Parathyroid Hormone Levels as an Independent Predictor of Ischemic Heart Disease in Stage 3–5 Non-Dialysis Chronic Kidney Disease: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data
2.2. Study Endpoints
2.3. Statistical Analysis
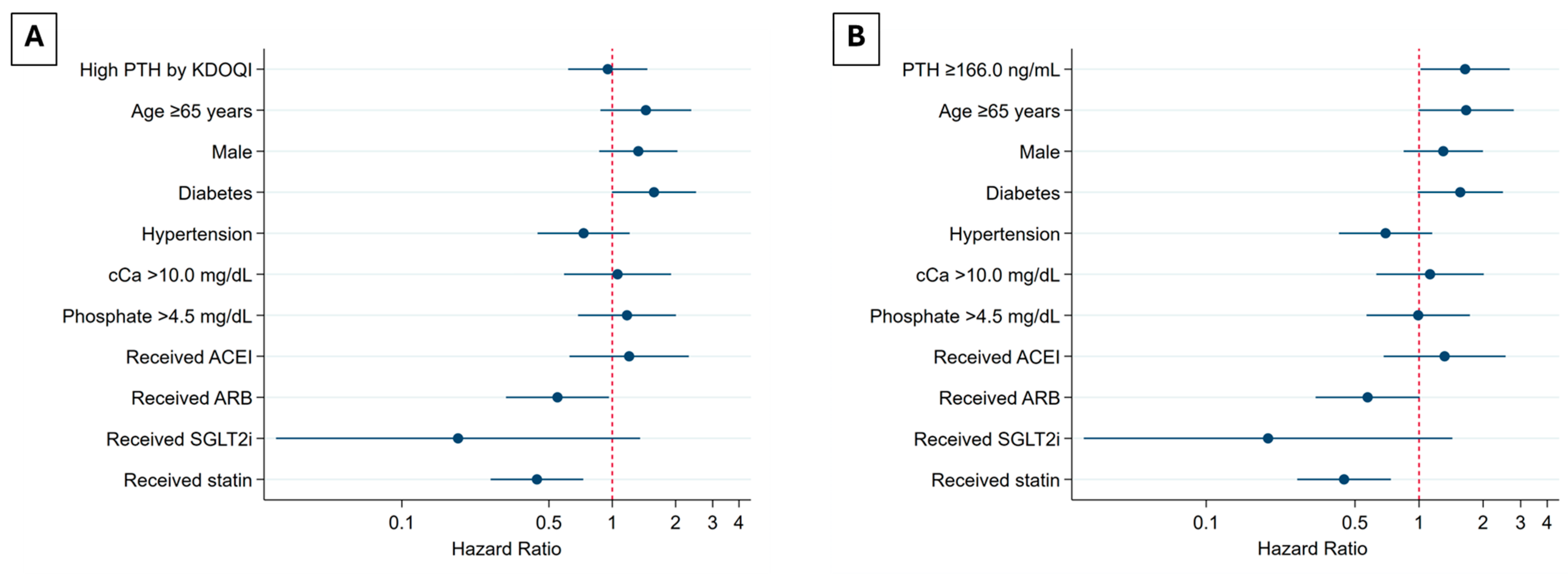
3. Results
3.1. Patient Characteristics
3.2. Risk of IHD and Minimum PTH Cut-Off Level
3.3. Risk of IHD in CKD Stage 3–5ND Using PTH KDOQI Cut-Offs and Tertile Cut-Offs
3.4. Joint Model to Evaluate Time-Varying PTH and the Risk of IHD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jadoul, M.; Aoun, M.; Imani, M.M. The major global burden of chronic kidney disease. Lancet Glob. Health 2024, 12, e342–e343. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Ballew, S.H.; Matsushita, K. Cardiovascular Risk Prediction in CKD. Semin. Nephrol. 2018, 38, 208–216. [Google Scholar] [CrossRef]
- Menon, V.; Gul, A.; Sarnak, M.J. Cardiovascular risk factors in chronic kidney disease. Kidney Int. 2005, 68, 1413–1418. [Google Scholar] [CrossRef]
- Rroji, M.; Figurek, A.; Spasovski, G. Should We Consider the Cardiovascular System While Evaluating CKD-MBD? Toxins 2020, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.-M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef]
- Bozic, M.; Diaz-Tocados, J.M.; Bermudez-Lopez, M.; Forné, C.; Martinez, C.; Fernandez, E.; Valdivielso, J.M. Independent effects of secondary hyperparathyroidism and hyperphosphataemia on chronic kidney disease progression and cardiovascular events: An analysis from the NEFRONA cohort. Nephrol. Dial. Transplant. 2022, 37, 663–672. [Google Scholar] [CrossRef]
- Lishmanov, A.; Dorairajan, S.; Pak, Y.; Chaudhary, K.; Chockalingam, A. Elevated serum parathyroid hormone is a cardiovascular risk factor in moderate chronic kidney disease. Int. Urol. Nephrol. 2012, 44, 541–547. [Google Scholar] [CrossRef]
- Xu, Y.; Evans, M.; Soro, M.; Barany, P.; Carrero, J.J. Secondary hyperparathyroidism and adverse health outcomes in adults with chronic kidney disease. Clin. Kidney J. 2021, 14, 2213–2220. [Google Scholar] [CrossRef]
- Kidney Disease: Improving, Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Bover, J.; Mazzaferro, S.; on behalf of the ERA CKD-MBD Working Group. Treatment of secondary hyperparathyroidism in non-dialysis CKD: An appraisal 2022s. Nephrol. Dial. Transplant. 2023, 38, 1397–1404. [Google Scholar] [CrossRef]
