The Future Diabetes Mortality: Challenges in Meeting the 2030 Sustainable Development Goal of Reducing Premature Mortality from Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Preparation
2.2. Forecasting Models
2.2.1. ARIMA (Autoregressive Integrated Moving Average)
2.2.2. GAM (Generalized Additive Model)
2.2.3. GLM (Generalized Linear Model)
2.2.4. Prophet (Developed by Meta/Facebook)
2.2.5. n-Sub-Epidemic Model
2.2.6. Ensemble Sub-Epidemic Models (Unweighted and Weighted)
2.3. Model Evaluation
2.4. Quantifying Forecast
3. Results
3.1. Forecasting by Type of Diabetes

3.2. Forecasting by Age Groups
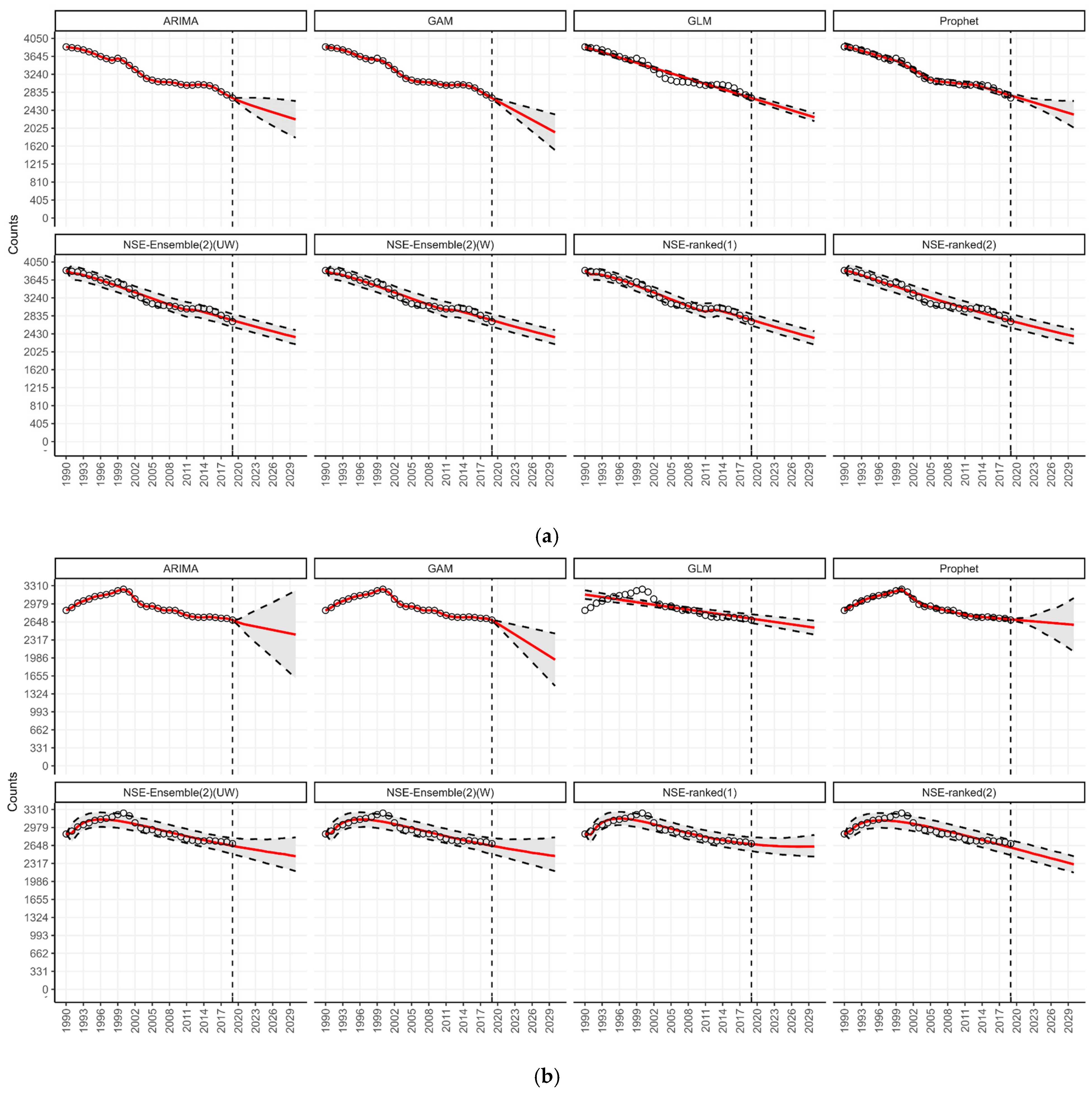
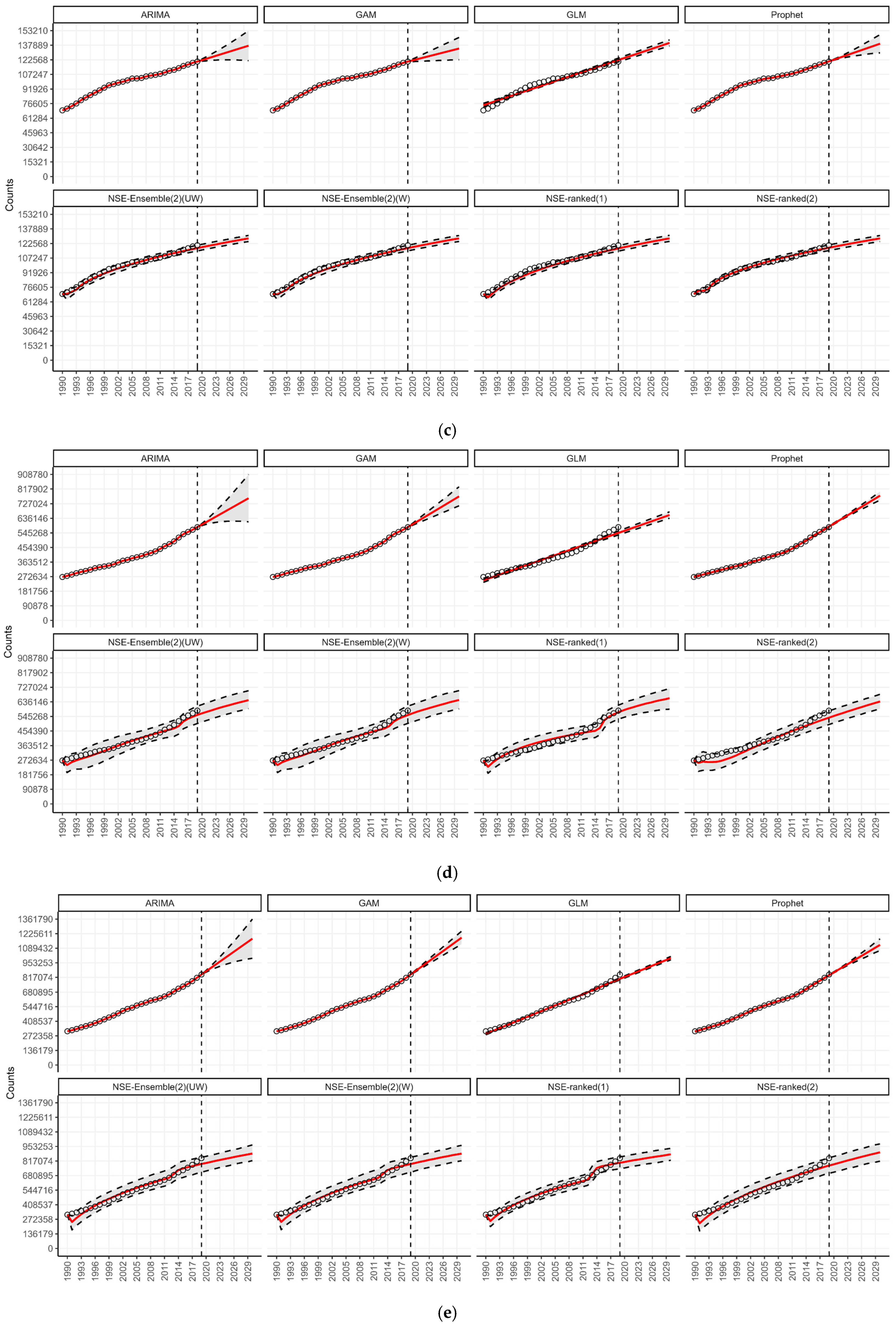
3.3. Forecasting by WHO Regions

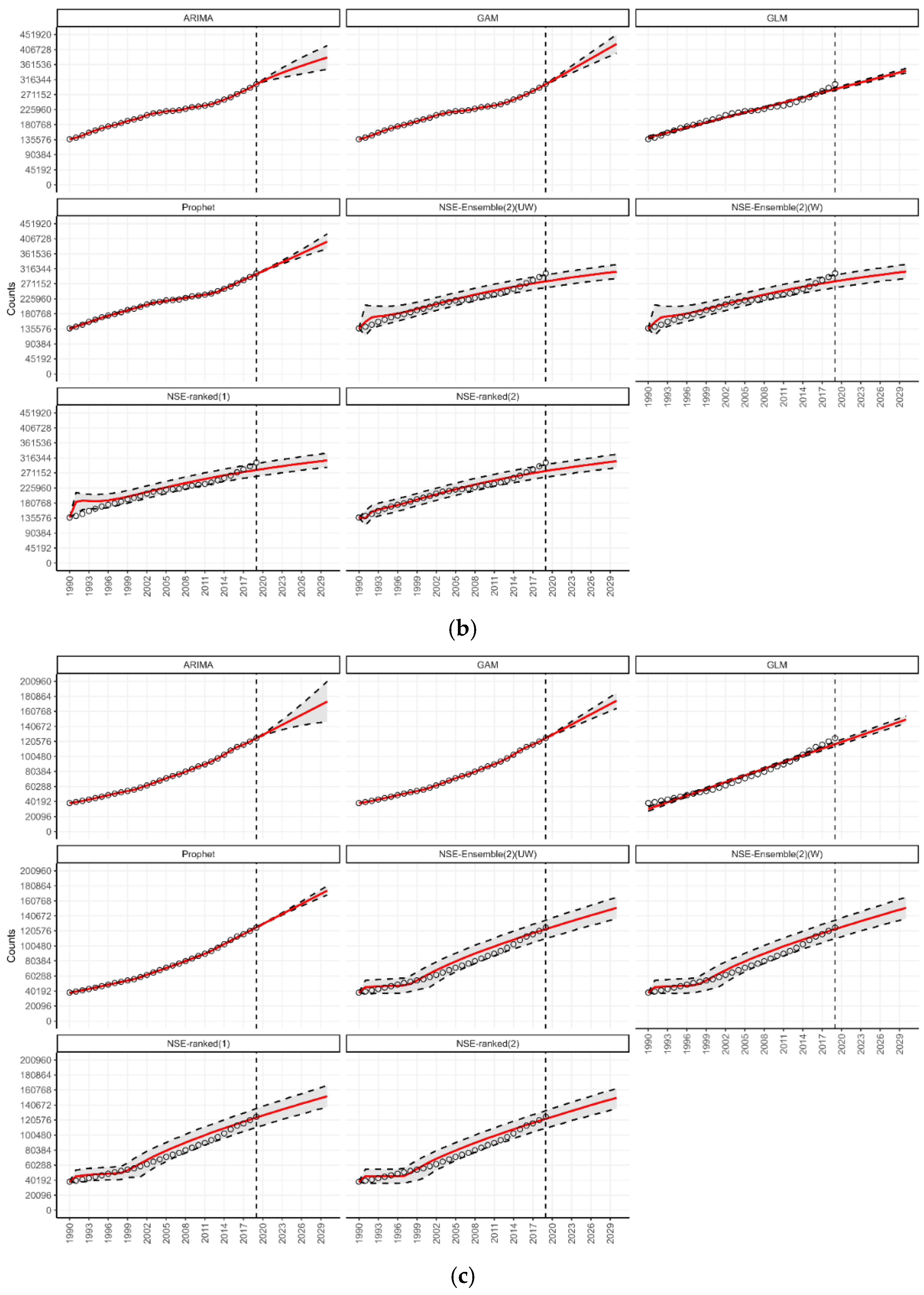
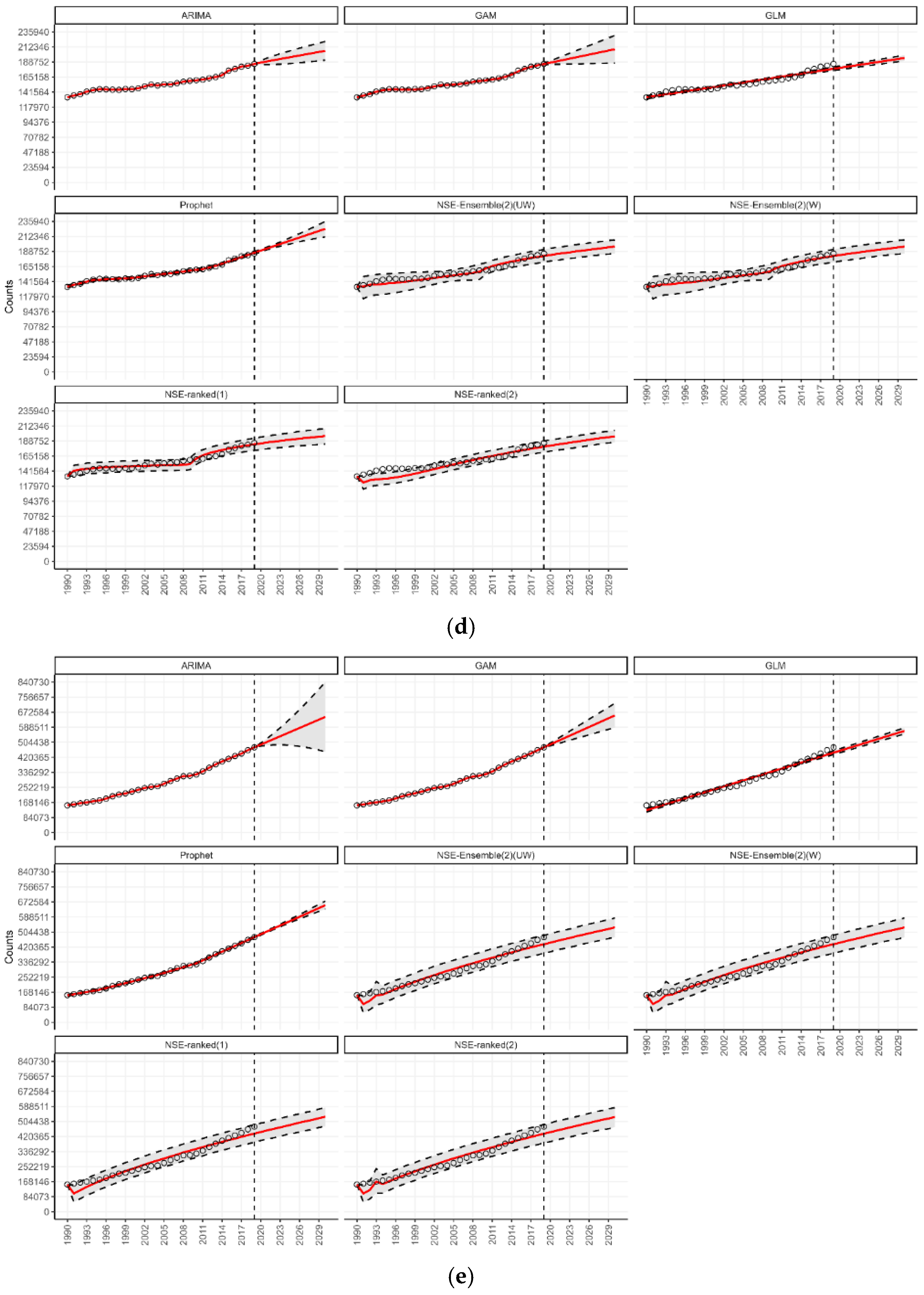
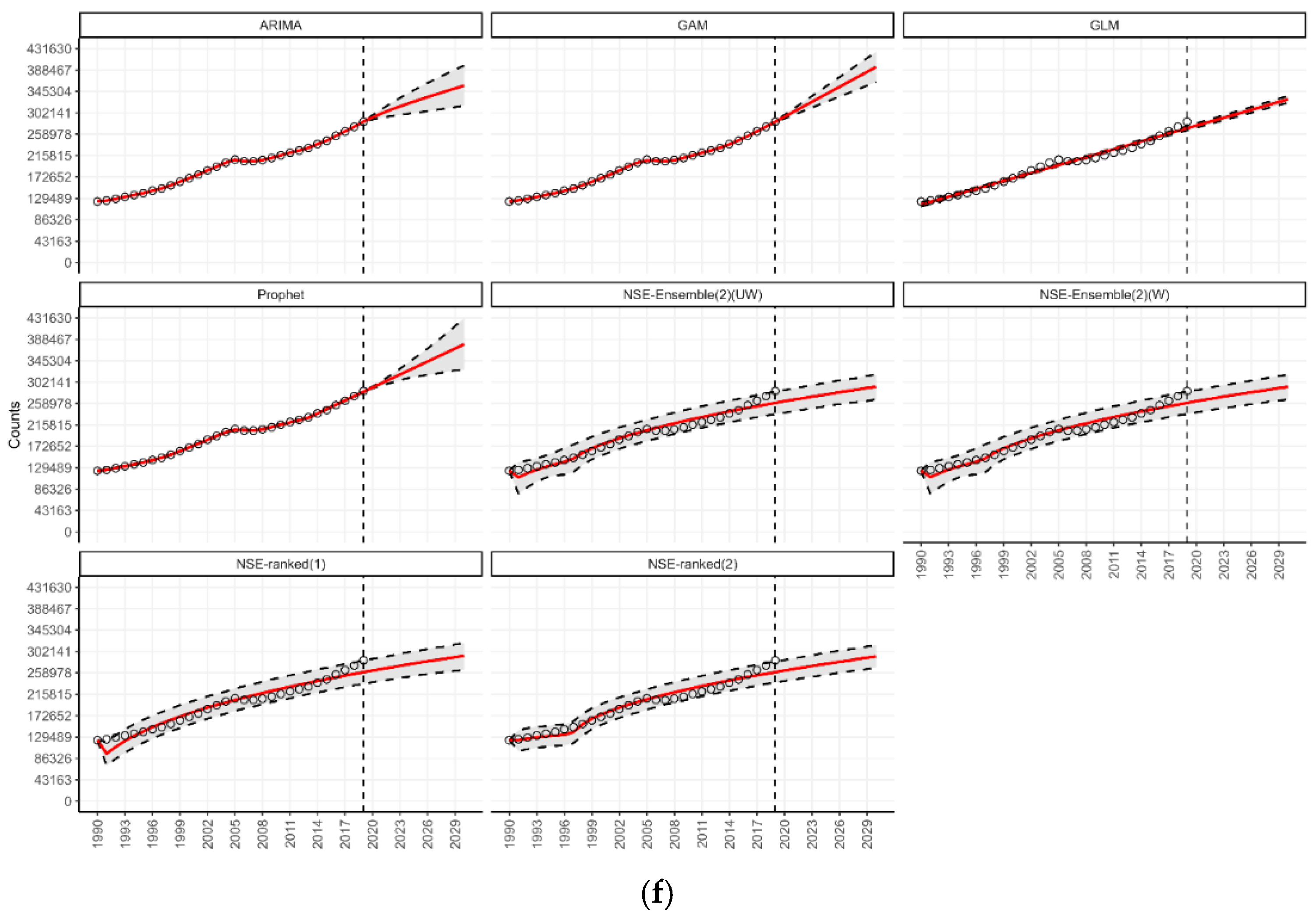
3.4. Forecasting for Countries by World Bank Income Classification
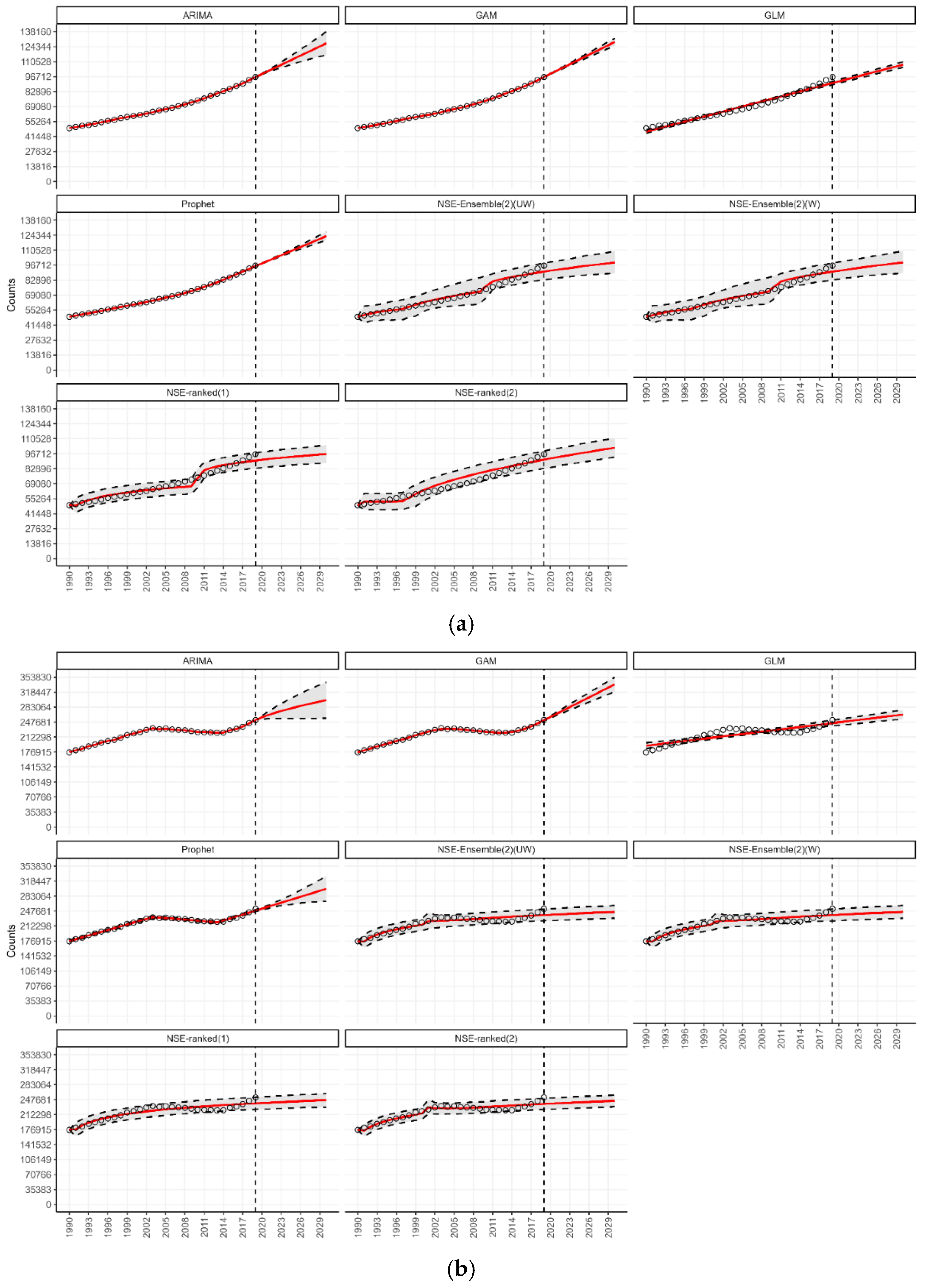
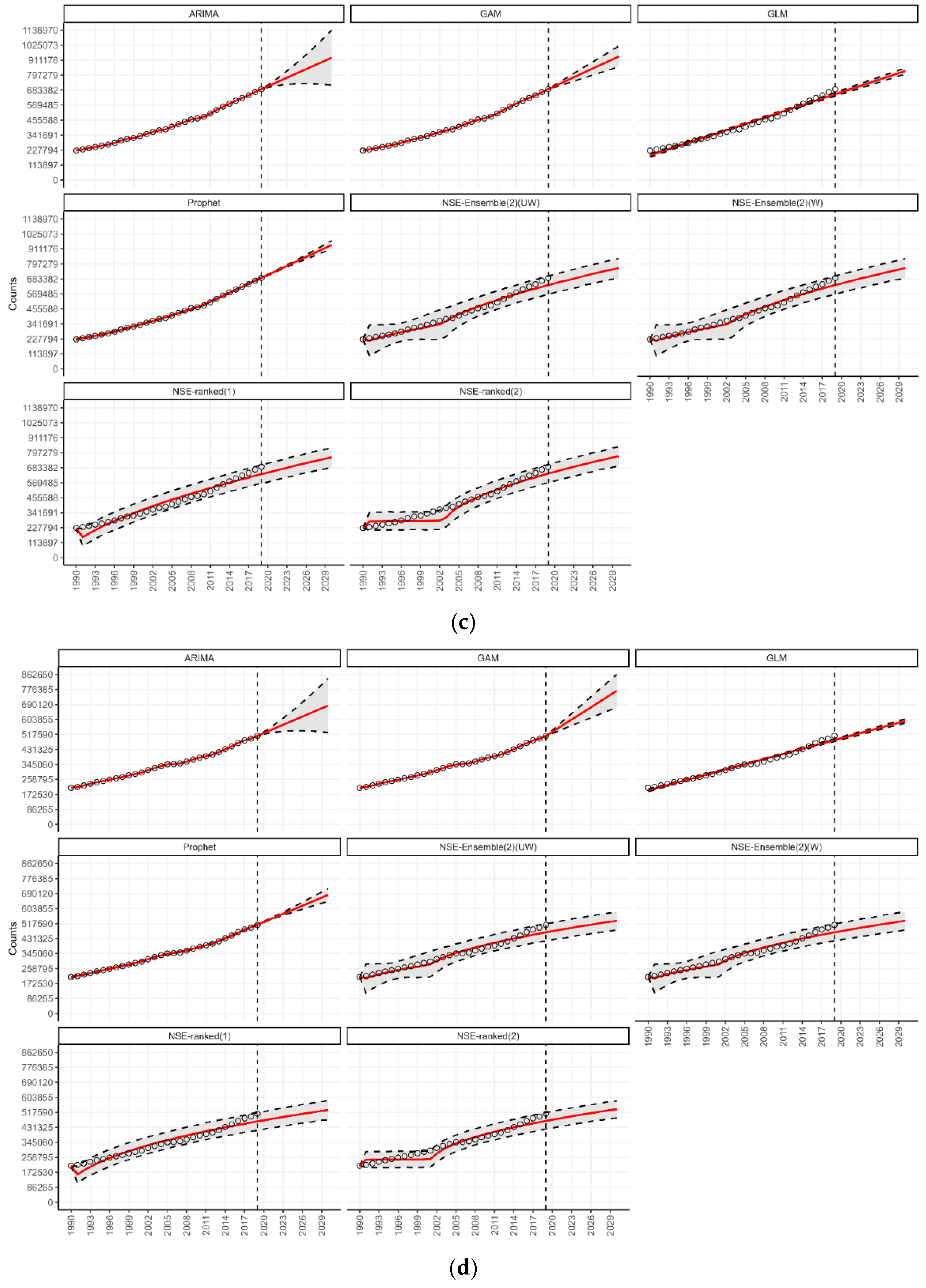
3.5. Forecasting in the Context of SDG 3.4
3.6. Summary of Main Findings
4. Discussion
4.1. Implications for Public Health and Healthcare Systems
