Cognitive Correlates of Functional Disruption at Psychosis Onset: Unique Relevance of Visual Cognition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.3.1. Clinical Assessment
2.3.2. Neuropsychological Assessment
2.3.3. Level of Functioning
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Association with Functional Impairment
3.3. Association with Symptoms Severity
3.4. Diagnostic Outcomes
3.5. Path Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, M.F.; Kern, R.S.; Heaton, R.K. Longitudinal Studies of Cognition and Functional Outcome in Schizophrenia: Implications for MATRICS. Schizophr. Res. 2004, 72, 41–51. [Google Scholar] [CrossRef]
- Watson, A.J.; Harrison, L.; Preti, A.; Wykes, T.; Cella, M. Cognitive Trajectories Following Onset of Psychosis: A Meta-Analysis—Corrigendum. Br. J. Psychiatry 2023, 222, 49. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive Impairment in Schizophrenia: Aetiology, Pathophysiology, and Treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef] [PubMed]
- Halverson, T.F.; Orleans-Pobee, M.; Merritt, C.; Sheeran, P.; Fett, A.K.; Penn, D.L. Pathways to Functional Outcomes in Schizophrenia Spectrum Disorders: Meta-Analysis of Social Cognitive and Neurocognitive Predictors. Neurosci. Biobehav. Rev. 2019, 105, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Montaner-Ferrer, M.J.; Gadea, M.; Sanjuán, J. Cognition and Social Functioning in First Episode Psychosis: A Systematic Review of Longitudinal Studies. Front. Psychiatry 2023, 14, 1055012. [Google Scholar] [CrossRef]
- Kadakia, A.; Fan, Q.; Shepherd, J.; Dembek, C.; Bailey, H.; Walker, C.; Williams, G.R. Healthcare Resource Utilization and Quality of Life by Cognitive Impairment in Patients with Schizophrenia. Schizophr. Res. Cogn. 2022, 28, 100233. [Google Scholar] [CrossRef]
- Galderisi, S.; Rucci, P.; Mucci, A.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Bozzatello, P.; et al. The Interplay among Psychopathology, Personal Resources, Context-related Factors and Real-life Functioning in Schizophrenia: Stability in Relationships after 4 Years and Differences in Network Structure between Recovered and Non-recovered Patients. World Psychiatry 2020, 19, 81–91. [Google Scholar] [CrossRef]
- Moura, B.M.; Isvoranu, A.-M.; Kovacs, V.; Van Rooijen, G.; Van Amelsvoort, T.; Simons, C.J.P.; Bartels-Velthuis, A.A.; Bakker, P.R.; Marcelis, M.; De Haan, L.; et al. The Puzzle of Functional Recovery in Schizophrenia-Spectrum Disorders—Replicating a Network Analysis Study. Schizophr. Bull. 2022, 48, 871–880. [Google Scholar] [CrossRef]
- Gilbert, E.; Mérette, C.; Jomphe, V.; Émond, C.; Rouleau, N.; Bouchard, R.-H.; Roy, M.-A.; Paccalet, T.; Maziade, M. Cluster Analysis of Cognitive Deficits May Mark Heterogeneity in Schizophrenia in Terms of Outcome and Response to Treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 333–343. [Google Scholar] [CrossRef]
- Green, M.J.; Cairns, M.J.; Wu, J.; Dragovic, M.; Jablensky, A.; Tooney, P.A.; Scott, R.J.; Carr, V.J. Genome-Wide Supported Variant MIR137 and Severe Negative Symptoms Predict Membership of an Impaired Cognitive Subtype of Schizophrenia. Mol. Psychiatry 2013, 18, 774–780. [Google Scholar] [CrossRef]
- Weinberg, D.; Lenroot, R.; Jacomb, I.; Allen, K.; Bruggemann, J.; Wells, R.; Balzan, R.; Liu, D.; Galletly, C.; Catts, S.V. Cognitive Subtypes of Schizophrenia Characterized by Differential Brain Volumetric Reductions and Cognitive Decline. JAMA Psychiatry 2016, 73, 1251–1259. [Google Scholar] [CrossRef]
