Can Double-Negative B Cells and Marginal Zone B Cells Have a Potential Impact on the Outcome of Kidney Transplantation?
Abstract
1. Introduction
2. Methods
2.1. Study Patients
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Study Schedule
2.3. Ethical Approval and Consent to Participate
2.4. Immunosuppressive Regimen
2.5. Flow Cytometry of B-Cell Subpopulations
- Anti-CD19 PC5.5 clone J3-119, Beckman Coulter, Beckman Coulter, Inc., Sykesville, MD, USA;
- Anti-CD27 PE-Dylight 594 clone LT27, EXBIO, Praha SA, Czech Republic;
- Anti-CD45-PC7 clone J33 Beckman Coulter, Beckman Coulter, Inc., Sykesville, MD, USA;
- Anti-IgD clone IA6-2 Thermo Scientific LSG, Lagoas Park, Porto Salvo, Portugal;
- Anti-IgM PE clone, SA-DA4 Beckman Coulter, Beckman Coulter, Inc., Sykesville, MD, USA.
2.6. Statistics
3. Results
3.1. Patient Characteristics
3.2. Differences in DN and MZB B-Cell Populations at Different Study Time Points: DN and MZ B Cells Underwent Changes During the 12-Month Follow-Up Period
3.3. How the DN and MZ Β Cell Populations Were Affected by Different Factors That Influence the Outcome of Kidney Transplantation
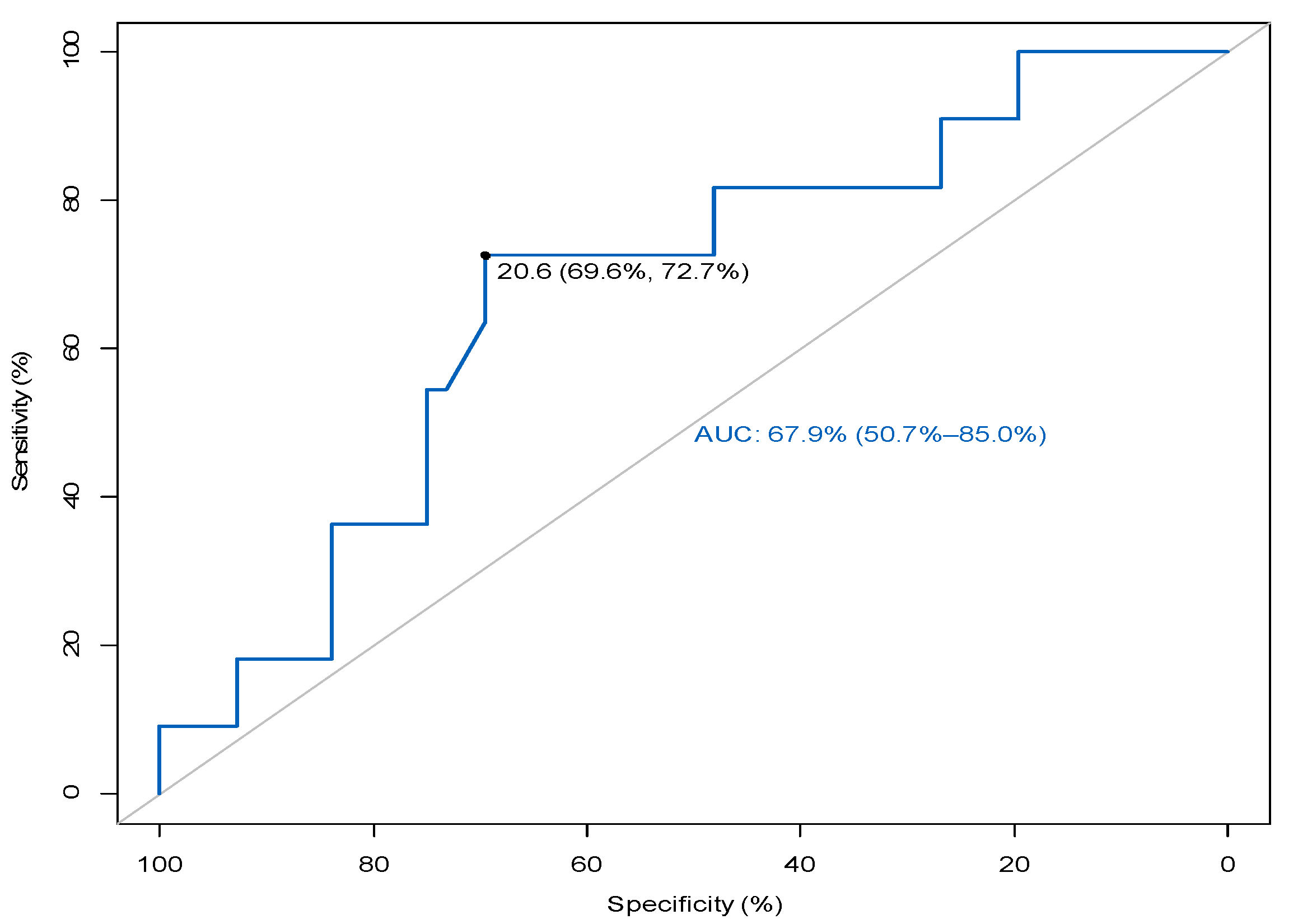
3.4. MZ B Cells in Relation to the Prediction of Rejection
3.5. Frequencies and Absolute Numbers of MZ and DN B Cells in Relation to Graft Function at Different Time Points During Study Follow-Up
3.6. Effect of Induction Immune Therapy on DN and MZ B Cells at Different Time Points
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, T.; Kurosaki, T. Memory B cells. Nat. Rev. Immunol. 2024, 24, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jung, J.; Sanz, I. OMIP-003: Phenotypic analysis of human memory B cells. Cytom. Part A J. Int. Soc. Anal. Cytol. 2011, 79, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Jenks, S.A.; Cashman, K.S.; Woodruff, M.C.; Lee, F.E.; Sanz, I. Extrafollicular responses in humans and SLE. Immunol. Rev. 2019, 288, 136–148. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E.-H. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Beckers, L.; Somers, V.; Fraussen, J. IgD− CD27− double negative (DN) B cells: Origins and functions in health and disease. Immunol. Lett. 2023, 255, 67–76. [Google Scholar] [CrossRef]
- Cervantes-Díaz, R.; Torres-Ruíz, J.; Romero-Ramírez, S.; Cañez-Hernández, M.; Pérez-Fragoso, A.; Páez-Franco, J.C.; Meza-Sánchez, D.E.; Pescador-Rojas, M.; Sosa-Hernández, V.A.; Gómez-Martín, D.; et al. Severity of SARS-CoV-2 infection is linked to double-negative (CD27− IgD−) B cell subset numbers. Inflamm. Res. 2022, 71, 131–140. [Google Scholar] [CrossRef]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 2018, 49, 725–739. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef]
