Thyroid Autoimmunity Impairs Oocyte Maturation, Fertilization, and Embryo Development in Assisted Reproductive Technology in Euthyroid Infertile Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Serum Assays
2.3. Controlled Ovarian Stimulation Protocol
2.4. Fertilization and Embryo Culture
2.5. Statistical Analysis
3. Results
3.1. Study Group Descriptions and Hormonal Profiles
3.2. Oocytes and Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMH | Anti-Mullerian hormone |
| ART | Assisted Reproductive Technology |
| COH | Controlled Ovarian Hyperstimulation |
| E2 | Estradiol |
| FR | Fertilization Rate |
| FSH | Follicle-Stimulating Hormone |
| IVF | In vitro Fertilization |
| ICSI | Intracytoplasmic Sperm Injection |
| LH | Luteinizing Hormone |
| OR | Ovarian Reserve |
| SD | Standard Deviation |
| TAI | Thyroid Autoimmunity |
| TgAbs | Thyroglobulin Antibodies |
| TPOAbs | Thyroperoxidase Antibodies |
| TSH | Thyrotropin |
References
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bucci, I.; Giuliani, C.; Di Dalmazi, G.; Formoso, G.; Napolitano, G. Thyroid Autoimmunity in Female Infertility and Assisted Reproductive Technology Outcome. Front. Endocrinol. 2022, 13, 768363. [Google Scholar] [CrossRef]
- Poppe, K. Management of Endocrine Disease: Thyroid and Female Infertility: More Questions Than Answers?! Eur. J. Endocrinol. 2021, 184, R123–R135. [Google Scholar] [CrossRef] [PubMed]
- Tańska, K.; Gietka-Czernel, M.; Glinicki, P.; Kozakowski, J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front. Endocrinol. 2023, 13, 1049665. [Google Scholar] [CrossRef]
- Venables, A.; Wong, W.; Way, M.; Homer, H.A. Thyroid autoimmunity and IVF/ICSI outcomes in euthyroid women: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2020, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Unuane, D.; Velkeniers, B. Impact of thyroid disease on fertility and assisted conception. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101378. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Zhang, Q.; Si, G.X.; Chen, Q.S.; Ye, E.L.; Yu, L.C.; Peng, M.M.; Yang, H.; Du, W.J.; Zhang, C.; et al. Associations between thyroid autoantibody status and abnormal pregnancy outcomes in euthyroid women. Endocrine 2015, 48, 924–928. [Google Scholar] [CrossRef]
