Renal and Safety Outcomes of SGLT2 Inhibitors in Patients with Type 2 Diabetes: A Nationwide Observational Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Study Population
2.3. Clinical Outcomes and Follow-Up
2.4. Statistical Analysis
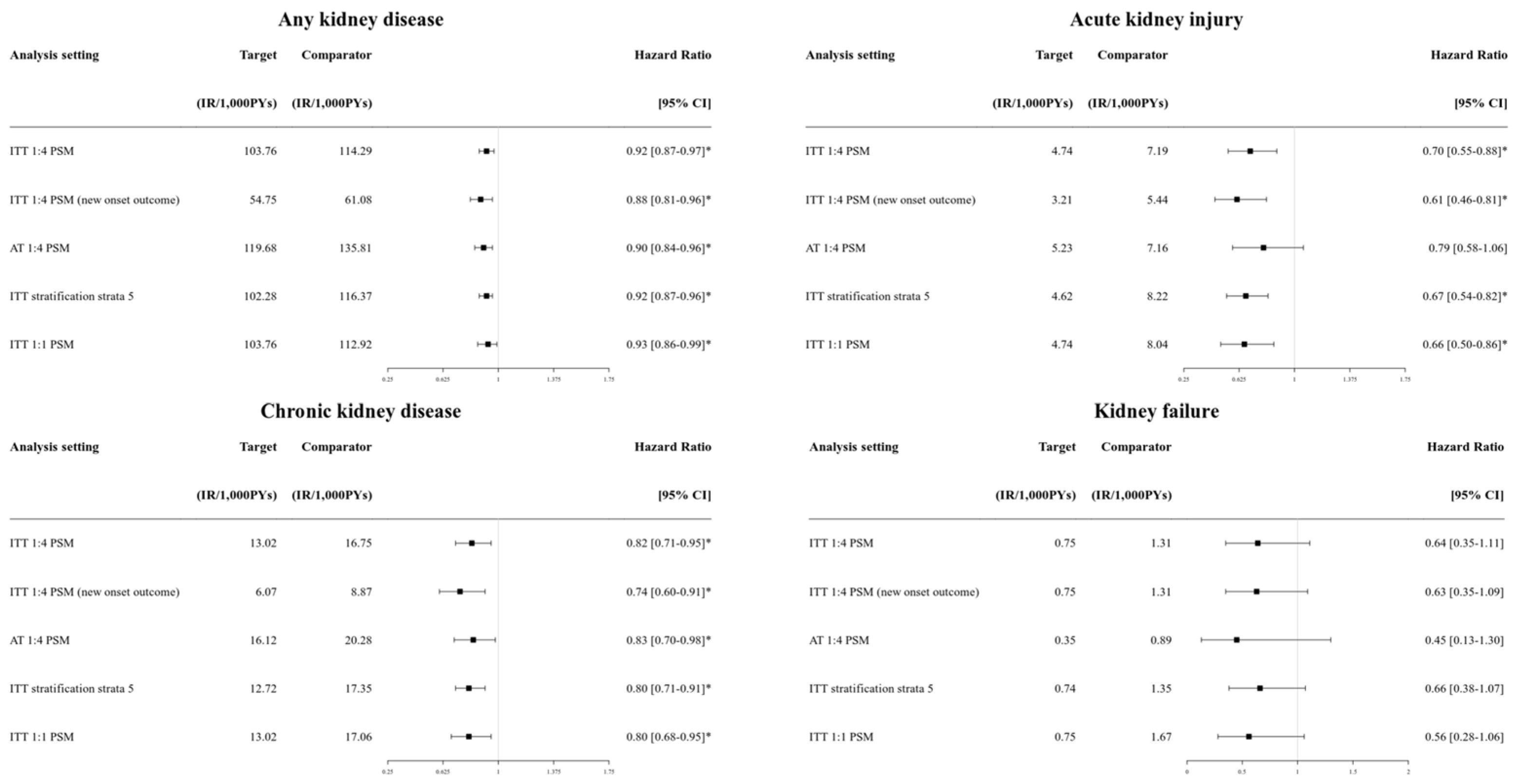
2.5. Sensitivity and Subgroup Analysis
3. Results
3.1. Cohort Characteristics
3.2. Outcome Assessment
3.3. Sensitivity Analysis
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SGLT2i | Sodium–glucose co-transporter 2 inhibitors |
| DPP4i | Dipeptidyl peptidase-4 inhibitors |
| T2DM | Type 2 diabetes mellitus |
| AKI | Acute kidney injury |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| CRRT | Continuous renal replacement therapy |
| AMI | Acute myocardial infarction |
| HHF | Hospitalization with heart failure |
| PS | Propensity score |
| PY | Person-year |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Karasik, A.; Thuresson, M.; Melzer-Cohen, C.; Chodick, G.; Khunti, K.; Wilding, J.P.H.; Rodriguez, L.A.G.; Cea-Soriano, L.; Kohsaka, S.; et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): A multinational observational cohort study. Lancet Diabetes Endocrinol. 2020, 8, 27–35. [Google Scholar] [CrossRef]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.-G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good Practices for Real-World Data Studies of Treatment and/or Comparative Effectiveness: Recommendations from the Joint ISPOR-ISPE Special Task Force on Real-World Evidence in Health Care Decision Making. Value Health 2017, 20, 1003–1008. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, C.; Kim, K.-H.; Lee, Y.; Yu, D.H.; Yun, J.; Baek, H.; Park, R.W.; You, S.C. Scalable Infrastructure Supporting Reproducible Nationwide Healthcare Data Analysis toward FAIR Stewardship. Sci. Data 2023, 10, 674. [Google Scholar] [CrossRef]
- Tian, Y.; Schuemie, M.J.; Suchard, M.A. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int. J. Epidemiol. 2018, 47, 2005–2014. [Google Scholar] [CrossRef]
- Schuemie, M.; Reps, J.; Black, A.; Defalco, F.; Evans, L.; Fridgeirsson, E.; Gilbert, J.P.; Knoll, C.; Lavallee, M.; Rao, G.A.; et al. Health-Analytics Data to Evidence Suite (HADES): Open-Source Software for Observational Research. Stud. Health Technol. Inform. 2024, 310, 966–970. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann. Intern. Med. 2007, 147, W-163–W-194. [Google Scholar] [CrossRef]