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes JDda, R.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Sun, J.; Hu, W.; Ye, S.; Deng, D.; Chen, M. The Description and Prediction of Incidence, Prevalence, Mortality, Disability-Adjusted Life Years Cases, and Corresponding Age-Standardized Rates for Global Diabetes. J. Epidemiol. Glob. Health 2023, 13, 566–576. [Google Scholar] [CrossRef]
- Rathmann, W.; Giani, G. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030: Response to Wild et al. Diabetes Care 2004, 27, 2568–2569. [Google Scholar] [CrossRef]
- Watkins, D.A.; Msemburi, W.T.; Pickersgill, S.J.; Kawakatsu, Y.; Gheorghe, A.; Dain, K.; Johansson, K.A.; Said, S.; Renshaw, N.; Tolla, M.T.; et al. NCD Countdown 2030: Efficient pathways and strategic investments to accelerate progress towards the Sustainable Development Goal target 3.4 in low-income and middle-income countries. Lancet 2022, 399, 1266–1278. [Google Scholar] [CrossRef]
- Rowley, W.R.; Bezold, C.; Arikan, Y.; Byrne, E.; Krohe, S. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul. Health Manag. 2017, 20, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, D.; Li, N.; Han, B. Predicting Diabetes and Estimating Its Economic Burden in China Using Autoregressive Integrated Moving Average Model. Int. J. Public Health 2022, 66, 1604449. [Google Scholar] [CrossRef] [PubMed]
- Goal 3 | Department of Economic and Social Affairs. 2024. Available online: https://sdgs.un.org/goals/goal3#targets_and_indicators (accessed on 15 April 2024).
- SDG Target 3.4 | Noncommunicable Diseases and Mental Health: By 2030, Reduce by One Third Premature Mortality from Non-Communicable Diseases Through Prevention and Treatment and Promote Mental Health and Well-Being. 2024. Available online: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/sdg-target-3.4-noncommunicable-diseases-and-mental-health (accessed on 15 April 2024).
