Insights into the Current Management Techniques for Peri-Implant Gaps: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Ethics
2.2. Source
2.3. Search Strategy
2.4. Eligibility Criteria
2.4.1. Participants
2.4.2. Interventions
2.4.3. Comparators
2.4.4. Outcomes
2.4.5. Study Design
2.4.6. Language
2.4.7. Study Location
2.4.8. Publication Year
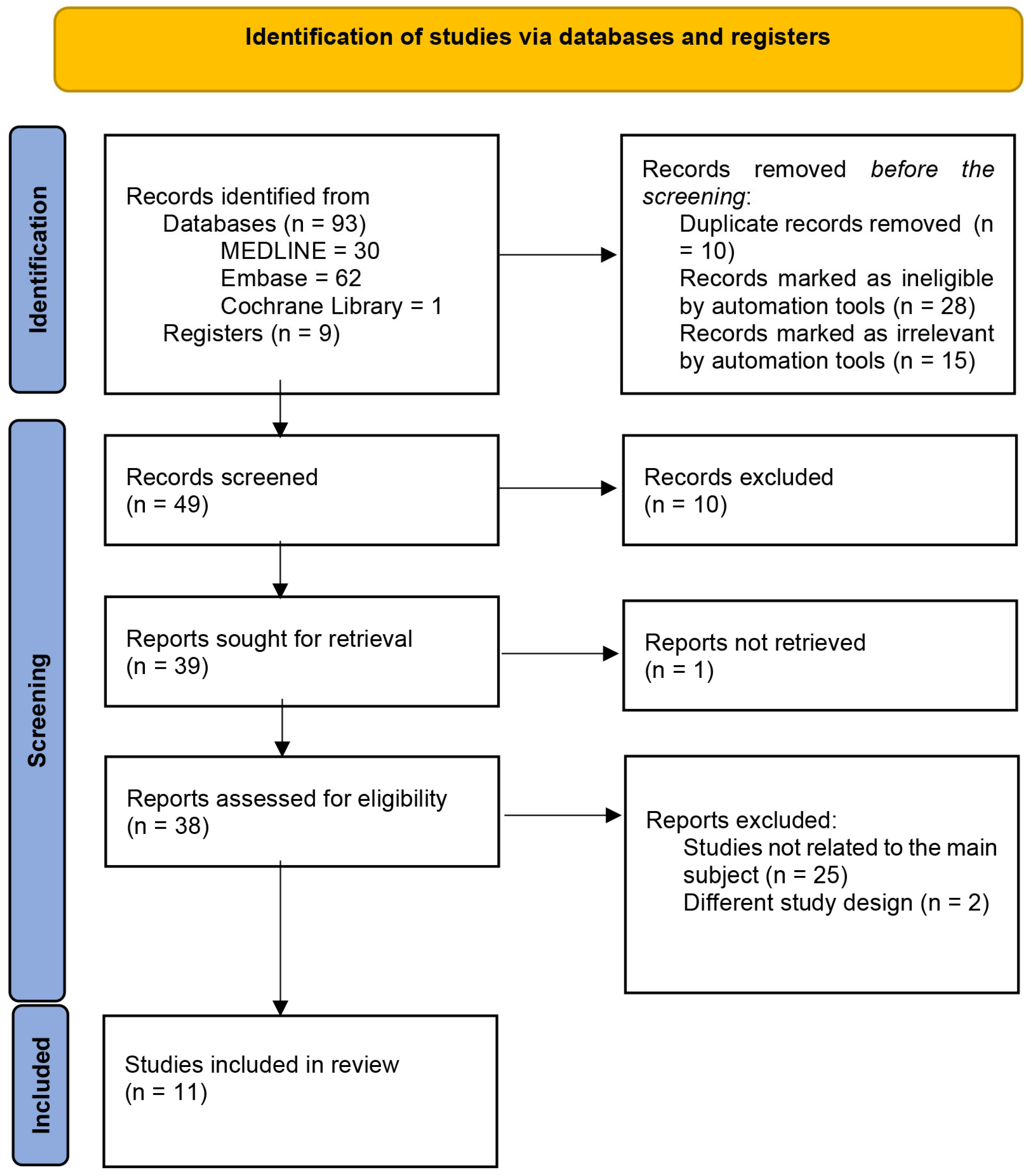
2.5. Study Selection
2.6. Data Extraction and Management
2.6.1. Study Characteristics
2.6.2. Outcome Data
2.6.3. Risk-of-Bias Assessment
3. Results
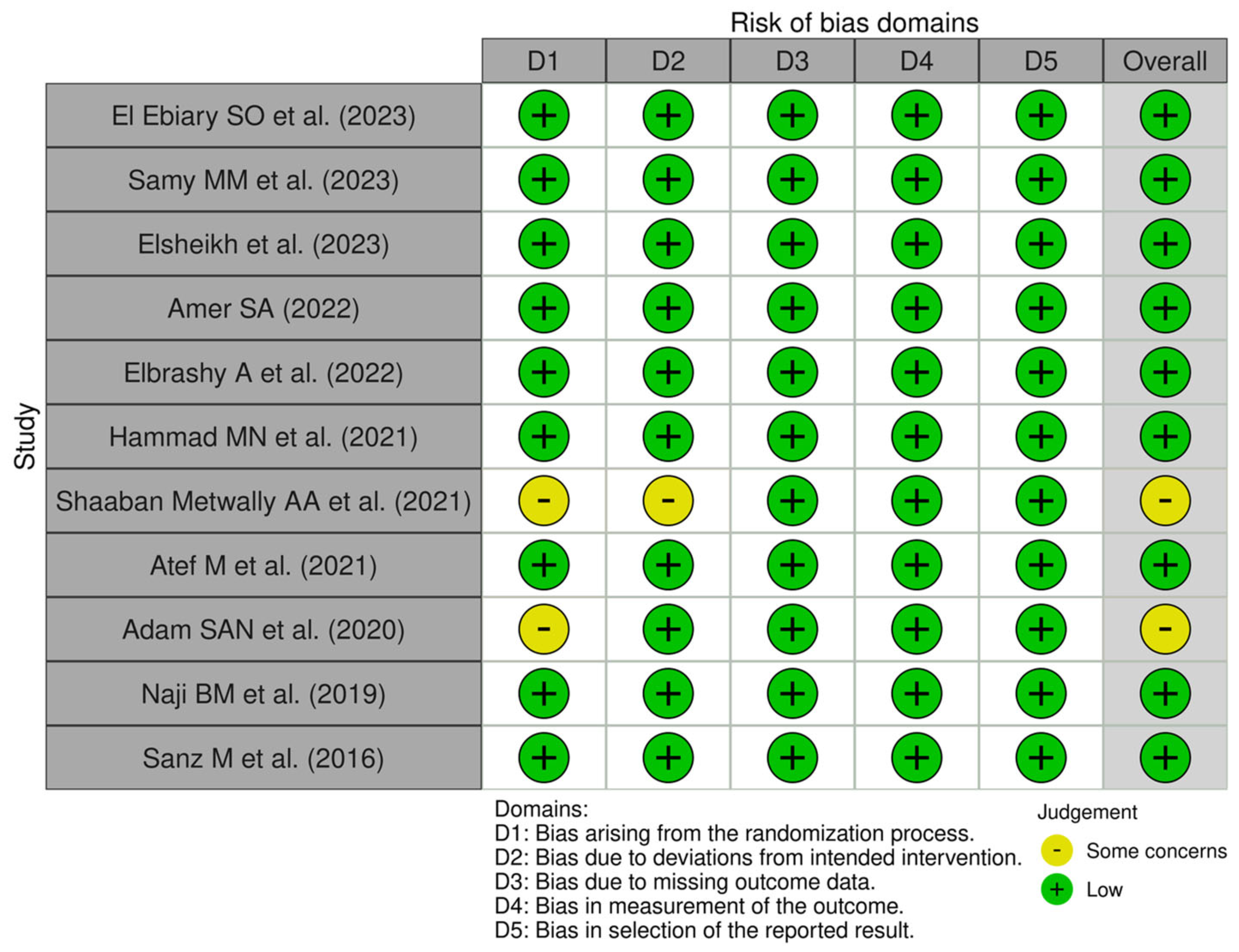
3.1. Risk-of-Bias Analysis
3.2. The Characteristics and Outcomes of the Included Studies
4. Discussion
- The inhibition of myofibroblast activities (especially in the first three weeks of soft tissue healing). This can be achieved via the fast, complete wound epithelialization of the socket by using the following:
- A free gingival graft to close the gap between the two portions of the flap that was designed during the implant surgery [43];
- Platelet concentrations with the aim of closing the gap between the two portions of the flap designed during the implant surgery and filling it in with a natural biomaterial [44].