- Inagaki, Y.; Takeshima, K.; Nishi, M.; Ariyasu, H.; Doi, A.; Kurimoto, C.; Uraki, S.; Morita, S.; Furukawa, Y.; Inaba, H.; et al. The influence of thyroid autoimmunity on pregnancy outcome in infertile women: A prospective study. Endocr. J. 2020, 67, 859–868. [Google Scholar] [CrossRef]
- Poppe, K.; Bisschop, P.; Fugazzola, L.; Minziori, G.; Unuane, D.; Weghofer, A. 2021 European Thyroid Association Guideline on Thyroid Disorders prior to and during Assisted Reproduction. Eur. Thyroid J. 2021, 9, 281–295. [Google Scholar] [CrossRef]
- Weghofer, A.; Himaya, E.; Kushnir, V.A.; Barad, D.H.; Gleicher, N. The Impact of Thyroid Function and Thyroid Autoimmunity on Embryo Quality in Women with Low Functional Ovarian Reserve: A Case-Control Study. Reprod. Biol. Endocrinol. 2015, 13, 43. [Google Scholar] [CrossRef]
- Andrisani, A.; Sabbadin, C.; Marin, L.; Ragazzi, E.; Dessole, F.; Armanini, D.; Donà, G.; Bordin, L.; Ambrosini, G. The influence of thyroid autoimmunity on embryo quality in women undergoing assisted reproductive technology. Gynecol. Endocrinol. 2018, 34, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Safarian, G.K.; Niauri, D.A.; Kogan, I.Y.; Bespalova, O.N.; Dzhemlikhanova, L.K.; Lesik, E.A.; Komarova, E.M.; Krikheli, I.O.; Obedkova, K.V.; Tkachenko, N.N.; et al. Impact of Antithyroperoxidase Antibodies (Anti-TPO) on Ovarian Reserve and Early Embryo Development in Assisted Reproductive Technology Cycles. Int. J. Mol. Sci. 2023, 24, 4705. [Google Scholar] [CrossRef] [PubMed]
- Cevher Akdulum, M.F.; Erdem, M.; Barut, G.; Demirdag, E.; İyidir, Ö.T.; Guler, I.; Erdem, A. The relationship between thyroid autoimmunity and poor response to ovarian stimulation in in vitro fertilization women with infertility. Endokrynol. Pol. 2022, 73, 699–705. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Y.; Qiu, W.; Fan, J.; Zhang, C.; Kwak-Kim, J. Does thyroid autoimmunity affect the reproductive outcome in women with thyroid autoimmunity undergoing assisted reproductive technology? Am. J. Reprod. Immunol. 2020, 84, e13321. [Google Scholar] [CrossRef]