- Ketteler, M.; Evenepoel, P.; Holden, R.M.; Isakova, T.; Jørgensen, H.S.; Komaba, H.; Nickolas, T.L.; Sinha, S.; Vervloet, M.G.; Cheung, M.; et al. Chronic kidney disease–mineral and bone disorder: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2025, 107, 405–423. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [Google Scholar] [CrossRef]
- Kidney Disease: Improving; Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, 117–314. [Google Scholar] [CrossRef]
- Yuan, J.; Zou, X.-R.; Han, S.-P.; Cheng, H.; Wang, L.; Wang, J.-W.; Zhang, L.-X.; Zhao, M.-H.; Wang, X.-Q. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: Results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol. 2017, 18, 23. [Google Scholar] [CrossRef]
- Iimori, S.; Noda, Y.; Okado, T.; Naito, S.; Toda, T.; Chida, Y.; Kuwahara, M.; Ando, R.; Nishio, Y.; Maeda, Y.; et al. Baseline characteristics and prevalence of cardiovascular disease in newly visiting or referred chronic kidney disease patients to nephrology centers in Japan: A prospective cohort study. BMC Nephrol. 2013, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Saeed, D.; Reza, T.; Shahzad, M.W.; Karim Mandokhail, A.; Bakht, D.; Qizilbash, F.H.; Silloca-Cabana, E.O.; Ramadhan, A.; Bokhari, S.F.H. Navigating the Crossroads: Understanding the Link Between Chronic Kidney Disease and Cardiovascular Health. Cureus 2023, 15, e51362. [Google Scholar] [CrossRef]
- Kimm, H.; Mok, Y.; Lee, S.J.; Lee, S.; Back, J.H.; Jee, S.H. The J-curve between Diastolic Blood Pressure and Risk of All-cause and Cardiovascular Death. Korean Circ. J. 2017, 48, 36–47. [Google Scholar] [CrossRef]
- Gaffney, B.; Jacobsen, A.P.; Pallippattu, A.W.; Leahy, N.; McEvoy, J.W. The Diastolic Blood Pressure J-Curve in Hypertension Management: Links and Risk for Cardiovascular Disease. Integr. Blood Press. Control 2021, 14, 179–187. [Google Scholar] [CrossRef]
- Shah, N.R.; Dumler, F. Hypoalbuminaemia—A Marker of Cardiovascular Disease in Patients with Chronic Kidney Disease Stages II–IV. Int. J. Med. Sci. 2008, 5, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.; Sarnak, M.J. Anemia as a risk factor for cardiovascular disease: Management of comorbidities in kidney disease in the 21st century: Anemia and bone disease. Kidney Int. 2003, 64, S32–S39. [Google Scholar] [CrossRef]
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 601. [Google Scholar] [CrossRef]
- Rosenstein, K.; Tannock, L.R. Dyslipidemia in Chronic Kidney Disease. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: http://www.ncbi.nlm.nih.gov/books/NBK305899/ (accessed on 2 September 2024).