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.T.; Buser, D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla: A systematic review. Int. J. Oral. Maxillofac. Implant. 2014, 29, 186–215. [Google Scholar] [CrossRef]
- Kabi, S.; Kar, R.; Samal, D.; Deepak, K.C.; Kar, I.B.; Mishra, N. Immediate dental implant placement with or without autogenous bone graft: A comparative study. Natl. J. Maxillofac. Surg. 2020, 11, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Liñares, A.; Dopico, J.; Magrin, G.; Blanco, J. Critical review on bone grafting during immediate implant placement. Periodontol. 2000 2023, 93, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Naiem, S.N.; Al-Nawas, B.; Tawfik, O.K.; El-Nahass, H. Jumping gap in immediate implant placement in the esthetic zone: A virtual implant planning using cone-beam computed tomography. J. Prosthodont. Res. 2023. ahead of print. [Google Scholar] [CrossRef]
- Kuchler, U.; Chappuis, V.; Gruber, R.; Lang, N.P.; Salvi, G.E. Immediate implant placement with simultaneous guided bone regeneration in the esthetic zone: 10-year clinical and radiographic outcomes. Clin. Oral. Implant. Res. 2016, 27, 253–257. [Google Scholar] [CrossRef]
- Santana, R.B.; Santana, C.M.; Dibart, S. Platelet-derived growth factor-mediated guided bone regeneration in immediate implant placement in molar sites with buccal bone defects. Int. J. Periodontics Restor. Dent. 2015, 35, 825–833. [Google Scholar] [CrossRef]
- Noelken, R.; Moergel, M.; Kunkel, M.; Wagner, W. Immediate and flapless implant insertion and provisionalization using autogenous bone grafts in the esthetic zone: 5-year results. Clin. Oral. Implant. Res. 2018, 29, 320–327. [Google Scholar] [CrossRef]
- Santos, P.L.; Gulinelli, J.L.; Telles, C.D.; Betoni Junior, W.; Okamoto, R.; Buchignani, V.C.; Queiroz, T.P. Bone substitutes for peri-implant defects of postextraction implants. Int. J. Biomater. 2013, 2013, 307136. [Google Scholar] [CrossRef]
- Colet, D.; Neiss, F.A.; Conci, R.A.; Griza, G.L. Using synthetic biomaterial to fill peri-implant defects (gap) in immediate implants. Dent. Press. Implantol. 2013, 7, 64–71. [Google Scholar]
- Noelken, R.; Pausch, T.; Wagner, W.; Al-Nawas, B. Peri-implant defect grafting with autogenous bone or bone graft material in immediate implant placement in molar extraction sites: 1- to 3-year results of a prospective randomized study. Clin. Oral. Implant. Res. 2020, 31, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral. Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, R.; Kundu, B.; Datta, S.; Kumar, M.; Chanda, A.; Kundu, D. Clinical outcome of hydroxyapatite coated, bioactive glass coated, and machined Ti6Al4V threaded dental implant in human jaws: A short-term comparative study. Implant. Dent. 2016, 25, 252–260. [Google Scholar] [CrossRef]
- Tabassum, A. Guided implant surgery and immediate implant placement in esthetic zone. Int. J. Med. Dent. Case Rep. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Kaushik, S.; Rathee, M.; Jain, P.; Malik, S.; Agarkar, V.; Alam, M. Effect of conventionally fabricated and three-dimensional printed provisional restorations on hard and soft peri-implant tissues in the mandibular posterior region: A randomized controlled clinical trial. Dent. Res. J. 2023, 20, 109. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, Y.E.; Kim, M.J.; Hwang, S.J. Peri-implant bone regeneration in hydroxyapatite block grafts with mesenchymal stem cells and bone morphogenetic protein-2. Tissue Eng. Regen. Med. 2016, 13, 437–445. [Google Scholar] [CrossRef]