- Seungdamrong, A.; Steiner, A.Z.; Gracia, C.R.; Legro, R.S.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Casson, P.; Christman, G.M.; Robinson, R.D.; et al. Preconceptional antithy-roid peroxidase antibodies, but not thyroid-stimulating hormone, are associated with decreased live birth rates in infertile women. Fertil. Steril. 2017, 104, 36–37. [Google Scholar] [CrossRef]
- Magri, F.; Schena, L.; Capelli, V.; Gaiti, M.; Zerbini, F.; Brambilla, E.; Rotondi, M.; De Amici, M.; Spinillo, A.; Nappi, R.E.; et al. Anti-Mullerian hormone as a predictor of ovarian reserve in ART protocols: The hidden role of thyroid autoimmunity. Reprod. Biol. Endocrinol. 2015, 13, 106. [Google Scholar] [CrossRef][Green Version]
- Poppe, K.; Autin, C.; Veltri, F.; Sitoris, G.; Kleynen, P.; Praet, J.P.; Rozenberg, S. Thyroid Disorders and In Vitro Outcomes of Assisted Reproductive Technology: An Unfortunate Combination? Thyroid 2020, 30, 1177–1185. [Google Scholar] [CrossRef]
- Poppe, K.; Autin, C.; Veltri, F.; Kleynen, P.; Grabczan, L.; Rozenberg, S.; Ameye, L. Thyroid autoimmunity and intracytoplasmic sperm injection outcome: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2018, 103, 1755–1766. [Google Scholar] [CrossRef]
- Grigoriadis, S.; Maziotis, E.; Simopoulou, M.; Sfakianoudis, K.; Giannelou, P.; Rapani, A.; Tsioulou, P.; Pantou, A.; Tzanakaki, D.; Pantos, K.; et al. The Impact of Thyroid Autoantibodies Positivity on In Vitro Fertilization Outcome: A Comprehensive Review. Int. Arch. Clin. Physiol. 2019, 1, 2. [Google Scholar] [CrossRef]
- Busnelli, A.; Paffoni, A.; Fedele, L.; Somigliana, E. The impact of thyroid autoimmunity on IVF/ICSI outcome: A systematic review and meta-analysis. Hum. Reprod. Update 2016, 22, 775–790. [Google Scholar] [CrossRef]
- Sakar, M.N.; Unal, A.; Atay, A.E.; Zebitay, A.G.; Verit, F.F.; Demir, S.; Turfan, M.; Omer, B. Is there an effect of thyroid autoimmunity on the outcomes of assisted reproduction? J. Obstet. Gynaecol. 2016, 36, 213–217. [Google Scholar] [CrossRef]
- Monteleone, P.; Faviana, P.; Artini, P.G. Thyroid Peroxidase Identified in Human Granulosa Cells: Another Piece to the Thyroid-Ovary Puzzle? Gynecol. Endocrinol. 2017, 33, 574–576. [Google Scholar] [CrossRef]