- Wells, R.; Swaminathan, V.; Sundram, S.; Weinberg, D.; Bruggemann, J.; Jacomb, I.; Cropley, V.; Lenroot, R.; Pereira, A.M.; Zalesky, A.; et al. The Impact of Premorbid and Current Intellect in Schizophrenia: Cognitive, Symptom, and Functional Outcomes. Npj Schizophr. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reser, M.P.; Allott, K.A.; Killackey, E.; Farhall, J.; Cotton, S.M. Exploring Cognitive Heterogeneity in First-Episode Psychosis: What Cluster Analysis Can Reveal. Psychiatry Res. 2015, 229, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, G.; Malla, A.; Joober, R.; Brodeur, M.B.; Lepage, M. Comparing Cognitive Clusters across First- and Multiple-Episode of Psychosis. Psychiatry Res. 2018, 269, 707–718. [Google Scholar] [CrossRef]
- Uren, J.; Cotton, S.M.; Killackey, E.; Saling, M.M.; Allott, K. Cognitive Clusters in First-Episode Psychosis: Overlap with Healthy Controls and Relationship to Concurrent and Prospective Symptoms and Functioning. Neuropsychology 2017, 31, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Haas, S.S.; Dwyer, D.B.; Ruef, A.; Oeztuerk, O.F.; Antonucci, L.A.; von Saldern, S.; Bonivento, C.; Garzitto, M.; Ferro, A.; et al. Cognitive Subtypes in Recent Onset Psychosis: Distinct Neurobiological Fingerprints? Neuropsychopharmacology 2021, 46, 1475–1483. [Google Scholar] [CrossRef]
- Crouse, J.J.; Moustafa, A.A.; Bogaty, S.E.R.; Hickie, I.B.; Hermens, D.F. Parcellating Cognitive Heterogeneity in Early Psychosis-Spectrum Illnesses: A Cluster Analysis. Schizophr. Res. 2018, 202, 91–98. [Google Scholar] [CrossRef]
- Lee, J.; Rizzo, S.; Altshuler, L.; Glahn, D.C.; Miklowitz, D.J.; Sugar, C.A.; Wynn, J.K.; Green, M.F. Deconstructing Bipolar Disorder and Schizophrenia: A Cross-Diagnostic Cluster Analysis of Cognitive Phenotypes. J. Affect. Disord. 2017, 209, 71–79. [Google Scholar] [CrossRef]
- Lee, R.S.C.; Hermens, D.F.; Naismith, S.L.; Lagopoulos, J.; Jones, A.; Scott, J.; Chitty, K.M.; White, D.; Robillard, R.; Scott, E.M.; et al. Neuropsychological and Functional Outcomes in Recent-Onset Major Depression, Bipolar Disorder and Schizophrenia-Spectrum Disorders: A Longitudinal Cohort Study. Transl. Psychiatry 2015, 5, e555. [Google Scholar] [CrossRef]
- Rheenen, T.E.V.; Lewandowski, K.E.; Tan, E.J.; Ospina, L.H.; Ongur, D.; Neill, E.; Gurvich, C.; Pantelis, C.; Malhotra, A.K.; Rossell, S.L.; et al. Characterizing Cognitive Heterogeneity on the Schizophrenia–Bipolar Disorder Spectrum. Psychol. Med. 2017, 47, 1848–1864. [Google Scholar] [CrossRef]
- Vaskinn, A.; Haatveit, B.; Melle, I.; Andreassen, O.A.; Ueland, T.; Sundet, K. Cognitive Heterogeneity across Schizophrenia and Bipolar Disorder: A Cluster Analysis of Intellectual Trajectories. J. Int. Neuropsychol. Soc. 2020, 26, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Van Rheenen, T.E.; Cropley, V.; Zalesky, A.; Bousman, C.; Wells, R.; Bruggemann, J.; Sundram, S.; Weinberg, D.; Lenroot, R.K.; Pereira, A.; et al. Widespread Volumetric Reductions in Schizophrenia and Schizoaffective Patients Displaying Compromised Cognitive Abilities. Schizophr. Bull. 2018, 44, 560–574. [Google Scholar] [CrossRef]
- Early Intervention in Psychosis Network (EIPN) Standards for Early Intervention in Psychosis Services 2019. Available online: https://www.rcpsych.ac.uk/docs/default-source/improving-care/ccqi/quality-networks/early-intervention-in-psychosis-teams-(eipn)/eipn-3rd-edition-standards---april-2025.pdf?sfvrsn=25c71a3d_3 (accessed on 21 March 2025).