- Shanbhag, S.; Shanbhag, V.; Stavropoulos, A. Genomic analyses of early peri-implant bone healing in humans: A systematic review. Int. J. Implant. Dent. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.Y.; Rungcharassaeng, K.; Deflorian, M.; Weinstein, T.; Wang, H.L.; Testori, T. Immediate implant placement and provisionalization of maxillary anterior single implants. Periodontol. 2000 2018, 77, 197–212. [Google Scholar] [CrossRef]
- El Ebiary, S.O.; Atef, M.; Abdelaziz, M.S.; Khashaba, M. Guided immediate implant with and without using a mixture of autogenous and xeno bone grafts in the dental esthetic zone: A randomized clinical trial. BMC Res. Notes 2023, 16, 331. [Google Scholar] [CrossRef]
- Samy, M.; Sharara, A.; El Halawani, G. Evaluation of mineralized plasmatic matrix as a grafting material versus beta tricalcium phosphate in immediate implant placement of mandibular molars: A randomized controlled clinical trial. Alex. Dent. J. 2024, 49, 53–60. [Google Scholar] [CrossRef]
- Elsheikh, H.A.; Abdelsameaa, S.E.; Elbahnasi, A.A.; Abdel-Rahman, F.H. Comparison between platelet rich fibrin as space filling material versus xenograft and alloplastic bone grafting materials in immediate implant placement: A randomized clinical trial. BMC Oral Health 2023, 23, 977. [Google Scholar] [CrossRef] [PubMed]
- Amer, S. Management of gap distance around immediate implants with topical melatonin gel and hyaluronic acid: A randomized clinical trial. Egypt. Dent. J. 2022, 68, 2209–2222. [Google Scholar] [CrossRef]
- Elbrashy, A.; Osman, A.H.; Shawky, M.; Askar, N.; Atef, M. Immediate implant placement with platelet rich fibrin as space filling material versus deproteinized bovine bone in maxillary premolars: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2022, 24, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.N.; El-Bialy, R.; Abdel Rasoul, M.A. Immediate implant placement with immediate provisionalization in the maxillary esthetic zone using mixture of allograft and xenograft vs xenografts to augment the jumping gap: A randomized clinical trial. Egypt. Dent. J. 2021, 67, 191–201. [Google Scholar] [CrossRef]
- Shaaban Metwally, A.A.; Fahmy, A.M.; El Khourazaty, N.S. Effects of platelet-rich fibrin on bone density in immediate implant placement and loading in esthetic zone: A randomized clinical trial. Egypt. Dent. J. 2021, 67, 669–677. [Google Scholar] [CrossRef]
- Atef, M.; El Barbary, A.; Dahrous, M.S.; Zahran, A.F. Comparison of the soft and hard peri-implant tissue dimensional changes around single immediate implants in the esthetic zone with socket shield technique versus using xenograft: A randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 456–465. [Google Scholar] [CrossRef]
- Adam, S.A.; Elarab, A.E.; Rahman, A.R.; Rahim, D.A. Evaluation of implant stability and marginal bone loss in immediate implant using nano bone versus autogenous bone: A randomized controlled trial. J. Osseointegr. 2020, 12, 8–17. [Google Scholar]
- Naji, B.M.; Abdelsameaa, S.S.; Alqutaibi, A.Y.; Ahmed, W.S. Immediate dental implant placement with a horizontal gap more than two millimetres: A randomized clinical trial. Int. J. Oral. Maxillofac. Surg. 2021, 50, 683–690. [Google Scholar] [CrossRef]
- Sanz, M.; Lindhe, J.; Alcaraz, J.; Sanz-Sanchez, I.; Cecchinato, D. The effect of placing a bone replacement graft in the gap at immediately placed implants: A randomized clinical trial. Clin. Oral. Implant. Res. 2017, 28, 902–910. [Google Scholar] [CrossRef]
- Simion, M.; Dahlin, C.; Trisi, P.; Piattelli, A. Qualitative and quantitative comparative study on different filling materials used in bone tissue regeneration: A controlled clinical study. Int. J. Periodontics Restor. Dent. 1994, 14, 198–215. [Google Scholar] [CrossRef]