- Arce, J.C.; Nyboe Andersen, A.; Fernández-Sánchez, M.; Visnova, H.; Bosch, E.; García-Velasco, J.A.; Barri de Sutter, P.; Klein, B.M.; Fauser, B.C. Ovarian response to recombinant human follicle-stimulating hormone: A randomized, antimüllerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil. Steril. 2014, 102, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.I.M.; Mínguez-Alarcón, L.; Messerlian, C.; de Poortere, R.A.; Williams, P.L.; Broeren, M.A.; Hauser, R.; Souter, I.C. Association of Thyroid Function and Autoimmunity with Ovarian Reserve in Women Seeking Infertility Care. Thyroid 2018, 28, 1349–1358. [Google Scholar] [CrossRef]
- Li, Z.; Xu, S.; Luo, W.; Hu, J.; Zhang, T.; Jiao, X.; Qin, Y. Association between thyroid autoimmunity and the decline of ovarian reserve in euthyroid women. Reprod. Biomed. Online 2022, 45, 615–622. [Google Scholar] [CrossRef]
- Chen, C.W.; Huang, Y.L.; Tzeng, C.R.; Huang, R.L.; Chen, C.H. Idiopathic Low Ovarian Reserve Is Associated with More Frequent Positive Thyroid Peroxidase Antibodies. Thyroid 2017, 27, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Kitahara, Y.; Osuka, S.; Tsukui, Y.; Kobayashi, M.; Iwase, A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 21, e12427. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, N.P.; Sakkas, E.; Vaiarelli, A.; Poppe, K.; Camus, M.; Tournaye, H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: A cross-sectional study of 5000 women based on age-specific AMH values. Hum. Reprod. 2015, 30, 1690–1696. [Google Scholar] [CrossRef]
- Osuka, S.; Iwase, A.; Goto, M.; Takikawa, S.; Nakamura, T.; Murase, T.; Kato, N.; Bayasula; Kotani, T.; Kikkawa, F. Thyroid Autoantibodies do not Impair the Ovarian Reserve in Euthyroid Infertile Women: A Cross-Sectional Study. Horm. Metab. Res. 2018, 50, 537–542. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Su, Z.; Ren, B.; Yu, S.; Li, W.; Xu, N.; Lou, H. Impaired embryo development potential associated with thyroid autoimmunity in euthyroid infertile women with diminished ovarian reserve. Front. Endocrinol. 2024, 15, 1376179. [Google Scholar] [CrossRef]
- Wei, S.X.; Wang, L.; Liu, Y.B.; Fan, Q.L.; Fan, Y.; Qiao, K. TPOAb positivity can impact ovarian reserve, embryo quality, and IVF/ICSI outcomes in euthyroid infertile women. Gynecol. Endocrinol. 2023, 39, 2266504. [Google Scholar] [CrossRef]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Brison, D.; Calderon, G.; Catt, J.; Conaghan, J.; Cowan, L.; Ebner, T.; Gardner, D.; Hardarson, T.; Lundin, K.; et al. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar]