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Bhuriya, R.; Li, S.; Chen, S.-C.; McCullough, P.A.; Bakris, G.L. Plasma parathyroid hormone level and prevalent cardiovascular disease in CKD stages 3 and 4: An analysis from the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2009, 53, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Magagnoli, L.; Cozzolino, M.; Caskey, F.J.; Evans, M.; Torino, C.; Porto, G.; Szymczak, M.; Krajewska, M.; Drechsler, C.; Stenvinkel, P.; et al. Association between CKD-MBD and mortality in older patients with advanced CKD—Results from the EQUAL study. Nephrol. Dial. Transplant. 2023, 38, 2562–2575. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Lin, Y.; Yao, K.; Xie, Y.; Zhou, T. Cardiovascular outcomes and safety of SGLT2 inhibitors in chronic kidney disease patients. Front. Endocrinol. 2023, 14, 1236404. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Hypertension in chronic kidney disease—Treatment standard 2023. Nephrol. Dial. Transplant. 2023, 38, 2694–2703. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am. J. Kidney Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef]
- Cho, M.; Choi, C.-Y.; Choi, Y.J.; Rhie, S.J. Clinical outcomes of renin angiotensin system inhibitor-based dual antihypertensive regimens in chronic kidney disease: A network meta-analysis. Sci. Rep. 2023, 13, 5727. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]


| Characteristics | Total | IHD | Non-IHD | p-Value |
|---|---|---|---|---|
| n = 1210 | n = 91 | n = 1119 | ||
| Age, years, mean (SD) | 70.2 (14.4) | 70.9 (12.6) | 70.2 (14.6) | 0.85 |
| Male, n (%) | 534 (44.1) | 44 (48.3) | 490 (43.8) | 0.23 |
| BMI, kg/m2, mean (SD) | 23.9 (4.8) | 22.9 (4.7) | 24.0 (4.8) | 0.03 |
| SBP, mmHg, mean (SD) | 136.8 (23.5) | 138.3 (24.7) | 136.6 (23.2) | 0.61 |
| DBP, mmHg, mean (SD) | 71.6 (15.4) | 68.4 (16.5) | 71.9 (15.3) | 0.01 |
| Comorbidities, n (%) | ||||
| Congestive heart failure | 89 (7.4) | 13 (14.3) | 76 (6.8) | 0.01 |
| Hypertension | 864 (71.4) | 59 (64.8) | 805 (71.9) | 0.15 |
| Paralysis | 52 (4.3) | 3 (3.3) | 49 (4.4) | 0.44 |
| Peripheral vascular disorder | 23 (1.9) | 2 (2.2) | 21 (1.9) | 0.53 |
| Diabetes mellitus | 555 (45.9) | 44 (48.3) | 511 (45.7) | 0.35 |
| Dyslipidemia | 661 (54.6) | 44 (48.3) | 617 (55.1) | 0.23 |
| eGFR, mL/min/1.73 m2, mean (SD) | 29.4 (17.4) | 22.7 (16.8) | 29.9 (17.3) | 0.001 |
| CKD stage, n (%) | ||||
| CKD stage 3a | 279 (23.1) | 13 (14.3) | 266 (23.8) | <0.001 |
| CKD stage 3b | 313 (25.9) | 13 (14.3) | 300 (26.8) | |
| CKD stage 4 | 269 (22.2) | 24 (26.4) | 245 (21.9) | |
| CKD stage 5 | 349 (28.8) | 41 (45.0) | 308 (27.5) | |
| Hb, g/dL, mean (SD) | 10.8 (2.1) | 10.2 (1.9) | 10.8 (2.1) | 0.004 |