- Nespoux, J.; Vallon, V. Renal effects of SGLT2 inhibitors: An update. Curr. Opin. Nephrol. Hypertens. 2020, 29, 190. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, C.; Kraut, E.; Whitlock, R.H.; Komenda, P.; Woo, V.; Rigatto, C.; Tangri, N. Acute Kidney Injury Events in Patients with Type 2 Diabetes Using SGLT2 Inhibitors Versus Other Glucose-Lowering Drugs: A Retrospective Cohort Study. Am. J. Kidney Dis. 2020, 76, 471–479.e1. [Google Scholar] [CrossRef] [PubMed]
- Idris, I.; Zhang, R.; Mamza, J.B.; Ford, M.; Morris, T.; Banerjee, A.; Khunti, K. Significant reduction in chronic kidney disease progression with sodium-glucose cotransporter-2 inhibitors compared to dipeptidyl peptidase-4 inhibitors in adults with type 2 diabetes in a UK clinical setting. Diabetes Obes. Metab. 2022, 24, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Au, P.C.; Tan, K.C.; Cheung, B.M.; Wong, I.C.; Li, H.-L.; Cheung, C.-L. Association between SGLT2 inhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2022, 107, e2962–e2970. [Google Scholar] [CrossRef]
- Arshad, M.; Hoda, F.; Siddiqui, N.A.; Najmi, A.K.; Ahmad, M. Genito Urinary Infection and Urinary Tract Infection in Patients with Type 2 Diabetes Mellitus Receiving SGLT2 Inhibitors: Evidence from a Systematic Literature Review of Landmark Randomized Clinical Trial. Drug Res. 2024, 74, 307–313. [Google Scholar] [CrossRef]
- Alkabbani, W.; Zongo, A.; Minhas-Sandhu, J.K.; Eurich, D.T.; Shah, B.R.; Alsabbagh, M.W.; Gamble, J.-M. Sodium-Glucose Cotransporter-2 Inhibitors and Urinary Tract Infections: A Propensity Score–matched Population-based Cohort Study. Can. J. Diabetes 2022, 46, 392–403.e13. [Google Scholar] [CrossRef]
- Neuen, B.L.; Oshima, M.; Agarwal, R.; Arnott, C.; Cherney, D.Z.; Edwards, R.; Langkilde, A.M.; Mahaffey, K.W.; McGuire, D.K.; Neal, B.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People with Type 2 Diabetes: A Meta-Analysis of Individual Participant Data from Randomized, Controlled Trials. Circulation 2022, 145, 1460–1470. [Google Scholar] [CrossRef]
- Ekanayake, P.; Hupfeld, C.; Mudaliar, S. Sodium-Glucose Cotransporter Type 2 (SGLT-2) Inhibitors and Ketogenesis: The Good and the Bad. Curr. Diabetes Rep. 2020, 20, 74. [Google Scholar] [CrossRef]
- Rong, X.; Zhu, Y.; Wen, B.; Liu, K.; Li, X.; Gou, Q.; Chen, X. Risk of hypovolemia associated with sodium–glucose cotransporter-2 inhibitors treatment: A meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 973129. [Google Scholar] [CrossRef]