- The National Centre of Excellence in Youth Mental Health. Early Psychosis Guidelines Writing Group and EPPIC National Support Program. In Australian Clinical Guidelines for Early Psychosis, 2nd ed.; Orygen: Melbourne, Australia, 2016. [Google Scholar]
- Andreasen, N.C.; Carpenter, W.T.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in Schizophrenia: Proposed Criteria and Rationale for Consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Samara, M.; Heres, S.; Davis, J.M. Dose Equivalents for Antipsychotic Drugs: The DDD Method. Schizophr. Bull. 2016, 42, 90–94. [Google Scholar] [CrossRef]
- Kay, S.R.; Flszbeln, A.; Qpjer, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1967, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Asberg, M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry J. Ment. Sci. 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. Young Mania Rating Scale. J. Affect. Disorders. 1978. [CrossRef]
- Elwood, R.W. The California Verbal Learning Test: Psychometric Characteristics and Clinical Application. Neuropsychol. Rev. 1995, 5, 173–201. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and Interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Grant, D.A.; Berg, E. A Behavioral Analysis of Degree of Reinforcement and Ease of Shifting to New Responses in a Weigl-Type Card-Sorting Problem. J. Exp. Psychol. 1948, 38, 404–411. [Google Scholar] [CrossRef]
- Blessing, A.; Wangelin, B.; Regehr, H.; Rudolph, N. Verbal Fluency Tasks in First Episode Psychosis. Clin. Neuropsychiatry 2009, 6, 21–28. [Google Scholar]
- Zhang, X.; Lv, L.; Min, G.; Wang, Q.; Zhao, Y.; Li, Y. Overview of the Complex Figure Test and Its Clinical Application in Neuropsychiatric Disorders, Including Copying and Recall. Front. Neurol. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Osterrieth, P.A. Le Test de Copie d’une Figure Complexe; Contribution à l’étude de La Perception et de La Mémoire. [Test of Copying a Complex Figure; Contribution to the Study of Perception and Memory]. Arch. Psychol. 1944, 30, 206–356. [Google Scholar]
- Taylor, E.M. Psychological Appraisal of Children with Cerebral Defects; Harvard University Press: Oxford, UK, 1959. [Google Scholar]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: New York, NY, USA, 2006; ISBN 0-19-515957-8. [Google Scholar]
- Seidman, L.J.; Lanca, M.; Kremen, W.S.; Faraone, S.V.; Tsuang, M.T. Organizational and Visual Memory Deficits in Schizophrenia and Bipolar Psychoses Using the Rey-Osterrieth Complex Figure: Effects of Duration of Illness. J. Clin. Exp. Neuropsychol. 2003, 25, 949–964. [Google Scholar] [CrossRef]
- Morosini, P.; Magliano, L.; Brambilla, L.; Ugolini, S.; Pioli, R. Development, Reliability and Acceptability of a New Version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to Assess Routine Social Functioning. Acta Psychiatr. Scand. 2000, 101, 323–329. [Google Scholar]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis. A Regression-Based Approach, 3rd ed.; Guilford Publications: New York, NY, USA, 2022. [Google Scholar]