- Seyssens, L.; Eeckhout, C.; Cosyn, J. Immediate implant placement with or without socket grafting: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2022, 24, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Natale, M.; Soardi, C.M.; Saleh, M.H.A.; Ponzi, A.; Tagliaferri, D.; Filannino, F.M.; Fontana, F.; Decker, A.; Marinotti, F.; d’Ambrosio, A.; et al. Immediate implant placement using the socket shield technique: Clinical, radiographic, and volumetric results using 3D digital techniques—A case series. Int. J. Periodontics Restor. Dent. 2024, 44, 187–195. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, R.; Crespi, G.; Cosola, S.; Covani, U. A retrospective digital analysis of contour changing after tooth extraction with or without using less traumatic surgical procedures. J. Clin. Med. 2022, 11, 922. [Google Scholar] [CrossRef]
- Covani, U.; Giammarinaro, E.; Panetta, D.; Salvadori, P.A.; Cosola, S.; Marconcini, S. Alveolar bone remodeling with or without collagen filling of the extraction socket: A high-resolution X-ray tomography animal study. J. Clin. Med. 2022, 11, 2493. [Google Scholar] [CrossRef] [PubMed]
- Menchini-Fabris, G.B.; Crespi, R.; Toti, P.; Crespi, G.; Rubino, L.; Covani, U. A 3-year retrospective study of fresh socket implants: CAD/CAM customized healing abutment vs cover screws. Int. J. Comput. Dent. 2020, 23, 109–117. [Google Scholar]
- Bäumer, D.; Zuhr, O.; Rebele, S.; Hürzeler, M. Socket shield technique for immediate implant placement: Clinical, radiographic and volumetric data after 5 years. Clin. Oral. Implant. Res. 2017, 28, 1450–1458. [Google Scholar] [CrossRef]
- Beretta, M.; Poli, P.P.; Pieriboni, S.; Tansella, S.; Manfredini, M.; Cicciu, M.; Maiorana, C. Peri-implant soft tissue conditioning by means of customized healing abutment: A randomized controlled clinical trial. Materials 2019, 12, 3041. [Google Scholar] [CrossRef] [PubMed]
- López Sacristán, H.; Del Canto Pingarrón, M.; Alobera Gracia, M.A.; de Elío Oliveros, J.; Díaz Pedrero, R.; Seco-Calvo, J. Use of autologous tooth-derived material as a graft in the post-extraction socket. Split-mouth study with radiological and histological analysis. BMC Oral Health 2024, 24, 832. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Cosola, S.; Toti, P.; Hwan Hwang, M.; Crespi, R.; Covani, U. Immediate implant and customized healing abutment for a periodontally compromised socket: 1-year follow-up retrospective evaluation. J. Clin. Med. 2023, 12, 2783. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, R.; Crespi, G.; Cosola, S.; Covani, U. Volume assessment of the external contour around immediate implant with or without immediate tooth-like crown provisionalization: A digital intraoral scans study. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124, 101418. [Google Scholar] [CrossRef]
- Crespi, R.; Toti, P.; Covani, U.; Crespi, G.; Menchini-Fabris, G.B. Guided tissue healing by preformed anatomical healing caps in the edentulous ridge: A 2-year retrospective case-control study. Int. J. Periodontics Restor. Dent. 2022, 42, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Chokaree, P.; Poovarodom, P.; Chaijareenont, P.; Rungsiyakull, P. Effect of Customized and Prefabricated Healing Abutments on Peri-Implant Soft Tissue and Bone in Immediate Implant Sites: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.E.; Bassetti, A. Flap design for guided tissue regeneration surgery in the esthetic zone: The whale’s tail technique. Int. J. Periodontics Restor. Dent. 2009, 29, 153–159. [Google Scholar]
- Niedzielska, I.; Ciapinski, D.; Bak, M.; Niedzielski, D. The assessment of the usefulness of platelet-rich fibrin in the healing process bone resorption. Coatings 2022, 12, 247. [Google Scholar] [CrossRef]

| Search Number | Query | Results |
|---|---|---|
| #7 | ((((soft tissue contour) OR (buccal bone level)) OR (implant stability)) OR (esthetics)) AND #6 | 30 |