- D’andrea, E.; Wexler, D.J.; Kim, S.C.; Paik, J.M.; Alt, E.; Patorno, E. Comparing Effectiveness and Safety of SGLT2 Inhibitors vs DPP-4 Inhibitors in Patients with Type 2 Diabetes and Varying Baseline HbA1c Levels. JAMA Intern. Med. 2023, 183, 242–254. [Google Scholar] [CrossRef]
- Ogawa, W.; Sakaguchi, K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: Possible mechanism and contributing factors. J. Diabetes Investig. 2016, 7, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Khan, R.A.; Kalam, A.; Venkata, S.K.; Kandhare, A.D.; Ghosh, P.; Sharma, M. Effect of anti-diabetic drugs on bone metabolism: Evidence from preclinical and clinical studies. Pharmacol. Rep. 2017, 69, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Zhang, Y.; Zhang, J.; Sheng, Y.; Wang, W.; Li, Y. Effect of SGLT2 inhibitors on fractures, BMD, and bone metabolism markers in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Osteoporos. Int. 2023, 34, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Filion, K.B.; Lix, L.M.; Yu, O.H.; Dell’aniello, S.; Douros, A.; Shah, B.R.; St-Jean, A.; Fisher, A.; Tremblay, E.; Bugden, S.C.; et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: Multi-database retrospective cohort study. BMJ 2020, 370, m3342. [Google Scholar] [CrossRef]
- Mascolo, A.; Scavone, C.; Scisciola, L.; Chiodini, P.; Capuano, A.; Paolisso, G. SGLT-2 inhibitors reduce the risk of cerebrovascular/cardiovascular outcomes and mortality: A systematic review and meta-analysis of retrospective cohort studies. Pharmacol. Res. 2021, 172, 105836. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Schuemie, M.J.; Blei, D.M.; Hripcsak, G. Adjusting for indirectly measured confounding using large-scale propensity score. J. Biomed. Inform. 2022, 134, 104204. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’Amato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025; in press. [Google Scholar] [CrossRef]

| Characteristics | Before PS Matching | After PS Matching | ||||
|---|---|---|---|---|---|---|
| SGLT2i (n = 16,736) | DPP4i (n = 67,463) | SMD | SGLT2i (n = 13,649) | DPP4i (n = 35,043) | SMD | |
| Female, n (%) | 6544 (39.1) | 29,279 (43.4) | −0.09 | 5473 (40.1) | 14,087 (40.2) | 0.00 |
| Age group, n (%) | ||||||
| <40 | 1523 (9.1) | 2361 (3.5) | 0.19 | 915 (6.7) | 2348 (6.7) | 0.04 |