- Carroll, J.B. The Three-Stratum Theory of Cognitive Abilities. In Contemporary Intellectual Assessment: Theories, Tests, and Issues; The Guilford Press: New York, NY, USA, 2005; pp. 69–76. ISBN 1-59385-125-1. [Google Scholar]
- Diamond, A.; Silverstein, S.M.; Keane, B.P. Visual System Assessment for Predicting a Transition to Psychosis. Transl. Psychiatry 2022, 12, 351. [Google Scholar] [CrossRef]
- Perez, V.B.; Shafer, K.M.; Cadenhead, K.S. Visual Information Processing Dysfunction across the Developmental Course of Early Psychosis. Psychol. Med. 2012, 42, 2167–2179. [Google Scholar] [CrossRef]
- McCleery, A.; Wynn, J.K.; Lee, J.; Reavis, E.A.; Ventura, J.; Subotnik, K.L.; Green, M.F.; Nuechterlein, K.H. Early Visual Processing Is Associated With Social Cognitive Performance in Recent-Onset Schizophrenia. Front. Psychiatry 2020, 11, 823. [Google Scholar] [CrossRef]
- Catalan, A.; Salazar De Pablo, G.; Aymerich, C.; Damiani, S.; Sordi, V.; Radua, J.; Oliver, D.; McGuire, P.; Giuliano, A.J.; Stone, W.S.; et al. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2021, 78, 859–867. [Google Scholar] [CrossRef]
- Hauser, M.; Zhang, J.P.; Sheridan, E.M.; Burdick, K.E.; Mogil, R.; Kane, J.M.; Correll, C.U. Neuropsychological Test Performance to Enhance Identification of Subjects at Clinical High Risk for Psychosis and to Be Most Promising for Predictive Algorithms for Conversion to Psychosis: A Meta-Analysi. J. Clin. Psychiatry 2017, 78, 12639. [Google Scholar] [CrossRef]
- Hui, C.L.M.; Li, Y.K.; Li, A.W.Y.; Lee, E.H.M.; Chang, W.C.; Chan, S.K.W.; Lam, S.Y.; Thornton, A.E.; Sham, P.; Honer, W.G.; et al. Visual Working Memory Deterioration Preceding Relapse in Psychosis. Psychol. Med. 2016, 46, 2435–2444. [Google Scholar] [CrossRef]
- Brasso, C.; Bellino, S.; Bozzatello, P.; Del Favero, E.; Montemagni, C.; Rocca, P. Inter-Relationships among Psychopathology, Cognition, and Real-Life Functioning in Early and Late Phase Schizophrenia: A Network Analysis Approach. Schizophr. Res. 2023, 256, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Hasson-Ohayon, I.; Goldzweig, G.; Lavi-Rotenberg, A.; Luther, L.; Lysaker, P.H. The Centrality of Cognitive Symptoms and Metacognition within the Interacting Network of Symptoms, Neurocognition, Social Cognition and Metacognition in Schizophrenia. Schizophr. Res. 2018, 202, 260–266. [Google Scholar] [CrossRef]
