean/Median | SD/Lower-Upper Quartiles |
|---|---|---|
| Cardiovascular risk assessment: | ||
| Systolic BP (mmHg) | 132.7 | ±15.341 |
| Diastolic BP (mmHg) | 81.85 | ±11.31 |
| Total cholesterol (mmol/L) | 5.718 | ±1.418 |
| HDL-C (mmol/L) | 1.566 | ±0.584 |
| Non-HDL cholesterol (mmol/L) | 4.175 | ±1.402 |
| LDL-C (mmol/L) | 3.438 | ±1.178 |
| Triglyceride (mmol/L) | 1.7 | 1.2–2.4 |
| HgbA1c (%) | 5.8 | 5.6–6.1 |
| Score2 score | 7.663 | ±5.178 |
| Assessment of atherosclerosis: | ||
| IMT right side average (mm) | 0.591 | ±0.142 |
| IMT left side average (mm) | 0.632 | ±0.136 |
| sVCAM-1 (ng/mL) | 995.3 | 812.1–1191.6 |
| sICAM-1 (ng/mL) | 256.3 | 217.9–311.35 |
| oxLDL (U/L) | 71.049 | ±21.103 |
| ApoB100 (g/L) | 1.111 | ±0.307 |
| ApoA1 (g/L) | 1.649 | ±0.359 |
| Assessment of disease activity: | ||
| hsCRP (mg/L) | 3.655 | 1.24–5.6 |
| CK (IU/mL) | 90.5 | 62–185 |
| Patient’s global activity (0–10 cm scale) | 2 | 0–3.5 |
| Physician global activity (0–10 cm scale) | 1 | 0–2.15 |
| Manual muscle test score (0–150) | 145 | 133.5–150 |
| Screening (n = 24) | 6-Month Follow-Up (n = 24) | p-Values | |||
|---|---|---|---|---|---|
| Mean/Median | SD/Lower-Upper Quartiles | Mean/Median | SD/Lower-Upper Quartiles | ||
| Lipid levels: | |||||
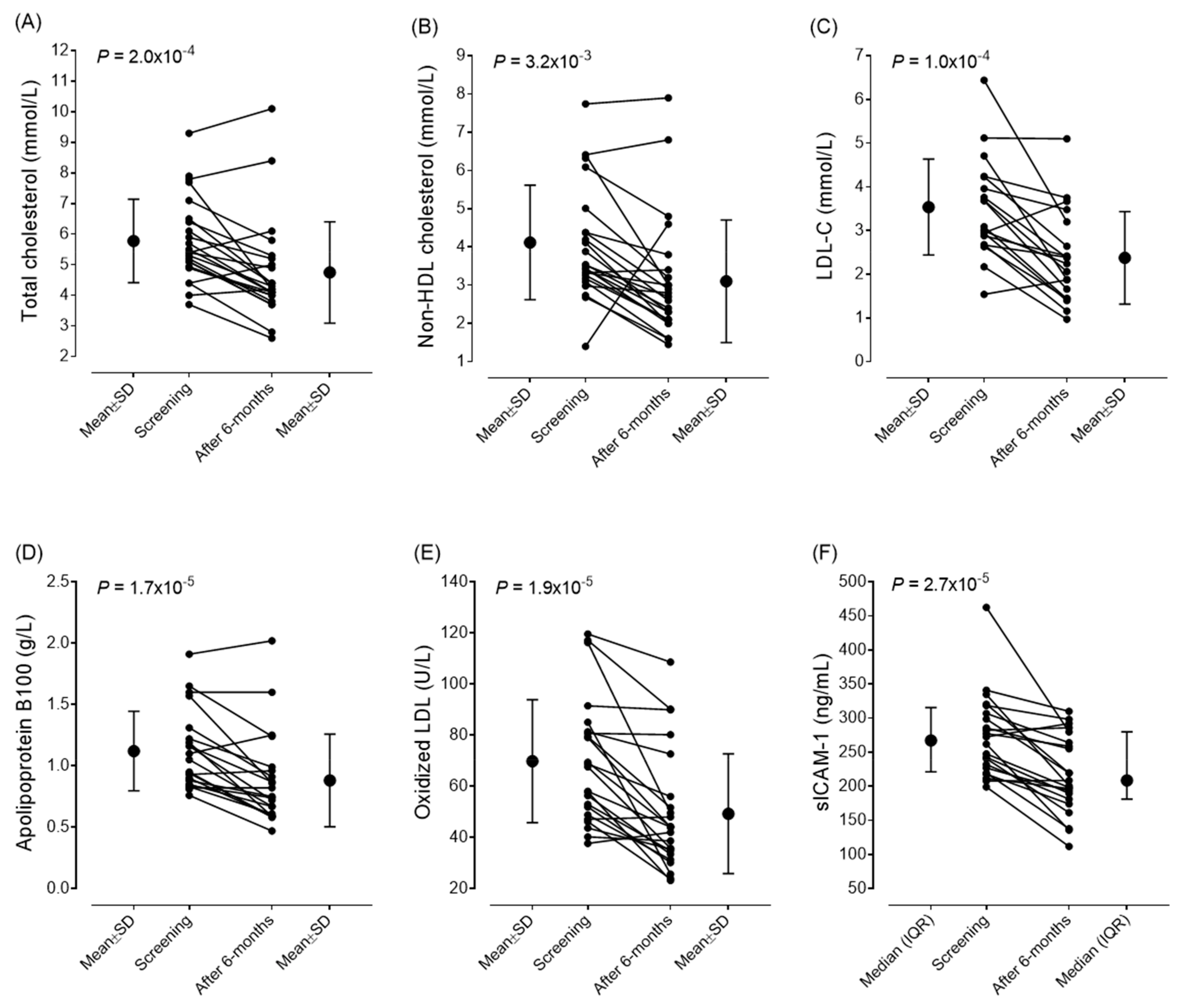
| Total cholesterol (mmol/L) | 5.78 | ±1.36 | 4.76 | ±1.69 | <0.001 † |
| HDL-C (mmol/L) | 1.66 | ±0.7 | 1.59 | ±0.56 | 0.452 |
| non-HDL cholesterol (mmol/L) | 4.11 | ±1.49 | 3.1 | ±1.64 | 0.003 |
| LDL-C (mmol/L) | 3.537 | ±1.095 | 2.377 | ±1.06 | <0.001 † |