- United Nations General Assembly. Resolution Adopted by the General Assembly on 6 July 2017—Work of the Statistical Commission pertaining to the 2030 Agenda for Sustainable Development; No. A/RES/71/313; Seventy-First Session; United Nations General Assembly: New York, NY, USA, 2017; Available online: https://docs.un.org/en/A/RES/71/313 (accessed on 15 April 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019); Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2019; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 15 March 2024).
- Bleichrodt, A.; Luo, R.; Kirpich, A.; Chowell, G.; Phan, A. A 2024 ARIMA, GAM, GLM, Prophet Forecasting Toolbox–BETA; GitHub: San Francisco, CA, USA, 2024; Available online: https://github.com/bleicham/ARIMA-GLM-GAM-Prophet-Beta (accessed on 25 February 2025).
- Box, G.E.P.; Jenkins, G.M.; Reinsel, G.C.; Ljung, G.M. Time Series Analysis: Forecasting and Control, 5th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Available online: https://www.wiley.com/en-us/Time+Series+Analysis%3A+Forecasting+and+Control%2C+5th+Edition-p-9781118675021 (accessed on 25 February 2025).
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice, 3rd ed.; Otexts: Carlton South, Australia, 2021. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Simpson, G.L. Modelling Palaeoecological Time Series Using Generalised Additive Models. Front. Ecol. Evol. 2018, 6, 149. [Google Scholar] [CrossRef]
- Agresti, A. Foundations of Linear and Generalized Linear Models, 1st ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Taylor, S.J.; Letham, B. Forecasting at Scale. Am. Stat. 2018, 72, 37–45. [Google Scholar] [CrossRef]
- Facebook Open Source. Prophet: Forecasting at Scale. 2023. Available online: http://facebook.github.io/prophet/ (accessed on 15 April 2024).
- Chowell, G.; Luo, R.; Sun, K.; Roosa, K.; Tariq, A.; Viboud, C. Real-time forecasting of epidemic trajectories using computational dynamic ensembles. Epidemics 2020, 30, 100379. [Google Scholar] [CrossRef]
- Chowell, G.; Hyman, J.M. (Eds.) Mathematical and Statistical Modeling for Emerging and Re-Emerging Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chowell, G.; Dahal, S.; Tariq, A.; Roosa, K.; Hyman, J.M.; Luo, R. An ensemble n-sub-epidemic modeling framework for short-term forecasting epidemic trajectories: Application to the COVID-19 pandemic in the USA. PLoS Comput. Biol. 2022, 18, e1010602. [Google Scholar] [CrossRef]
- Bleichrodt, A.; Luo, R.; Kirpich, A.; Chowell, G. Retrospective evaluation of short-term forecast performance of ensemble sub-epidemic frameworks and other time-series models: The 2022–2023 mpox outbreak across multiple geographical scales, July 14th, 2022, through February 26th, 2023. medRxiv 2023. [Google Scholar] [CrossRef]
- Endocrinology, T.L.D. Alarming rise in young-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2024, 12, 433. [Google Scholar] [CrossRef]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ 2022, 379, e072385. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Tang, Y.; Zhang, S.; Zhuang, Z.; Ni, Q. Rising tide: The growing global burden and inequalities of early-onset type 2 diabetes among youths aged 15–34 years (1990–2021). Diabetol. Metab. Syndr. 2025, 17, 103. [Google Scholar] [CrossRef]
- Salehidoost, R.; Mansouri, A.; Amini, M.; Yamini, S.A.; Aminorroaya, A. Diabetes and all-cause mortality, a 18-year follow-up study. Sci. Rep. 2020, 10, 3183. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Conway, R.; Mayer-Davis, E.; Dabelea, D. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care 2023, 46, 490–499. [Google Scholar] [CrossRef] [PubMed]
- CDC Newsroom. CDC. Future Surge in Diabetes Could Dramatically Impact People Under 20 in U.S. 1 January 2016. Available online: https://archive.cdc.gov/www_cdc_gov/media/releases/2022/p1229-future-diabetes-surge.html (accessed on 15 April 2024).
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef]
- Cho, N.H. Diabetes burden and prevention in Korea and the Western Pacific Region. Diabetes Res. Clin. Pract. 2014, 106 (Suppl. S2), S282–S287. [Google Scholar] [CrossRef]
- Ye, J.; Wu, Y.; Yang, S.; Zhu, D.; Chen, F.; Chen, J.; Ji, X.; Hou, K. The global, regional and national burden of type 2 diabetes mellitus in the past, present and future: A systematic analysis of the Global Burden of Disease Study 2019. Front. Endocrinol. 2023, 14, 1192629. [Google Scholar] [CrossRef]
- Bernabe-Ortiz, A.; Carrillo-Larco, R.M. The burden of diabetes in the Americas. Lancet Diabetes Endocrinol. 2022, 10, 613–614. [Google Scholar] [CrossRef]
- Redmond, M.L.; Bimali, M.; Ablah, E.; Mayes, P.; Dugan, K. A Geo-Stratified Analysis of Associations Between Socio-Economic Factors and Diabetes Risk. Kans. J. Med. 2022, 15, 175–183. [Google Scholar] [CrossRef]
- Liu, J.; Bai, R.; Chai, Z.; Cooper, M.E.; Zimmet, P.Z.; Zhang, L. Low- and middle-income countries demonstrate rapid growth of type 2 diabetes: An analysis based on Global Burden of Disease 1990–2019 data. Diabetologia 2022, 65, 1339–1352. [Google Scholar] [CrossRef]
- Dagenais, G.R.; Gerstein, H.C.; Zhang, X.; McQueen, M.; Lear, S.; Lopez-Jaramillo, P.; Mohan, V.; Mony, P.; Gupta, R.; Kutty, V.R.; et al. Variations in Diabetes Prevalence in Low-, Middle-, and High-Income Countries: Results From the Prospective Urban and Rural Epidemiological Study. Diabetes Care 2016, 39, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Flood, D.; Seiglie, J.A.; Dunn, M.; Tschida, S.; Theilmann, M.; Marcus, M.E.; Brian, G.; Norov, B.; Mayige, M.T.; Singh Gurung, M.; et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: A cross-sectional study of nationally representative, individual-level data in 680 102 adults. Lancet Healthy Longev. 2021, 2, e340–e351. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Diabetes Atlas. 2024. Available online: https://diabetesatlas.org/ (accessed on 4 April 2025).
- Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; Stevens, G.A.; Danaei, G.; Lhoste, V.P.; Phelps, N.H.; Heap, R.A.; Jain, L.; Brisis, Y.D.D.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Lv, F.; Gao, X.; Huang, A.H.; Zu, J.; He, X.; Sun, X.; Liu, J.; Gao, N.; Jiao, Y.; Keane, M.G.; et al. Excess diabetes mellitus-related deaths during the COVID-19 pandemic in the United States. eClinicalMedicine 2022, 54, 101671. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Cousin, E.; Duncan, B.B.; Stein, C.; Ong, K.L.; Vos, T.; Abbafati, C.; Abbasi-Kangevari, M.; Abdelmasseh, M.; Abdoli, A.; Abd-Rabu, R.; et al. Diabetes mortality and trends before 25 years of age: An analysis of the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol. 2022, 10, 177–192. [Google Scholar] [CrossRef]
- Mondal, P.; Shit, L.; Goswami, S. Study of Effectiveness of Time Series Modeling (Arima) in Forecasting Stock Prices. Int. J. Comput. Sci. Eng. Appl. 2014, 4, 13–29. [Google Scholar] [CrossRef]
- Dimri, T.; Ahmad, S.; Sharif, M. Time series analysis of climate variables using seasonal ARIMA approach. J. Earth Syst. Sci. 2020, 129, 149. [Google Scholar] [CrossRef]
- Tektas, M. Weather Forecasting Using ANFIS and ARIMA MODELS: Case study for İstanbul. Environ. Res. Eng. Manag. 2010, 51, 5–10, ISSN: 1392-1649. [Google Scholar]
- Satrio, C.B.A.; Darmawan, W.; Nadia, B.U.; Hanafiah, N. Time series analysis and forecasting of coronavirus disease in Indonesia using ARIMA model and PROPHET. Procedia Comput. Sci. 2021, 179, 524–532. [Google Scholar] [CrossRef]
- Bleichrodt, A.; Phan, A.; Luo, R.; Kirpich, A.; Chowell-Puente, G. StatModPredict: A User-Friendly R-Shiny Interface for Fitting and Forecasting with Statistical Models; SSRN Scholarly Paper No. 4849702; Social Science Research Network: Atlanta, GA, USA, 2014. [Google Scholar] [CrossRef]
- Long, B.; Tan, F.; Newman, M. Forecasting the Monkeypox Outbreak Using ARIMA, Prophet, NeuralProphet, and LSTM Models in the United States. Forecasting 2023, 5, 127–137. [Google Scholar] [CrossRef]
- A ARIMA modelling in, R. In Forecasting: Principles and Practice, 2nd ed.; OTexts: Melbourne, Australia, 2024; Available online: https://otexts.com/fpp2/arima-r.html (accessed on 10 April 2025).
- Auto.Arima Function—RDocumentation, Version 8.21.1; DataCamp Inc.: New York, NY, USA, 2024. Available online: https://www.rdocumentation.org/packages/forecast/versions/8.21.1/topics/auto.arima (accessed on 10 April 2025).
- Forecast Function—RDocumentation, Version 8.24.0; DataCamp Inc.: New York, NY, USA, 2024. Available online: https://www.rdocumentation.org/packages/forecast/versions/8.4/topics/forecast (accessed on 15 April 2024).
- Shafi, A. What is a Generalised Additive Model? Medium. 18 May 2021. Available online: https://towardsdatascience.com/generalised-additive-models-6dfbedf1350a (accessed on 15 April 2024).
- Wood, S.N.; Fasiolo, M. A Generalized Fellner-Schall Method for Smoothing Parameter Optimization with Application to Tweedie Location, Scale and Shape Models. Biometrics 2017, 73, 1071–1081. [Google Scholar] [CrossRef]
- Predict Function—RDocumentation, Version 3.6.2; DataCamp Inc.: New York, NY, USA, 2024. Available online: https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/predict (accessed on 15 April 2024).
- Ampofo, A.G.; Boateng, E.B. Beyond 2020: Modelling obesity and diabetes prevalence. Diabetes Res. Clin. Pract. 2020, 167, 108362. [Google Scholar] [CrossRef]
- Prophet Package—RDocumentation, version 1.0; DataCamp Inc.: New York, NY, USA, 2024; Available online: https://www.rdocumentation.org/packages/prophet/versions/1.0 (accessed on 15 April 2024).