- Lundsgaard, J.; Kristensen, T.D.; Wenneberg, C.; Gregersen, M.; Nordentoft, M.; Glenthøj, L.B. Premorbid Adjustment Associates with Cognitive and Functional Deficits in Individuals at Ultra-High Risk of Psychosis. Schizophr. Heidelb. Ger. 2022, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, L.; La Cascia, C.; La Barbera, D.; Sanchez-Gutierrez, T.; Tripoli, G.; Seminerio, F.; Sartorio, C.; Marrazzo, G.; Sideli, L.; Arango, C.; et al. The Relationship of Symptom Dimensions with Premorbid Adjustment and Cognitive Characteristics at First Episode Psychosis: Findings from the EU-GEI Study. Schizophr. Res. 2021, 236, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Stefanatou, P.; Karatosidi, C.-S.; Tsompanaki, E.; Kattoulas, E.; Stefanis, N.C.; Smyrnis, N. Premorbid Adjustment Predictors of Cognitive Dysfunction in Schizophrenia. Psychiatry Res. 2018, 267, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Allott, K.A.; Cotton, S.M.; Chinnery, G.L.; Baksheev, G.N.; Massey, J.; Sun, P.; Collins, Z.; Barlow, E.; Broussard, C.; Wahid, T.; et al. The Relative Contribution of Neurocognition and Social Cognition to 6-Month Vocational Outcomes Following Individual Placement and Support in First-Episode Psychosis. Schizophr. Res. 2013, 150, 136–143. [Google Scholar] [CrossRef]
- Caruana, E.; Cotton, S.; Killackey, E.; Allott, K.; Caruana, E.; Cotton, S.; Killackey, E.; Allott, K. The Relationship between Cognition, Job Complexity, and Employment Duration in First-Episode Psychosis. Psychiatr. Rehabil. J. 2015, 38, 210–217. [Google Scholar] [CrossRef]
- Rassovsky, Y.; Horan, W.P.; Lee, J.; Sergi, M.J.; Green, M.F. Pathways between Early Visual Processing and Functional Outcome in Schizophrenia. Psychol. Med. 2011, 41, 487. [Google Scholar] [CrossRef]
- Sasabayashi, D.; Takahashi, T.; Takayanagi, Y.; Nemoto, K.; Ueno, M.; Furuichi, A.; Higuchi, Y.; Mizukami, Y.; Kobayashi, H.; Yuasa, Y.; et al. Resting state hyperconnectivity of the default mode network in schizophrenia and clinical high-risk state for psychosis. Cereb Cortex. 2023, 33, 8456–8464. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Li, L.; Zhou, Y.; Lin, D.; Zhang, M.; Zhang, L.; Huang, G.; Liu, X.; Becker, B.; et al. Functional connectivity profiles of the default mode and visual networks reflect temporal accumulative effects of sustained naturalistic emotional experience. Neuroimage 2023, 269, 119941. [Google Scholar] [CrossRef]
- Waters, F.; Collerton, D.; Ffytche, D.H.; Jardri, R.; Pins, D.; Dudley, R.; Blom, J.D.; Mosimann, U.P.; Eperjesi, F.; Ford, S.; et al. Visual Hallucinations in the Psychosis Spectrum and Comparative Information From Neurodegenerative Disorders and Eye Disease. Schizophr. Bull. 2014, 40, S233–S245. [Google Scholar] [CrossRef] [PubMed]
- Kogata, T.; Iidaka, T. A Review of Impaired Visual Processing and the Daily Visual World in Patients with Schizophrenia. Nagoya J. Med. Sci. 2018, 80, 317. [Google Scholar]
- Subdoh, B.; Grover, S. Depression in Schizophrenia: Prevalence and Its Impact on Quality of Life, Disability, and Functioning. Asian J. Psychiatry 2020, 54, 102425. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Thika, S.; Anderson, G.; Galecki, P.; Maes, M. Affective Symptoms in Schizophrenia Are Strongly Associated with Neurocognitive Deficits Indicating Disorders in Executive Functions, Visual Memory, Attention and Social Cognition. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.J.; Garety, P.; Hardy, A. The Relationship between Depressive Symptoms and Negative Symptoms in People with Non-Affective Psychosis: A Meta-Analysis. Psychol. Med. 2019, 49, 2486–2498. [Google Scholar] [CrossRef]