| Triglyceride (mmol/L) | 1.515 | 1.2–2.4 | 1.535 | 0.94–2.13 | 0.976 |
| Assessment of atherosclerosis: | |||||
| sVCAM-1 (ng/mL) | 988.95 | 812.1–1191.6 | 1063.2 | 775.2–1236.2 | 0.465 |
| sICAM-1 (ng/mL) | 267.2 | 217.9–311.35 | 208.7 | 181.2–279.7 | <0.001 † |
| oxLDL (U/L) | 69.83 | ±24.01 | 49.29 | ±23.39 | <0.001 † |
| ApoB100 (g/L) | 1.12 | ±0.32 | 0.88 | ±0.39 | <0.001 † |
| ApoA1 (g/L) | 1.61 | ±0.29 | 1.68 | ±0.33 | 0.182 |
| IMT right side average (mm) | 0.599 | ±0.088 | 0.647 | ±0.123 | 0.079 |
| IMT left side average (mm) | 0.683 | ±0.124 | 0.653 | ±0.124 | 0.505 |
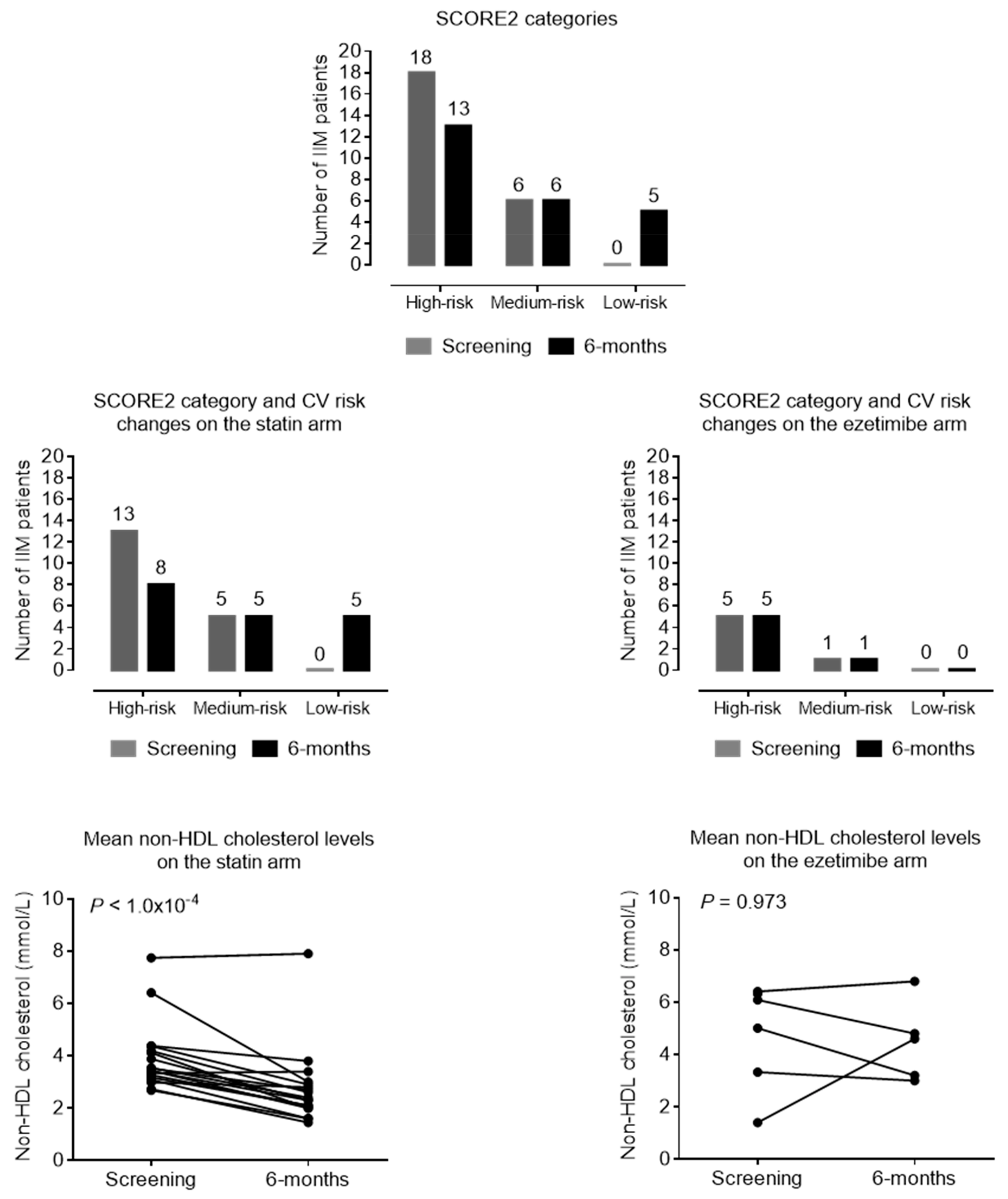
| Change in SCORE2: (primary endpoint) | |||||
| Score2 point | 9.75 | ±4.36 | 8.478 | ±3.58 | 0.014 |
| Screening (n = 24) | 6-Month Follow-Up (n = 24) | p-Values | |||
|---|---|---|---|---|---|
| Mean/Median | SD/Lower-Upper Quartiles | Mean/Median | SD/Lower-Upper Quartiles | ||
| Metabolic parameters | |||||
| HgbA1c (%): | 5.9 | 5.6–6.1 | 5.9 | 5.7–6.4 | 0.614 |
| AST (U/L) | 22 | 17.5–27.5 | 22 | 17–27 | 0.754 |
| ALT (U/L) | 20 | 10.55–30.5 | 25 | 17–31 | 0.126 |
| GGT (U/L) | 35.5 | 20–77 | 33 | 17–102 | 0.339 |
| Disease activity: | |||||
| hsCRP (mg/L) | 2.68 | 1.24–5.6 | 2.83 | 1.64–5.32 | 0.321 |
| CK (IU/mL) | 84 | 62–185 | 90.5 | 68–175 | 0.212 |
| Patient’s global activity | 2 | 0–2 | 0.5 | 0–2 | 0.197 |
| Physician global Activity (0–10 cm) | 0.75 | 0–1.15 | 1 | 0–1 | 0.594 |
| Muscle pain VAS score (0–10 cm) | 0 | 0–1.2 | 0 | 0–1 | 0.463 |