- Gardner, D.K.; Schoolcraft, W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999, 11, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Meggyes, M.; Doba, K.; Farkas, N.; Bogar, B.; Barakonyi, A.; Szereday, L.; Szekeres-Bartho, J.; Mezosi, E. Charac-teristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J. Reprod. Immunol. 2017, 124, 62–70. [Google Scholar] [CrossRef]
- Huang, N.; Liu, D.; Lian, Y.; Chi, H.; Qiao, J. Immunological Microenvironment Alterations in Follicles of Patients with Autoimmune Thyroiditis. Front. Immunol. 2021, 12, 770852. [Google Scholar] [CrossRef]
- Herman, T.; Török, P.; Laganà, A.S.; Chiantera, V.; Venezia, R.; Jakab, A. Hashimoto’s Thyroiditis Negatively Influences Intracytoplasmic Sperm Injection Outcome in Euthyroid Women on T4 Substitution Therapy: A Retrospective Study. Gynecol. Obstet. Investig. 2024, 89, 150–158. [Google Scholar] [CrossRef]
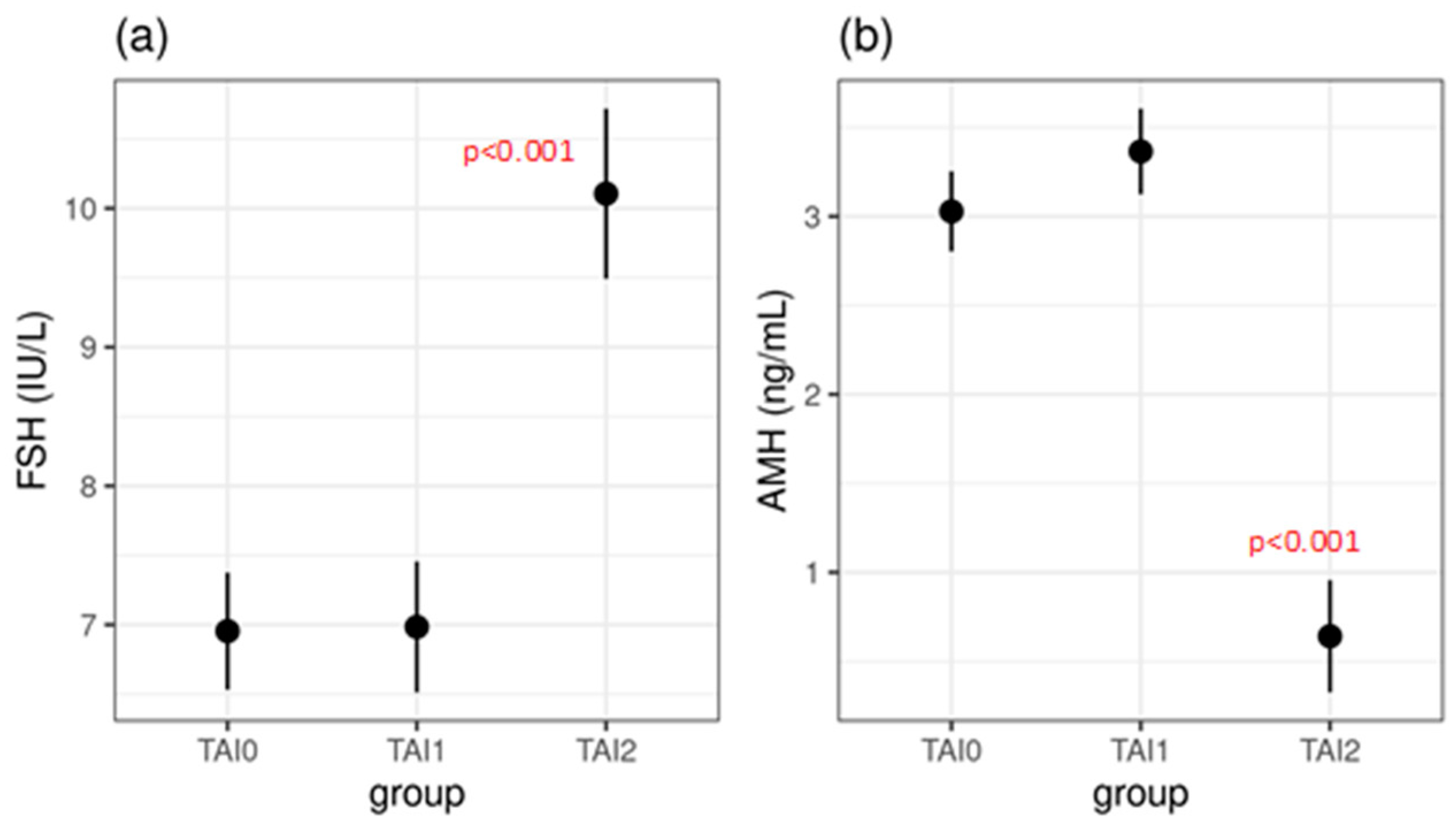

| TAI0 | TAI1 | TAI2 | TAI1 vs. TAI0 | TAI2 vs. TAI0 | TAI2 vs. TAI1 | |
|---|---|---|---|---|---|---|
| (n = 150) | (n = 120) | (n = 71) | ||||
| Mean (±SD) | Mean (±SD) | Mean (±SD) | p Value | p Value | p Value | |
| Female age | 35.0 ± 3.2 | 34.9 ± 3.0 | 35.9 ± 2.2 | 0.977 | 0.065 | 0.053 |
| TPOAbs (IU/mL) | 23.7 ± 10.7 | 584.8 ± 781.3 | 414.0 ± 521.4 | - | - | 0.107 |
| TGAbs (IU/mL) | 2.7 ± 1.8 | 144.6 ± 224.2 | 167.8 ± 219.9 | - | - | 0.673 |
| TSH (mIU/L) | 1.9 ± 0.7 | 2.3 ± 1.3 | 2.1 ± 1.2 | 0.011 * | 0.295 | 0.613 |
| FSH (IU/L) | 7.0 ± 1.4 | 7.0 ± 2.0 | 10.1 ± 4.7 | 0.995 | <0.001 * | <0.001 * |
| LH (IU/L) | 6.5 ± 2.0 | 6.5 ± 2.9 | 6.4 ± 2.7 | 0.998 | 0.881 | 0.913 |
| E2 (pmol/L) | 176.1 ± 69.7 | 172.0 ± 94.7 | 195.6 ± 184.8 | 0.951 | 0.449 | 0.338 |
| AMH (ng/mL) | 3.0 ± 1.3 | 3.4 ± 1.7 | 0.6 ± 0.3 | 0.112 | <0.001 * | <0.001 * |
| TAI0 (n = 150) Mean/SD | TAI1 (n = 120) Mean/SD | TAI2 (n = 71) Mean/SD | TAI1 vs. TAI0 p Value | TAI2 vs. TAI0 p Value | TAI2 vs. TAI1 p Value | |
|---|---|---|---|---|---|---|
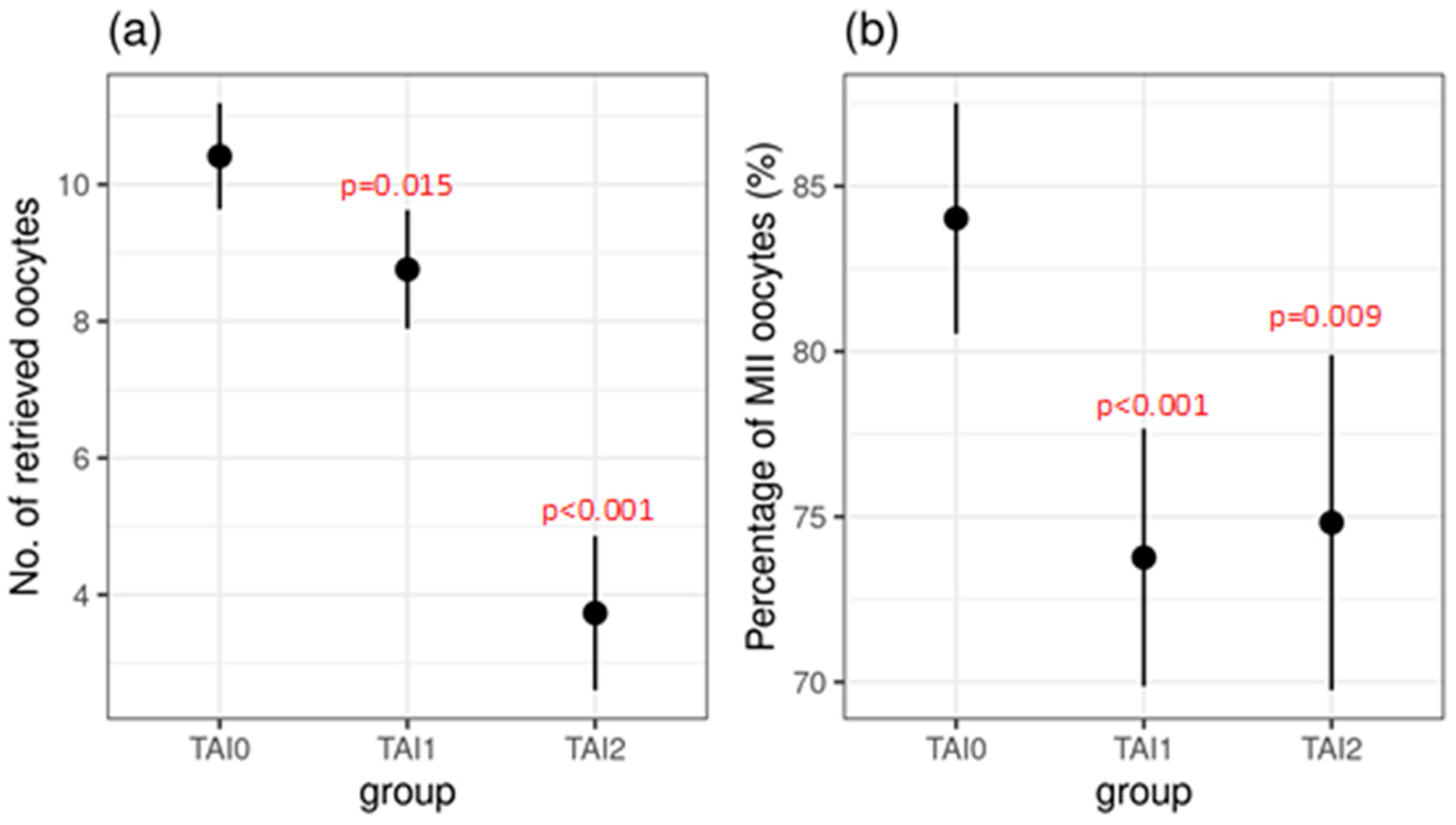
| Oocytes retrieved (n) | 10.4 ± 4.7 | 8.8 ± 6.0 | 3.7 ± 2.5 | 0.015 * | <0.001 * | <0.001 * |