- Adhan, I.; Lizano, P.; Bannai, D.; Lutz, O.; Dhaliwal, K.; Zeng, V.; Miewald, J.; Montrose, D.; Keshavan, M. Visual Cortical Alterations and Their Association with Negative Symptoms in Antipsychotic-Naïve First Episode Psychosis. Psychiatry Res. 2020, 288, 112957. [Google Scholar] [CrossRef]
- Sevy, S.; Lindenmayer, J.P.; Khan, A.; Ljuri, I.; Kulsa, M.K.C.; Jones, O. Differential Improvement of Negative-Symptom Subfactors after Cognitive Remediation in Low-Functioning Individuals with Schizophrenia. Schizophr. Res. Cogn. 2020, 19, 100145. [Google Scholar] [CrossRef]
- Bodapati, A.S.; Jenkins, L.M.; Sharma, R.P.; Rosen, C. Visual Memory Uniquely Predicts Anhedonia in Schizophrenia but Not Bipolar Disorder. J. Neuropsychol. 2019, 13, 136–146. [Google Scholar] [CrossRef]
- Cohen, A.S.; Leung, W.W.; Saperstein, A.M.; Blanchard, J.J. Neuropsychological Functioning and Social Anhedonia: Results from a Community High-Risk Study. Schizophr. Res. 2006, 85, 132–141. [Google Scholar] [CrossRef]
- Howes Vallis, E.; MacKenzie, L.E.; Zwicker, A.; Drobinin, V.; Rempel, S.; Abidi, S.; Uher, R. Visual Memory and Psychotic Symptoms in Youth. Cogn. Neuropsychiatry 2020, 25, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Scala, S.; Pousada, A.; Stone, W.S.; Thermenos, H.W.; Manschreck, T.C.; Tsuang, M.T.; Faraone, S.V.; Seidman, L.J. Verbal and Visual-Spatial Memory Impairment in Youth at Familial Risk for Schizophrenia or Affective Psychosis: A Pilot Study. Schizophr. Res. 2013, 144, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, J.M.; Meyhoefer, I.; Antonucci, L.A.; Kambeitz-Ilankovic, L.; Surmann, M.; Bienek, O.; Romer, G.; Dannlowski, U.; Hahn, T.; Korda, A.; et al. The Impact of Visual Disfunction in Recent-Onset Psychosis Amd Clinical High-Risk for Psychosis. Neuropsychopharmacology 2022, 47, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Badde, L.; Haas, S.S.; Bonivento, C.; Van Rheenen, T.E.; Antonucci, L.A.; Ruef, A.; Penzel, N.; Rosen, M.; Lichtenstein, T.; et al. Transdiagnostic Subgroups of Cognitive Impairment in Early Affective and Psychotic Illness. Neuropsychopharmacology 2024, 49, 573–583. [Google Scholar] [CrossRef]
| Variables | Mean (SD)/N (%) | |||
|---|---|---|---|---|
| Demographics | ||||
| Gender, male | 80 (75.7) | |||
| Age | 24 (5.6) | |||
| Ethnicity | ||||
| White Caucasian Portuguese | 86 (80.5) | |||
| 78 (73) | ||||
| Other European | 8 (7.5) | |||
| Ethnic minorities | 21 (19.5) | |||
| Years of Education | 12 (3.1) | |||
| Duration of Illness (months) | 14.4 (21) a | |||
| Chlorpromazine Equivalent DDD | 423 mg (229 mg) b | |||