| 40–59 | 8853 (52.9) | 25,906 (38.4) | 0.12 | 6920 (50.7) | 17,802 (50.8) | 0.01 |
| 60–74 | 5372 (32.1) | 28,065 (41.6) | 0.09 | 4886 (35.8) | 12,370 (35.3) | 0.01 |
| ≥75 | 988 (5.9) | 11,131 (16.5) | −0.17 | 928 (6.8) | 2523 (7.2) | −0.03 |
| Medical history, n (%) | ||||||
| Hyperlipidemia | 14,410 (86.1) | 56,265 (83.4) | 0.07 | 11,738 (86.0) | 30,242 (86.3) | −0.01 |
| Hypertensive disorder | 10,996 (65.7) | 42,975 (63.7) | 0.04 | 8885 (65.1) | 22,848 (65.2) | 0.00 |
| Cerebrovascular disease | 1004 (6.0) | 5060 (7.5) | −0.06 | 846 (6.2) | 2208 (6.3) | 0.00 |
| Heart disease | 4720 (28.2) | 13,763 (20.4) | 0.18 | 3535 (25.9) | 9251 (26.4) | −0.01 |
| Atrial fibrillation | 435 (2.6) | 1214 (1.8) | 0.05 | 328 (2.4) | 841 (2.4) | 0.00 |
| Heart failure | 1807 (10.8) | 4588 (6.8) | 0.14 | 1283 (9.4) | 3364 (9.6) | −0.01 |
| Ischemic heart disease | 3029 (18.1) | 8096 (12.0) | 0.17 | 2238 (16.4) | 5852 (16.7) | −0.01 |
| Peripheral vascular disease | 2544 (15.2) | 13,155 (19.5) | −0.11 | 2211 (16.2) | 5817 (16.6) | −0.01 |
| Osteoporosis | 1439 (8.6) | 9512 (14.1) | −0.18 | 1297 (9.5) | 3434 (9.8) | −0.01 |
| Medication use, n (%) | ||||||
| ACE inhibitor/ARB | 6661 (39.8) | 23,477 (34.8) | 0.10 | 5255 (38.5) | 13,386 (38.2) | 0.00 |
| Antithrombotic agents | 7950 (47.5) | 32,113 (47.6) | 0.00 | 6442 (47.2) | 16,540 (47.2) | 0.00 |
| Calcium channel blockers | 5322 (31.8) | 21,319 (31.6) | 0.00 | 4313 (31.6) | 11,039 (31.5) | 0.00 |
| Diuretics | 3381 (20.2) | 13,358 (19.8) | 0.01 | 2689 (19.7) | 7044 (20.1) | −0.01 |
| Insulins | 1205 (7.2) | 5195 (7.7) | −0.02 | 983 (7.2) | 2523 (7.2) | 0.00 |
| Lipid-modifying agents | 8853 (52.9) | 32,653 (48.4) | 0.09 | 7111 (52.1) | 18,292 (52.2) | 0.00 |
| Outcomes | SGLT2i (n = 13,649) | DPP4i (n = 35,043) | HR [95% CI] | ||
|---|---|---|---|---|---|
| Events, n | IR | Events, n | IR | ||
| Renal outcomes | |||||
| Any kidney outcomes | 950 | 54.75 | 2722 | 61.08 | 0.88 [0.81–0.96] * |
| Acute kidney injury | 72 | 3.21 | 320 | 5.44 | 0.61 [0.46–0.81] * |
| Chronic kidney disease | 134 | 6.07 | 511 | 8.87 | 0.74 [0.60–0.91] * |
| Dialysis | 25 | 1.11 | 105 | 1.77 | 0.64 [0.39–1.01] |
| Kidney failure | 17 | 0.75 | 78 | 1.31 | 0.63 [0.35–1.09] |
| Kidney transplantation | 0 | 0.00 | <5 | <0.08 | 0.20 [NA–2.06] |
| Safety outcomes | |||||
| Urinary tract infection | 901 | 49.41 | 2408 | 50.70 | 0.97 [0.89–1.06] |
| Genital infection | 686 | 837 | 34.44 | 15.46 | 2.38 [2.12–2.68] * |