- Isnardi, I.; Ng, Y.-S.; Menard, L.; Meyers, G.; Saadoun, D.; Srdanovic, I.; Samuels, J.; Berman, J.; Buckner, J.H.; Cunningham-Rundles, C.; et al. Complement receptor 2/CD21− human naive B cells contain mostly autoreactive unresponsive clones. Blood 2010, 115, 5026–5036. [Google Scholar] [CrossRef]
- Moir, S.; Ho, J.; Malaspina, A.; Wang, W.; DiPoto, A.C.; O’Shea, M.A.; Roby, G.; Kottilil, S.; Arthos, J.; Proschan, M.A.; et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008, 205, 1797–1805. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Blomberg, B.B. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp. Gerontol. 2017, 87 Pt A, 113–120. [Google Scholar] [CrossRef]
- Schuller, M.; Pfeifer, V.; Kirsch, A.H.; Klötzer, K.A.; Mooslechner, A.A.; Rosenkranz, A.R.; Stiegler, P.; Schemmer, P.; Sourij, H.; Eller, P.; et al. B Cell Composition Is Altered After Kidney Transplantation and Transitional B Cells Correlate with SARS-CoV-2 Vaccination Response. Front. Med. 2022, 9, 818882. [Google Scholar] [CrossRef] [PubMed]
- Fouza, A.; Tagkouta, A.; Daoudaki, M.; Stangou, M.; Fylaktou, A.; Bougioukas, K.; Xochelli, A.; Vagiotas, L.; Kasimatis, E.; Nikolaidou, V.; et al. Exploring Perturbations in Peripheral B Cell Memory Subpopulations Early after Kidney Transplantation Using Unsupervised Machine Learning. J. Clin. Med. 2023, 12, 6331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Uduman, M.; Siu, J.H.Y.; Tull, T.J.; Sanderson, J.D.; Wu, Y.-C.B.; Zhou, J.Q.; Petrov, N.; Ellis, R.; Todd, K.; et al. Spatiotemporal segregation of human marginal zone and memory B cell populations in lymphoid tissue. Nat. Commun. 2018, 21, 3857. [Google Scholar] [CrossRef]
- Palm, A.E.; Kleinau, S. Marginal zone B cells: From housekeeping function to autoimmunity? J. Autoimmun. 2021, 119, 102627. [Google Scholar] [CrossRef]
- Appelgren, D.; Eriksson, P.; Ernerudh, J.; Segelmark, M. Marginal-Zone B-Cells Are Main Producers of IgM in Humans, and Are Reduced in Patients with autoimmune Vasculitis. Front. Immunol. 2018, 9, 2242. [Google Scholar] [CrossRef]
- Asano, Y.; Daccache, J.; Jain, D.; Ko, K.; Kinloch, A.; Veselits, M.; Wolfgeher, D.; Chang, A.; Josephson, M.; Cunningham, P.; et al. Innate-like self-reactive B cells infiltrate human renal allografts during transplant rejection. Nat. Commun. 2021, 16, 4372. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.K.; Friedrich, H.C.; Kleinau, S. Nodal marginal zone B cells in mice: A novel subset with dormant self-reactivity. Sci. Rep. 2016, 6, 27687. [Google Scholar] [CrossRef]
- Gorbacheva, V.; Fan, R.; Gaudette, B.; Baldwin, W.M.; Fairchild, R.L.; Valujskikh, A. Marginal zone B cells are required for optimal humoral responses to allograft. Am. J. Transplant. 2025, 25, 48–59. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, H.; Yu, M.; Peng, B.; Liu, S.; Luo, M.; Stefano, G.B.; Kream, R.M.; Ming, Y. Profiles of B-cell subsets in immunologically stable renal allograft recipients and end-stage renal disease patients. Transpl. Immunol. 2020, 58, 101249. [Google Scholar] [CrossRef]