| AP’s class c | ||||
| First Generation Second Generation | 3 (3.2) 98 (97.3) | |||
| Of which Clozapine | 5 (5.6) | |||
| Lifetime Substance use d | 74 (69.1) | |||
| Age at First Use (any substance) | 15.7 (3.6) | |||
| Cannabis | 65 (60.7) | |||
| Other substances † | 28 (26.2) | |||
| Clinical Symptoms | ||||
| PANSS Total e | 80.3 (16.1) | |||
| PANSS Positive | 24.3 (6.5) | |||
| PANSS Negative | 16.5 (7.3) | |||
| PANSS General | 39.5 (9.4) | |||
| SANS Total f | 26.9 (15.4) | |||
| Diagnosis at discharge g | ||||
| Schizophrenia (F.20.0) Brief Psychotic Disorder (F23.0)/Drug Induced Psychosis (F.12.25) Depressive/Manic episode with psychotic symptoms (F.32.3; F.30.2) | 45 (44.5) 35 (34.6) 20 | |||
| Pre-morbid adjustment h PAS General PAS Infancy PAS Primary Adolescence PAS Secondary Adolescence PAS Adulthood | 21.8 (11.04) 6.9 (4.6) 10.1 (5.4) 10.7 (5.9) 7.3 (4.6) | |||
| Current functioning i | ||||
| PSP | 38.2 (15.8) | |||
| Employment Status (Unemployed) j | 42 (41.6) | |||
| Cognitive domains and measures ‡ | Mean(SD)/Frequencies (%) | Cohen’s d * FEP vs. Normative | 95%CI * | |
| Lower | Upper | |||
| Attention | ||||
| Tulouse Pieron—Dispersion Index (%) | 40 (32.5) | 2.1 | 0.0207 | 0.7300 |
| Verbal Memory and Learning CVLT | ||||
| Total Free Recall (A1–A5) | 41.6 (9.7) | 2.7 | 0.1541 | 1.0084 |
| Total Long Delayed Free Recall (20 min.) | 8 (3.2) | 3.2 | 0.3006 | 1.2814 |
| Executive Functioning | ||||
| TMT A (time in seconds) TMT B | 42.6 (20.2) 111 (61.4) | 0.06 1.2 | −0.1916 −0.1025 | 0.2036 0.4164 |
| WCST Preservatives Errors | 15.2 (11.7) | 0.5 | −0.1567 | 0.2623 |
| WCST N. correct categories | 4.3 (2.1) | 1.8 | −0.0297 | 0.6113 |
| Digit Span backwards | 3.7 (1.3) | 0.9 | −0.1280 | 0.3406 |
| Verbal Fluency (1 min) | 17 (5.6) | 0.9 | −0.1280 | 0.3406 |
| Visual Memory and Visual Spatial Abilities | ||||
| ROCFT—Memory recall (4 min) | 15.2 | 1.5 | 0.0704 | 0.5067 |
| ROCFT—Copy Type | Category’s Percentile | |||
| Patients | Normative Controls | |||
| 10th | I | III/IV | ||
| 25th | II | II | ||
| 50th | III | I | ||
| 75th | IV | I | ||
| 95th | V | I | ||
| Functional Impairment | ||||||||
| Current Social Functioning (time of Hospitalization and Month Prior) | ||||||||
| Stepwise Multivariate Regression Model | ||||||||
| Predictors | Effect Size | Unstand. B | S.E. | ß | t | p Value | 95% CI | |
| Lower | Upper | |||||||
| aR2 = 0.378 | ||||||||
| Visual Mem. | 0.616 | 0.226 | 0.297 | 2.732 | 0.008 | 0.165 | 1.067 | |
| Educ. Level | 1.656 | 0.571 | 0.322 | 2.900 | 0.005 | 0.514 | 2.799 | |
| Medic. Level | −0.017 | 0.007 | −0.263 | −2.550 | 0.013 | −0.031 | −0.004 | |
| Employment Status (time of hospitalization and month prior) | ||||||||
| Stepwise Logistic Regression Model | ||||||||
| Predictors | Effect size | Unstand. B | S.E. | Exp(B) | Wald | p Value | 95% CI | |
| aR2 = 0.275 | Lower | Upper | ||||||