- Chowell, G.; Dahal, S.; Bleichrodt, A.; Tariq, A.; Hyman, J.M.; Luo, R. SubEpiPredict: A tutorial-based primer and toolbox for fitting and forecasting growth trajectories using the ensemble n-sub-epidemic modeling framework. Infect. Dis. Model. 2024, 9, 411–436. [Google Scholar] [CrossRef]
- Chowell, G.; Hincapie-Palacio, D.; Ospina, J.; Pell, B.; Tariq, A.; Dahal, S.; Moghadas, S.; Smirnova, A.; Simonsen, L.; Viboud, C. Using Phenomenological Models to Characterize Transmissibility and Forecast Patterns and Final Burden of Zika Epidemics. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Pell, B.; Kuang, Y.; Viboud, C.; Chowell, G. Using phenomenological models for forecasting the 2015 Ebola challenge. Epidemics 2018, 22, 62–70. [Google Scholar] [CrossRef]
- Roosa, K.; Lee, Y.; Luo, R.; Kirpich, A.; Rothenberg, R.; Hyman, J.M.; Yan, P.; Chowell, G. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect. Dis. Model. 2020, 5, 256–263. [Google Scholar] [CrossRef]
- Roosa, K.; Lee, Y.; Luo, R.; Kirpich, A.; Rothenberg, R.; Hyman, J.M.; Yan, P.; Chowell, G. Short-term Forecasts of the COVID-19 Epidemic in Guangdong and Zhejiang, China: February 13–23, 2020. J. Clin. Med. 2020, 9, 596. [Google Scholar] [CrossRef]
- Hurvich, C.M.; Tsai, C.-L. Regression and time series model selection in small samples. Biometrika 1989, 76, 297–307. [Google Scholar] [CrossRef]
- Sugiura, N. Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun. Stat.-Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]

| Category | ARIMA | GAM | GLM | Prophet | NSE (UW) | NSE (W) |
|---|---|---|---|---|---|---|
| Type of diabetes | ||||||
| Type 1 | 88.3 (81.9, 94.7) | 90.3 (86.0, 94.6) | 82.9 (81.4, 84.5) | 92.9 (85.6, 100.3) | 86.5 (83.6, 89.2) | 80.1 (76.9, 83.3) |
| Type 2 * | 1.9 (1.8, 2.1) | 2.0 (1.9, 2.1) | 1.7 (1.6, 1.7) | 1.9 (1.8, 2.0) | 1.5 (1.3, 1.6) | 1.5 (1.3, 1.6) |
| Age Group | ||||||
| <5 | 2.23 (1.82, 2.64) | 1.94 (1.53, 2.34) | 2.28 (81.33, 84.46) | 92.87 (85.6, 100.27) | 2.36 (2.2, 2.52) | 2.36 (2.2, 2.52) |
| 5–14 | 2.42 (1.61, 3.23) | 1.96 (1.48, 2.44) | 2.55 (2.42, 2.68) | 2.6 (2.11, 3.09) | 2.46 (2.18, 2.8) | 2.46 (2.18, 2.8) |
| 15–49 | 137.5 (121.7, 153.2) | 134.5 (122.8, 146.1) | 140.4 (137.3, 143.6) | 139.6 (130.0, 149.1) | 128.1 (124.7, 131.1) | 128 (124.7, 131.0) |
| 50–69 | 761.6 (614.4, 908.8) | 773.1 (714.5, 831.7) | 657 (637.1, 676.9) | 777.9 (750.5, 801.5) | 646.8 (594, 705.8) | 647.7 (593, 706.4) |
| 70+ | 1178.7 (995.7, 1361.8) | 1188.4 (1127.9, 1249) | 996.9 (981.2, 1012.6) | 1120.7 (1067.5, 1177.3) | 887.4 (821, 967.8) | 886.5 (820.1, 963.8) |
| WHO Region | ||||||
| AFR | 209.2 (614.4, 908.8) | 205.8 (714.5, 831.7) | 205.6 (637.1, 676.9) | 212.3 (750.5, 801.5) | 186.9 (594, 705.8) | 187 (593, 706.4) |
| AMR | 383.2 (347.7, 418.7) | 423.9 (395.9, 451.9) | 343.2 (336.4, 350) | 398.2 (377, 422.9) | 307.8 (286.9, 329.2) | 308 (287.2, 329.3) |
| EMR | 174 (147, 201) | 175.2 (164.8, 185.5) | 149.9 (145.1, 154.7) | 174.6 (167.9, 180.9) | 151.5 (136.9, 165.3) | 151.4 (136.9, 165.4) |
| EUR | 206.3 (191.7, 221) | 208.8 (187.2, 230.4) | 195 (190.7, 199.4) | 224.3 (212.5, 236.7) | 196.6 (185.7, 207.1) | 196.4 (185.7, 207.1) |
| SEAR | 646 (451.4, 840.7) | 654.4 (586.7, 722.1) | 568 (551.1, 584.9) | 654.4 (630.2, 677.8) | 530.6 (474.4, 582.4) | 530.2 (474.2, 582.3) |
| WPR | 357.2 (316.7, 397.7) | 394.7 (364.3, 425.2) | 329.5 (322.7, 336.3) | 378.7 (328.5, 436.6) | 292.8 (267.4, 317.4) | 292.6 (267, 317.1) |
| World Bank Income Classification | ||||||
| High-Income | 299.7 (614.4, 908.8) | 336.9 (714.5, 831.7) | 265.7 (637.1, 676.9) | 300.2 (750.5, 801.5) | 245.7 (594, 705.8) | 245.9 (593, 706.4) |
| Low-Income | 127.5 (116.7, 138.2) | 128.7 (125.4, 132) | 107.9 (105.3, 110.4) | 123.5 (119.5, 127.6) | 99.2 (89.6, 109.7) | 99.2 (89.7, 109.7) |
| Low-Middle | 930.1 (721.3, 1139) | 938.8 (860, 1017.5) | 828.8 (806.2, 851.3) | 941.6 (909.6, 973.8) | 765.9 (690.9, 838.3) | 766.6 (691.3, 838.2) |
| Upper-Middle | 684.2 (528.8, 839.5) | 768.2 (673.7, 862.7) | 595 (583, 607) | 682.6 (645.8, 722.2) | 532.7 (480.2, 585.1) | 533.7 (481.4, 584) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagh, K.; Kirpich, A.; Chowell, G. The Future Diabetes Mortality: Challenges in Meeting the 2030 Sustainable Development Goal of Reducing Premature Mortality from Diabetes. J. Clin. Med. 2025, 14, 3364. https://doi.org/10.3390/jcm14103364
Wagh K, Kirpich A, Chowell G. The Future Diabetes Mortality: Challenges in Meeting the 2030 Sustainable Development Goal of Reducing Premature Mortality from Diabetes. Journal of Clinical Medicine. 2025; 14(10):3364. https://doi.org/10.3390/jcm14103364
Chicago/Turabian StyleWagh, Kaustubh, Alexander Kirpich, and Gerardo Chowell. 2025. "The Future Diabetes Mortality: Challenges in Meeting the 2030 Sustainable Development Goal of Reducing Premature Mortality from Diabetes" Journal of Clinical Medicine 14, no. 10: 3364. https://doi.org/10.3390/jcm14103364
APA StyleWagh, K., Kirpich, A., & Chowell, G. (2025). The Future Diabetes Mortality: Challenges in Meeting the 2030 Sustainable Development Goal of Reducing Premature Mortality from Diabetes. Journal of Clinical Medicine, 14(10), 3364. https://doi.org/10.3390/jcm14103364