| #6 | #4 AND #5 | 46 |
| #5 | (((bone grafting) OR (guided bone regeneration)) OR (connective tissue graft)) OR (provisionalization) | 90,733 |
| #4 | ((immediate implant placement) AND (((implant jumping gap) OR (jumping distance)) OR (peri-implant gap)) AND ((gap management) AND (2013:2024[pdat])) | 11 |
| #3 | ((immediate implant placement) AND (2013:2024[pdat])) AND (((implant jumping gap) OR (jumping distance)) OR (peri-implant gap) AND (2013:2024[pdat])) | 220 |
| #2 | ((implant jumping gap) OR (jumping distance)) OR (peri-implant gap) | 1437 |
| #1 | (immediate implant placement) | 5851 |
| Study | Method | Participants | Interventions (n) | Outcomes | Results | Conclusion |
|---|---|---|---|---|---|---|
| El Ebiary SO et al. (2023) [19] | RCliT (6 months) | 24 (9 males, 15 females) patients were indicated for extraction and immediate implant installation therapy with non-restorable upper anterior teeth in the esthetic zone. | Group 1: bone grafts with 50% xenografts and 50% autogenous grafts (12). Group 2: conventional implant without grafting (12). | Pink esthetic score (immediately post-intervention, 6 months). | Baseline: Study: 11.58 (1.16). Control: 11.75 (1.71). p-value: 0.746. After 6 months: Study: 12.42 (1.44). Control: 11.17 (1.53). p-value: 0.048. | Grafting the jumping gap results in higher esthetic outcomes in the anterior maxilla. |
| Samy MM et al. (2023) [20] | RCliT (6 months) | 16 patients indicated for immediate dental implant following badly decayed molars. | Group 1: mineralized plasmatic matrix as graft (8). Group 2: β-tricalcium phosphate as graft (8). |
| Implant stability immediately post-operation: Group 1: 58. Group 2: 62.5. p-value: 0.224. After 6 months: Group 1: 70.5. Group 2: 70. p-value: 0.557. Marginal bone loss: Group 1: 0.3. Group 2: 0.35. p-value: 0.040. Pre-operative bone density: Group 1: 632.5. Group 2: 735. p-value: 0.040. Immediately post-operation: Group 1: 637.5. Group 2: 745. p-value: 0.058. After 6 months: Group 1: 850. Group 2: 815. p-value: 0.635. | A mineralized plasmatic matrix promotes better bone growth and implant stability in the mandibular region than β-tricalcium phosphate as a graft. |
| Elsheikh HA et al. (2023) [21] | RCliT (18 months) | 36 patients (19 females and 17 males) seeking immediate implant replacement for non-restorable maxillary anterior and premolar teeth in the esthetic zone. | Group 1: platelet-rich fibrin placed into the jumping gap (12). Group 2: xenograft (12). Group 3: alloplastic β-tricalcium phosphate bone grafting (12). |
| Implant stability at surgery: Group 1: 64.33 (2.77). Group 2: 65.08 (2.27). Group 3: 66.33 (2.57). p-value: 0.114. After 6 months: Group 1: 71.83 (2.41). Group 2: 72.35 (2.35). Group 3: 73.83 (3.16). p-value: 0.119. After 18 months: Group 1: 73.50 (2.07). Group 2: 73.92 (2.39). Group 3: 74.92 (1.38). p-value: 0.216. Peri-implant pocket depth after 6 months: Group 1: 1.54 (0.23). Group 2: 1.40 (0.25). Group 3: 1.57 (0.43). p-value: 0.396. After 18 months: Group 1: 2.42 (0.46). Group 2: 2.32 (0.33). Group 3: 2.49 (0.27). p-value: 0.533. Buccal bone changes: Group 1: 1.56 (0.52). Group 2: 0.65 (0.31). Group 3: 0.69 (0.32). p-value: <0.001. | Xenograft and alloplastic bone grafts better preserve the bone around immediate implants than platelet-rich fibrin. |
| Amer SA (2022) [22] | RCliT (6 months) | 32 patients needing immediate implants in the premolar region of the maxillary teeth. | Group 1: no filling material (8). Group 2: 1.2% topical hyaluronic acid gel as a filling material (8). Group 3: 1.2% hyaluronic acid gel plus melatonin gel as a filling material (8). Group 4: melatonin gel as a filling material (8). | Clinical evaluation.