| Manual muscle test score (0–150) | 147 | 143–150 | 146 | 138–150 | 0.593 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szinay, D.; Szabó, K.; Molnár, H.; Béldi, T.; Bencs, V.; Lőrincz, H.; Harangi, M.; Griger, Z.; Nagy-Vincze, M. Assessment of Efficacy and Safety of Lipid-Lowering Treatment and Its Importance in Risk Assessment and Prevention in a Hungarian Myositis Cohort. J. Clin. Med. 2025, 14, 3404. https://doi.org/10.3390/jcm14103404
Szinay D, Szabó K, Molnár H, Béldi T, Bencs V, Lőrincz H, Harangi M, Griger Z, Nagy-Vincze M. Assessment of Efficacy and Safety of Lipid-Lowering Treatment and Its Importance in Risk Assessment and Prevention in a Hungarian Myositis Cohort. Journal of Clinical Medicine. 2025; 14(10):3404. https://doi.org/10.3390/jcm14103404
Chicago/Turabian StyleSzinay, Dorottya, Katalin Szabó, Henrik Molnár, Tibor Béldi, Viktor Bencs, Hajnalka Lőrincz, Mariann Harangi, Zoltán Griger, and Melinda Nagy-Vincze. 2025. "Assessment of Efficacy and Safety of Lipid-Lowering Treatment and Its Importance in Risk Assessment and Prevention in a Hungarian Myositis Cohort" Journal of Clinical Medicine 14, no. 10: 3404. https://doi.org/10.3390/jcm14103404
APA StyleSzinay, D., Szabó, K., Molnár, H., Béldi, T., Bencs, V., Lőrincz, H., Harangi, M., Griger, Z., & Nagy-Vincze, M. (2025). Assessment of Efficacy and Safety of Lipid-Lowering Treatment and Its Importance in Risk Assessment and Prevention in a Hungarian Myositis Cohort. Journal of Clinical Medicine, 14(10), 3404. https://doi.org/10.3390/jcm14103404