- Segundo, D.S.; Rodrigo, E.; Fernández-Fresnedo, G. Decrease in Marginal Zone B-cells (CD27+IgD+CD38–) as Surrogate Marker of Rejection in Kidney Transplant Recipients. Trends Transpl. 2013, 7, 051–055. [Google Scholar]
- Alfaro, R.; Legaz, I.; González-Martínez, G.; Jimenez-Coll, V.; Martínez-Banaclocha, H.; Galián, J.A.; Botella, C.; de la Peña-Moral, J.; Moya-Quiles, M.R.; Campillo, J.A.; et al. Monitoring of B Cell in Kidney Transplantation: Development of a Novel Clusters Analysis and Role of Transitional B Cells in Transplant Outcome. Diagnostics 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rondaan, C.; de Joode, A.A.E.; Raveling-Eelsing, E.; Bos, N.A.; Westra, J. Changes in T and B cell subsets in end stage renal disease patients before and after kidney transplantation. Immun. Ageing 2021, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Stranavova, L.; Hruba, P.; Slatinska, J.; Sawitzki, B.; Reinke, P.; Volk, H.-D.; Viklicky, O. Dialysis therapy is associated with peripheral marginal zone B-cell augmentation. Transpl. Immunol. 2020, 60, 101289. [Google Scholar] [CrossRef] [PubMed]
- Fouza, A.; Fylaktou, A.; Tagkouta, A.; Daoudaki, M.; Vagiotas, L.; Kasimatis, E.; Xochelli, A.; Nikolaidou, V.; Katsanos, G.; Tsoulfas, G.; et al. Frequencies or Absolute Numbers? Cluster Analysis of Frequencies and Absolute Numbers of B-Cell Subsets in Dialysis Patients Who Are Candidates for Kidney Transplantation Reveals Different Profiles. J. Clin. Med. 2024, 13, 6454. [Google Scholar] [CrossRef]
- Hendricks, J.; Bos, N.A.; Kroese, F.G.M. Heterogeneity of Memory Marginal Zone B Cells. Crit. Rev. Immunol. 2018, 38, 145–158. [Google Scholar] [CrossRef]
- Kallarakal, M.A.B.; Cohen, G.S.B.; Ibukun, F.I.M.; Krummey, S.M. Marginal Zone B Cells Are Necessary for the Formation of Anti-donor IgG After Allogeneic Sensitization. Transplantation 2024, 108, 1357–1367. [Google Scholar] [CrossRef]

| Study Sample | N: 71 |
|---|---|
| Characteristics of recipients | |
| Sex | Female: 20/Male: 51 28.17/71.83% |
| Age in years | 48.5 (39, 60) |
| Type of donors | Deceased/brain death: 50 (70%) Living: 21 (30%) |
| Preemptive recipients | 7 (9.86%) |
| Dialysis patient candidates for transplantation | |
| Type of dialysis | HD: 64 (81%) CAPD: 7 (19%) |
| Duration of dialysis (months) | 87 (34–127) |
| Distribution of underlying kidney disease | |
| Polycystic kidney disease | 14 (19.7%) |
| Primary glomerulonephritis IgA nephropathy Membranous nephropathy Focal segmental glomerulosclerosis Membranoproliferative glomerulonephritis | 12 (17%) 5 (7%) 3 (4.25%) 2 (2.83%) 2 (2.83%) |