| Baseline gingival index: Group 1: 1.65 (0.37). Group 2: 1.70 (0.27). Group 3: 1.70 (0.38). Group 4: 1.74 (0.29). p-value: 0.889. Probing depth: Group 1: 3.71 (0.61). Group 2: 3.64 (0.67). Group 3: 3.73 (0.64). Group 4: 3.79 (0.63). p-value: 0.949. Gingival index after 6 months: Group 1: 1.23 (0.69). Group 2: 1 (0.52). Group 3: 0.35 (0.54). Group 4: 0.5 (0.55). p-value: <0.05. Probing depth: Group 1: 3.54 (0.41). Group 2: 3.61 (0.31). Group 3: 2.67 (0.24). Group 4: 2.61 (0.21). p-value: 0.001. (Group C,D) | Neither melatonin gel nor hyaluronic acid prevented bone loss around the implants. |
| Elbrashy A et al. (2022) [23] | RCliT (6 months) | 20 patients (11 males and 9 females) seeking immediate implant replacement, suffering from non-restorable maxillary premolars. | Group 1: bovine cancellous xenograft (10). Group 2: platelet-rich fibrin to graft (10). |
| Crestal bone loss: Study: 1.85 (0.89). Control: 0.77 (0.32). p-value: 0.002. Buccopalatal dimensions change: Study: 1.63. Control: 0.59. p-value: <0.001. Implant stability: Study: 74 (9.0). Control: 64 (9.0). p-value: 0.023. Pink esthetic zone: Study: 10.9 (1.52). Control: 11.9 (1.60). p-value: 0.169. | A xenograft as a gap distance-filling material significantly maintained the crestal bone level surrounding the implant. |
| Hammad MN et al. (2021) [24] | RCliT (6 months) | 17 (7 males and 10 females) patients indicated for immediate implant placement with non-restorable maxillary teeth on 20 extraction sockets. | Group 1: mixture of allograft and xenograft (10 extractions in 9 patients). Group 2: xenograft (10 extractions in 8 patients). | Marginal bone loss (6 months). Pink esthetic score (immediately post-intervention, 6 months). | Marginal bone loss: Group 1: 0.43 (0.2). Group 2: 0.34 (0.1). p-value: 0.219. Pink esthetic zone at baseline: Study: 12.4 (1.07). Control: 12.7 (0.82). p-value: 0.49. After 6 months: Study: 11.7 (1.05). Control: 12.1 (10.73). p-value: 0.336. | Both bone graft types showed minimal bone loss, slightly more in the mixed graft group, and a similar gum appearance after implant placement. |
| Shaaban Metwally AA et al. (2021) [25] | RCliT (9 months) | Patients with unrestorable teeth, indicated for implant placement. | Group 1: platelet-rich fibrin. Group 2: without protein-rich fibrin. | Bone density (immediately post-intervention, 3 months, 6 months, 9 months). | Baseline: Study: 572.77 (33.29). Control: 568.38 (47.18). p-value: 0.413. After 3 months: Study: 712.12 (32.70). Control: 663.97 (34.12). p-value: 0.136. After 6 months: Study: 979.57 (82.86). Control: 800.05 (53.88). p-value: 0.732. After 9 months: Study: 1139.2 (65.51). Control: 972.45 (64.18). p-value: 0.039. | Protein-rich fibrin effectively enhances bone density around immediate implants placed in the esthetic zone. |
| Atef M et al. (2021) [26] | RCliT (12 months) | 42 patients (12 males and 30 females), each with a single non-restorable tooth in the esthetic zone to be replaced with an immediate implant. | Group 1: ungrafted socket shield method (21). Group 2: bovine cancellous xenograft (21). | Esthetic outcomes.
| Pink esthetic score: Study: 12.2 (0.64). Control: 11.86 (0.35). p-value: 0.333. Midfacial mucosal alteration: Study: 0.45 (0.75). Control: −0.47 (0.58). p-value: 0.017. | The socket shield method preserved bone and soft tissue better than traditional grafting after immediate implant placement without affecting gum appearance or patient satisfaction. |
| Adam SAN et al. (2020) [27] | RCT (6 months) | 18 (8 males, 10 females) patients were indicated for immediate implant placement with an unrestorable single tooth. | Group 1: NanoBone grafts (9). Group 2: autogenous bone from the chin (9). | Marginal bone level (immediate post-intervention, 6 months). Implant stability quotient (6 months). | Marginal bone level at baseline:
Group 2: 0.97 (0.06). p-value: 0.009.