| MII oocytes (%) | 84.0 ± 14.2 | 73.8 ± 25.1 | 73.8 ± 25.1 | <0.001 * | 0.009 * | 0.943 |
| Fertilized oocytes (n) | 6.5 ± 2.3 | 4.5 ± 2.8 | 2.2 ± 1.8 | <0.001 * | <0.001 * | <0.001 * |
| Fertilization rate (%) | 80.8 ± 15.0 | 70.3 ± 29.3 | 73.9 ± 33.1 | 0.002 * | 0.139 | 0.608 |
| Cleavage-stage embryos (3rd day) per MII oocytes (%) | 74.4 ± 15.6 | 63.6 ± 30.2 | 65.0 ± 35.6 | 0.002 * | 0.037 * | 0.933 |
| Blastocyst per MII oocytes (%) | 47.1 ± 18.4 | 26.8 ± 25.8 | 18.0 ± 30.1 | <0.001 * | <0.001 * | 0.038 * |
| Cleavage rate (%) | 92.6 ± 11.9 | 84.3 ± 30.3 | 79.1 ± 34.9 | 0.020 * | <0.001 * | 0.362 |
| Blastocyst rate (%) | 58.2 ± 19.9 | 35.7 ± 32.5 | 21.3 ± 34.7 | <0.001 * | <0.001 * | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sušanj Šepić, T.; Čavlović, K.; Dević Pavlić, S.; Smajla, N.; Višnić, A.; Radojčić Badovinac, A.; Smiljan Severinski, N. Thyroid Autoimmunity Impairs Oocyte Maturation, Fertilization, and Embryo Development in Assisted Reproductive Technology in Euthyroid Infertile Patients. J. Clin. Med. 2025, 14, 3385. https://doi.org/10.3390/jcm14103385
Sušanj Šepić T, Čavlović K, Dević Pavlić S, Smajla N, Višnić A, Radojčić Badovinac A, Smiljan Severinski N. Thyroid Autoimmunity Impairs Oocyte Maturation, Fertilization, and Embryo Development in Assisted Reproductive Technology in Euthyroid Infertile Patients. Journal of Clinical Medicine. 2025; 14(10):3385. https://doi.org/10.3390/jcm14103385
Chicago/Turabian StyleSušanj Šepić, Tina, Kristina Čavlović, Sanja Dević Pavlić, Nataša Smajla, Alenka Višnić, Anđelka Radojčić Badovinac, and Neda Smiljan Severinski. 2025. "Thyroid Autoimmunity Impairs Oocyte Maturation, Fertilization, and Embryo Development in Assisted Reproductive Technology in Euthyroid Infertile Patients" Journal of Clinical Medicine 14, no. 10: 3385. https://doi.org/10.3390/jcm14103385
APA StyleSušanj Šepić, T., Čavlović, K., Dević Pavlić, S., Smajla, N., Višnić, A., Radojčić Badovinac, A., & Smiljan Severinski, N. (2025). Thyroid Autoimmunity Impairs Oocyte Maturation, Fertilization, and Embryo Development in Assisted Reproductive Technology in Euthyroid Infertile Patients. Journal of Clinical Medicine, 14(10), 3385. https://doi.org/10.3390/jcm14103385