| Reflux nephropathy | 6 (8.4%) |
| Diabetes mellitus | 6 (8.4%) |
| Nephrosclerosis/hypertension | 8 (11.25%) |
| Urinary tract infections/stones | 5 (7%) |
| Other | 12 (17%) |
| Unknown | 8 (11.25%) |
| Information on transplantation, graft function | |
| Delayed graft function | Yes: 12 (24%) No: 38 (76%) |
| Cold ischemia time (hours) | 19.2 (4.6) |
| eGFR (mL/min/1.73 m2) | 52 (36–89) |
| Recipients with rejection | 11 (15.5%) |
| Induction therapy | |
| Basiliximab, N (%) | 63 (88.7%) |
| Anti-thymocyte globulin, N(%) | 8 (11.3%) |
| T0 1 | T3 1 | T6 1 | T12 1 | p 2 | Post Hoc 3 Comparison | |
|---|---|---|---|---|---|---|
| %Double negative, (CD19+IgD−CD27), DN | 11.9 (7.8, 18.6) | 10.1 (7.7, 13.8) | 13.1 (8.8, 18.8) | 13.6 (9.2, 17.7) | 0.004 | ns |
| #Double negative (CD19+IgD−CD27−), DN | 10 (5.8, 19) | 11 (5.3, 18) | 11 (6.1, 17.5) | 12 (7.9, 21) | ns | ns |
| %Marginal zone B cells (CD19+IgD+IgM+CD27+), MZB | 26.6 (11.2, 38.1) | 25.6 (14.4, 42.2) | 22.8 (11.3, 41.6) | 30 (19.5, 44.3) | ns | ns |
| #(CD19+IgD+IgM+CD27+), MZB | 5 (1.7, 10.4) | 6 (2.8, 15) | 3 (1, 10) | 7 (5, 11) | 0.005 | ns |
| Univariate Regression | |||||
|---|---|---|---|---|---|
| Frequency of Cell Population | 95% Confidence Interval | ||||
| β | Ν | p | Lower | Upper | |
| Double negative, DN | |||||
| Age of the recipient | −0.04 | 71 | 0.5 | −0.17 | 0.09 |
| Type of donor (deceased/living) | 1.6 | 71 | 0.3 | −1.8 | 5 |
| Cold ischemia time, CIT | −0.04 | 71 | 0.7 | −0.20 | 0.13 |
| Delayed graft function, DGF | 2.8 | 71 | 0.093 | −0.49 | 6.1 |
| Dialysis vintage | −0.01 | 64 | 0.05 | −0.04 | 0.02 |
| Marginal zone B cells, MZB | |||||
| Age of the recipient | 0.07 | 71 | 0.7 | −0.27 | 0.42 |
| Type of donor (deceased/living) | −1.2 | 71 | 0.8 | −10 | 8 |
| Cold ischemia time, CIT | −0.19 | 71 | 0.5 | −0.28 | 0.6 |
| DGF | −4.1 | 71 | 0.4 | −13 | 4.7 |
| Dialysis vintage | 0.04 | 64 | 0.4 | −0.04 | 0.12 |
| Univariate Regression | Multivariate Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute Numbers of Cells | 95% Confidence Interval | 95% Confidence Interval | |||||||
| β | Ν | p | Lower | Upper | β | p | Lower | Upper | |
| Double negative, DN | |||||||||
| Age of the recipient | −0.47 | 71 | 0.01 | −0.47 | −0.82 | −0.27 | 0.3 | −0.75 | 0.21 |
| Type of donor (deceased/living) | 11 | 71 | 0.023 | 1.5 | 20 | 5.9 | 0.6 | −15 | 27 |
| Cold ischemia time, CIT | −0.49 | 71 | 0.031 | −0.94 | −0.05 | −0.1 | 0.8 | −1 | 0.84 |
| Delayed graft function, DGF | −2.0 | 71 | 0.7 | −12 | 7.5 | ||||
| Dialysis vintage | −0.12 | 64 | 0.005 | −0.12 | −0.21 | −0.05 | 0.4 | −0.17 | 0.07 |
| Marginal zone B cells, MZB | |||||||||
| Age of the recipient | −0.19 | 71 | 0.079 | −0.19 | 0.02 | −0.11 | 0.3 | −0.35 | 0.12 |
| Type of donor (deceased/living) | 5.6 | 71 | 0.052 | −0.05 | 11 | 6.4 | 0.3 | −5 | 18 |