Group 2: 0.88 (0.07). p-value: 0.0001. After 6 months:
Group 2: 0.53 (0.06). p-value: 0.0001.
Group 2: 0.32 (0.06). p-value: 0.0001. Implant stability quotient:
Group 2: 63.36 (10.91). p-value: 0.833. | Both methods resulted in similarly stable implants, but NanoBone caused less harm to the patients. |
| Naji BM et al. (2019) [28] | RCliT (6 months) | 48 patients (18 males and 30 females) were indicated for dental implant placement for unrestorable maxillary premolar. | Group 1: mucoperiosteal flap with alloplastic nanocrystalline calcium sulfate bone graft (16). Group 2: flap without graft (16). Group 3: flapless without graft (16). | The horizontal dimension of the buccal alveolar bone (immediately post-intervention, 6 months). | Baseline–6 months: Group 1: 0.37 (0.09). Group 2: 0.91 (0.54). Group 3: 0.24 (0.11). p-value: 0.003. | Both “flapless without graft” and “flap with graft” showed similar bone healing after implant placement in premolars with adequate bone thickness. |
| Sanz M et al. (2016) [29] | RCliT (16 weeks) | 86 (41 males and 45 females) patients who needed at least one tooth in the anterior maxilla to be removed and replaced with implants. | Group 1: deproteinized bovine bone mineral with 10% collagen (DBBM-C) filling (43). Group 2: no graft (43). | Crest dimensions.
| Baseline–4 months: Buccolingual dimension: Study: −2.19 (2.10). Control: −2.65 (1.81). p-value: 0.149. Alveolar crest width: Study: −1.26 (1.75). Control: 1.71 (1.36). p-value: 0.187. Horizontal crest dimension: Study: −1.07 (1.10). Control: 1.59 (1.05). p-value: 0.029. Horizontal gap dimension: Study: −1.57 (−1.27). Control: −2.23 (1.22). p-value: 0.018. Vertical defect dimension: Study: −6.97 (2.68). Control: −6.45 (3.24). p-value: 0.43. | Using DBBM-C bone graft helped prevent horizontal bone resorption around newly placed implants. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamed, S.K.; Menchini-Fabris, G.B.; Alqarni, A.; Alarabi, S.M.; Alharbi, A.A.; Alshamrani, A.; Covani, U.; Cosola, S. Insights into the Current Management Techniques for Peri-Implant Gaps: A Systematic Review. J. Clin. Med. 2025, 14, 3351. https://doi.org/10.3390/jcm14103351
Ahamed SK, Menchini-Fabris GB, Alqarni A, Alarabi SM, Alharbi AA, Alshamrani A, Covani U, Cosola S. Insights into the Current Management Techniques for Peri-Implant Gaps: A Systematic Review. Journal of Clinical Medicine. 2025; 14(10):3351. https://doi.org/10.3390/jcm14103351
Chicago/Turabian StyleAhamed, Syed Kowsar, Giovanni Battista Menchini-Fabris, Ali Alqarni, Shaimaa Mohammed Alarabi, Abdulaziz Abdullah Alharbi, Ammar Alshamrani, Ugo Covani, and Saverio Cosola. 2025. "Insights into the Current Management Techniques for Peri-Implant Gaps: A Systematic Review" Journal of Clinical Medicine 14, no. 10: 3351. https://doi.org/10.3390/jcm14103351
APA StyleAhamed, S. K., Menchini-Fabris, G. B., Alqarni, A., Alarabi, S. M., Alharbi, A. A., Alshamrani, A., Covani, U., & Cosola, S. (2025). Insights into the Current Management Techniques for Peri-Implant Gaps: A Systematic Review. Journal of Clinical Medicine, 14(10), 3351. https://doi.org/10.3390/jcm14103351