| −0.572 | 0.220 | 0.564 | 6.774 | 0.009 | 0.367 | 0.868 | ||
| Visual-Spatial | ||||||||
| General Pre-morbid Adj. | −0.067 | 0.027 | 935 | 6.039 | 0.014 | 0.887 | 0.987 | |
| Symptom Severity | ||||||||
| Stepwise Multivariate Regression Model | ||||||||
| Predictors | Effect size | Unstand. B | S.E. | ß | t | pValue | 95% CI | |
| aR2 = 0.255 | ||||||||
| Medic. level | 0.024 | 0.006 | 0.339 | 3.754 | <0.0005 | 0.011 | 0.036 | |
| Depressive Symptoms | 0.272 | 0.106 | 0.225 | 2.563 | 0.012 | 0.061 | 0.482 | |
| Visual Mem. | −0.458 | 0.192 | −0.214 | −2.380 | 0.019 | −0.840 | −0.076 | |
| Diagnostic Outcome (non-affective vs. affective psychosis) | ||||||||
| Stepwise Logistic Regression Model | ||||||||
| Predictors | Effect size | Unstand. B | S.E. | Exp(B) | Wald | p Value | 95% CI | |
| aR2 = 0.376 | Lower | Upper | ||||||
| Current. Func. | 0.061 | 0.031 | 1.063 | 3.831 | 0.049 | 1.000 | 1.129 | |
| Visual-Spatial | −0.697 | 0.365 | 0.498 | 3.647 | 0.036 | 244 | 1.018 | |
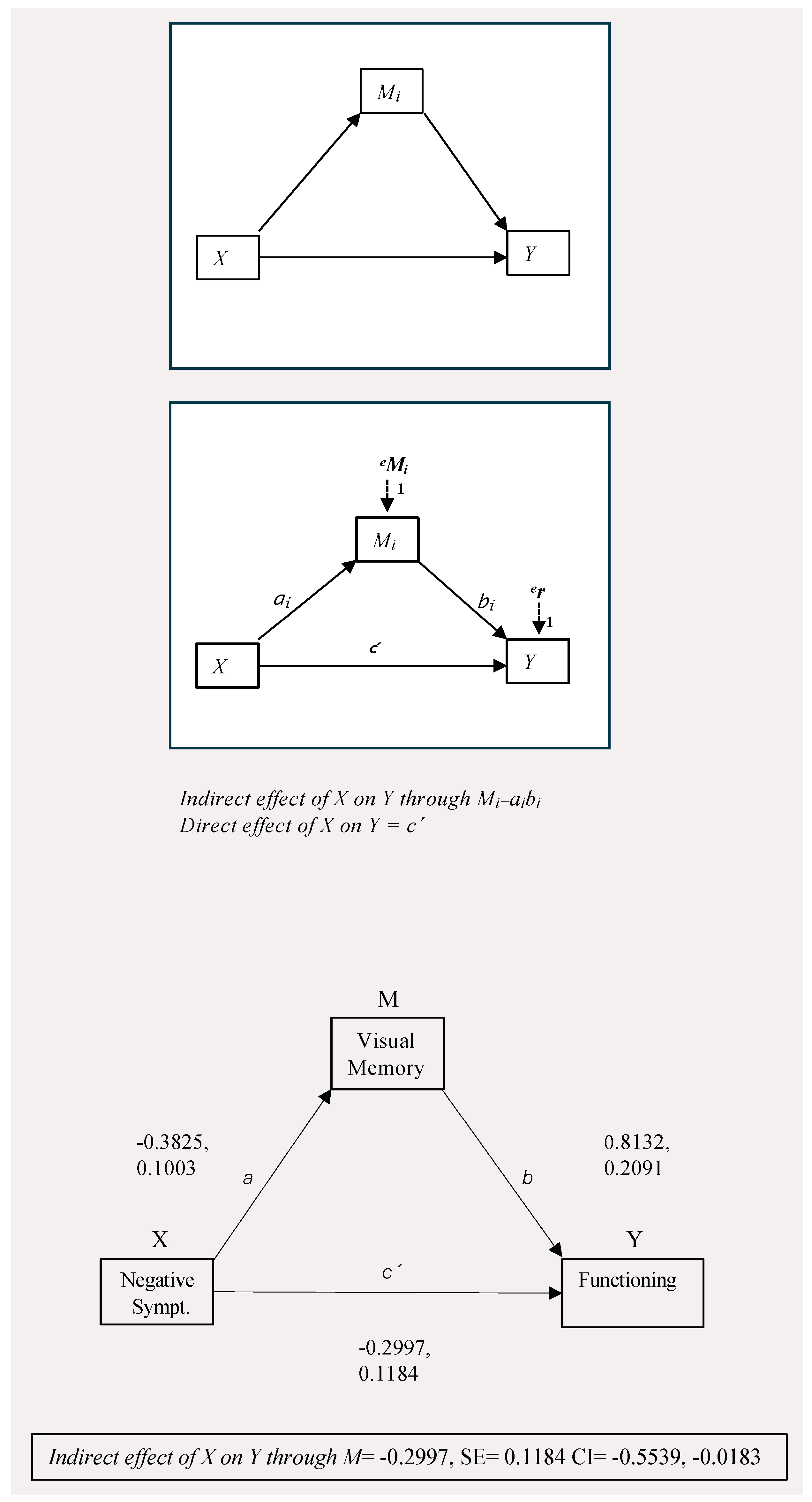
| M (Visual Memory) | Y (Functioning) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß | SE | p | 95% CI | ß | SE | p | 95% CI | |||||
| Antecedent | upper | lower | upper | lower | ||||||||
| X (Negative Symptoms) | a | −0.3825 | 0.1003 | 0.0015 | −0.5278 | −0.1292 | c’ | −0.3768 | 0.2083 | 0.073 | −0.7908 | 0.0373 |
| M (Visual Memory) | - | - | - | - | - | b | 0.9123 | 0.2091 | <0.001 | 0.4968 | 10.3279 | |
| R2 = 1087 | R2 = 0.2619 | |||||||||||
| F(1, 100) = 10.733 p0 = 0.0015 | F(2, 98) = 15.436 p = <0.001 | |||||||||||
| Total Effect | SE | p | 95%CI | |||||||||
| C = c’+ a*b | upper | lower | ||||||||||
| −0.6764 | 0.2159 | 0.0023 | −1.1055 | −0.2475 | ||||||||
| Indir. Effect | SE | p | 95%CI | |||||||||
| a*b | upper | lower | ||||||||||
| −0.2997 | 0.1184 | - | −0.5539 | −0.0183 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, A.; Coentre, R.; Mendes, T.; Levy, P.; Cella, M.; Novais, F. Cognitive Correlates of Functional Disruption at Psychosis Onset: Unique Relevance of Visual Cognition. J. Clin. Med. 2025, 14, 3308. https://doi.org/10.3390/jcm14103308
Avila A, Coentre R, Mendes T, Levy P, Cella M, Novais F. Cognitive Correlates of Functional Disruption at Psychosis Onset: Unique Relevance of Visual Cognition. Journal of Clinical Medicine. 2025; 14(10):3308. https://doi.org/10.3390/jcm14103308
Chicago/Turabian StyleAvila, Alessia, Ricardo Coentre, Tiago Mendes, Pedro Levy, Matteo Cella, and Filipa Novais. 2025. "Cognitive Correlates of Functional Disruption at Psychosis Onset: Unique Relevance of Visual Cognition" Journal of Clinical Medicine 14, no. 10: 3308. https://doi.org/10.3390/jcm14103308
APA StyleAvila, A., Coentre, R., Mendes, T., Levy, P., Cella, M., & Novais, F. (2025). Cognitive Correlates of Functional Disruption at Psychosis Onset: Unique Relevance of Visual Cognition. Journal of Clinical Medicine, 14(10), 3308. https://doi.org/10.3390/jcm14103308