| Cold ischemia time, CIT | −0.20 | 71 | 0.2 | −0.47 | 0.07 | 0.2 | 0.5 | −0.34 | 0.74 |
| DGF | −5.4 | 71 | 0.047 | −11 | −0.07 | −4.4 | 0.12 | −10 | 1.2 |
| Dialysis vintage | 0.00 | 64 | >0.9 | −0.04 | 0.03 | ||||
| Kidney Transplant Recipients (N: 71) | Recipients Experiencing Rejection (N: 11) | Recipients Free of Rejection (N: 60) | p-Value * | |
|---|---|---|---|---|
| Age of recipients | 49 (40, 57) | 49 (38.25, 57) | 48 (43, 52) | 0.968 |
| Sex (female/male) | 19/52 | 3/8 | 16/44 | 1.000 |
| Donor type (deceased/living) | 50/21 | 7/4 | 43/17 | 0.721 |
| DGF | 21/71 | 6/11 | 15/60 | 0.072 |
| Frequency and Absolute Number of Cells at Pre-Transplant Time (T0) | Rejection | p-Value Wilcoxon Rank Sum Test | |
|---|---|---|---|
| Rejection Free, (N = 60) Median (IQR) | Rejection, (N = 11) Median (IQR) | ||
| % DN | 12 (8, 19) | 9 (7, 15) | 0.5 |
| # DN | 10 (6, 19) | 9 (6, 18) | 0.8 |
| % MZB | 31 (9, 39) | 17 (9, 27) | 0.5 |
| # MZB | 5.2 (1.1, 11.8) | 4.2 (1.8, 6.3) | 0.7 |
| Cell Population at 12 Months Post-Transplant | Rejection | p-Value Wilcoxon Rank Sum Test | |
|---|---|---|---|
| Rejection Free, N = 60 Median (IQR) | Rejection, N = 11 Median (IQR) | ||
| % DN | 12.9 (8.7, 17.9) | 14.4 (12.7, 17.6) | 0.2 |
| # DN | 12 (8, 21) | 11 (9, 25) | 0.7 |
| % MZB | 31 (20, 45) | 20 (13, 26) | 0.064 |
| # MZB | 7 (5, 11) | 9 (6, 10) | >0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouza, A.; Fylaktou, A.; Daoudaki, M.; Talimtzi, P.; Tagkouta, A.; Vagiotas, L.; Katsanos, G.; Tsoulfas, G.; Antoniadis, N. Can Double-Negative B Cells and Marginal Zone B Cells Have a Potential Impact on the Outcome of Kidney Transplantation? J. Clin. Med. 2025, 14, 3312. https://doi.org/10.3390/jcm14103312
Fouza A, Fylaktou A, Daoudaki M, Talimtzi P, Tagkouta A, Vagiotas L, Katsanos G, Tsoulfas G, Antoniadis N. Can Double-Negative B Cells and Marginal Zone B Cells Have a Potential Impact on the Outcome of Kidney Transplantation? Journal of Clinical Medicine. 2025; 14(10):3312. https://doi.org/10.3390/jcm14103312
Chicago/Turabian StyleFouza, Ariadni, Asimina Fylaktou, Maria Daoudaki, Persefoni Talimtzi, Anneta Tagkouta, Lampros Vagiotas, Georgios Katsanos, Georgios Tsoulfas, and Nikolaos Antoniadis. 2025. "Can Double-Negative B Cells and Marginal Zone B Cells Have a Potential Impact on the Outcome of Kidney Transplantation?" Journal of Clinical Medicine 14, no. 10: 3312. https://doi.org/10.3390/jcm14103312
APA StyleFouza, A., Fylaktou, A., Daoudaki, M., Talimtzi, P., Tagkouta, A., Vagiotas, L., Katsanos, G., Tsoulfas, G., & Antoniadis, N. (2025). Can Double-Negative B Cells and Marginal Zone B Cells Have a Potential Impact on the Outcome of Kidney Transplantation? Journal of Clinical Medicine, 14(10), 3312. https://doi.org/10.3390/jcm14103312