| Diabetic ketoacidosis | 21 | 0.93 | 35 | 0.59 | 1.27 [0.68–2.30] |
| Hyperkalemia | 58 | 2.58 | 306 | 5.19 | 0.49 [0.36–0.67] * |
| Hypokalemia | 73 | 265 | 3.25 | 4.49 | 0.82 [0.61–1.09] |
| Hypovolemia | 214 | 627 | 9.83 | 11.01 | 0.92 [0.77–1.09] |
| Hypoglycemia | 46 | 134 | 2.04 | 2.26 | 0.97 [0.65–1.42] |
| Bone fracture | 610 | 1714 | 30.54 | 32.95 | 0.91 [0.82–1.02] |
| Renal Outcomes | SGLT2i with CV Risk (n = 12,980) | DPP4i with CV Risk (n = 33,362) | HR [95% CI] | SGLT2i with Renal Risk (n = 2678) | DPP4i with Renal Risk (n = 7202) | HR [95% CI] |
|---|---|---|---|---|---|---|
| Event, (IR) | Event, (IR) | Event, (IR) | Event, (IR) | |||
| Any kidney outcomes | 904 (55.48) | 2602 (62.36) | 0.90 [0.83–0.98] * | – | – | – |
| Acute kidney injury | 66 (3.10) | 309 (5.54) | 0.53 [0.39–0.72] * | 15 (3.83) | 104 (9.67) | 0.40 [0.21–0.70] * |
| Chronic kidney disease | 139 (6.65) | 499 (9.15) | 0.81 [0.66–0.998] * | 37 (10.29) | 177 (18.2) | 0.68 [0.45–0.98] * |
| Dialysis | 25 (1.17) | 98 (1.74) | 0.72 [0.44–1.14] | 7 (1.72) | 44 (3.91) | 0.46 [0.17–1.05] |
| Kidney failure | 17 (0.79) | 65 (1.15) | 0.75 [0.40–1.32] | 0 (0.00) | <5 (<0.44) | 0.17 [NA–2.70] |
| Kidney transplantation | 0 (0.00) | <5 (<0.09) | 0.25 [NA–5.13] | 0 (0.00) | <5 (<0.44) | 0.17 [NA–2.70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Kim, C.; Choi, H.; Park, R.W.; Lee, S. Renal and Safety Outcomes of SGLT2 Inhibitors in Patients with Type 2 Diabetes: A Nationwide Observational Cohort Study. J. Clin. Med. 2025, 14, 3349. https://doi.org/10.3390/jcm14103349
Chang J, Kim C, Choi H, Park RW, Lee S. Renal and Safety Outcomes of SGLT2 Inhibitors in Patients with Type 2 Diabetes: A Nationwide Observational Cohort Study. Journal of Clinical Medicine. 2025; 14(10):3349. https://doi.org/10.3390/jcm14103349
Chicago/Turabian StyleChang, Junhyuk, Chungsoo Kim, Heejung Choi, Rae Woong Park, and Sukhyang Lee. 2025. "Renal and Safety Outcomes of SGLT2 Inhibitors in Patients with Type 2 Diabetes: A Nationwide Observational Cohort Study" Journal of Clinical Medicine 14, no. 10: 3349. https://doi.org/10.3390/jcm14103349
APA StyleChang, J., Kim, C., Choi, H., Park, R. W., & Lee, S. (2025). Renal and Safety Outcomes of SGLT2 Inhibitors in Patients with Type 2 Diabetes: A Nationwide Observational Cohort Study. Journal of Clinical Medicine, 14(10), 3349. https://doi.org/10.3390/jcm14103349