| Albumin, g/dL, mean (SD) | 3.6 (0.5) | 3.4 (0.5) | 3.6 (0.5) | <0.001 |
| LDL, mg/dL, mean (SD) | 100.0 (37.1) | 97.3 (37.4) | 100.2 (37.1) | 0.36 |
| Triglycerides, mg/dL, mean (SD) | 136.7 (81.7) | 144.4 (70.2) | 136.1 (82.6) | 0.14 |
| Cholesterol, mg/dL, mean (SD) | 175.8 (52.2) | 171.7 (50.1) | 176.2 (52.4) | 0.35 |
| HDL, mg/dL, mean (SD) | 51.6 (16.5) | 46.3 (13.0) | 52.0 (16.7) | 0.008 |
| Calcium (corrected), mg/dL, mean (SD) | 9.5 (0.8) | 9.4 (0.7) | 9.5 (0.8) | 0.91 |
| ≤10 mg/dL, n (%) | 987 (85.4) | 73 (83.9) | 914 (85.6) | 0.38 |
| >10 mg/dL, n (%) | 168 (14.5) | 14 (16.1) | 154 (14.4) | |
| Phosphate, mg/dL, mean (SD) | 3.9 (1.2) | 4.0 (1.6) | 3.9 (1.2) | 0.44 |
| ≤4.5 mg/dL, n (%) | 954 (81.3) | 67 (75.3) | 887 (81.8) | 0.08 |
| >4.5 mg/dL, n (%) | 219 (18.7) | 22 (24.7) | 197 (18.2) | |
| PTH, ng/L, median (IQR) | 115.5 (70.1–213.1) | 144.2 (84.6–287.5) | 114.1 (70–209.2) | 0.02 |
| <84.6 ng/L, n (%) | 403 (33.3) | 22 (24.2) | 381 (34.1) | 0.038 |
| 84.6–165.9 ng/L, n (%) | 403 (33.3) | 28 (30.8) | 375 (33.5) | |
| ≥166 ng/L, n (%) | 404 (33.4) | 41 (45.0) | 363 (32.4) | |
| Medication, n (%) | ||||
| SGLT2i | 75 (6.2) | 1 (1.1) | 74 (6.6) | 0.02 |
| ACEI | 126 (10.4) | 11 (12.1) | 115 (10.3) | 0.34 |
| ARBs | 373 (30.8) | 16 (17.6) | 357 (31.9) | 0.002 |
| Statin | 459 (37.9) | 21 (23.1) | 438 (39.1) | 0.001 |
| Raw Effect | HR | 95% CI | p-Value |
|---|---|---|---|
| PTH level, ng/L, n (%) | |||
| <84.6 | ref | ||
| 84.6–165.9 | 1.36 | 0.78–2.38 | 0.28 |
| ≥166 | 1.87 | 1.11–3.13 | 0.02 |
| Hypercalcemia | 1.19 | 0.67–2.11 | 0.55 |
| Hyperphosphatemia | 1.27 | 0.79–2.06 | 0.32 |
| Model 1 | HR | 95% CI | p-value |
| PTH level, ng/L, n (%) | |||
| <84.6 | ref | ||
| 84.6–165.9 | 1.22 | 0.68–2.20 | 0.50 |
| ≥166 | 1.84 | 1.05–3.20 | 0.03 |
| Hypercalcemia | 1.22 | 0.68–2.16 | 0.50 |
| Hyperphosphatemia | 0.96 | 0.56–1.64 | 0.88 |
| Model 2 | HR | 95% CI | p-value |
| PTH level, ng/L, n (%) | |||
| <84.6 | ref | ||
| 84.6–165.9 | 1.24 | 0.69–2.23 | 0.5 |
| ≥166 | 2.01 | 1.13–3.56 | 0.02 |
| Hypercalcemia | 1.26 | 0.71–2.24 | 0.43 |
| Hyperphosphatemia | 1.08 | 0.61–1.89 | 0.79 |
| Age ≥65 years | 1.52 | 0.90–2.54 | 0.11 |
| Male | 1.32 | 0.86–2.03 | 0.20 |
| Model 3 | HR | 95% CI | p-value |
| PTH level, ng/L, n (%) | |||
| <84.6 | ref | ||
| 84.6–165.9 | 1.29 | 0.72–2.33 | 0.39 |
| ≥166 | 2.07 | 1.16–3.68 | 0.01 |
| Hypercalcemia | 1.25 | 0.70–2.23 | 0.45 |
| Hyperphosphatemia | 1.03 | 0.58–1.81 | 0.92 |
| Age ≥65 years | 1.61 | 0.95–2.71 | 0.07 |
| Male | 1.34 | 0.88–2.07 | 0.17 |
| HT | 0.61 | 0.37–1.00 | 0.051 |
| DM | 1.37 | 0.86–2.17 | 0.18 |
| Model 4 | HR | 95% CI | p-value |
| PTH level, ng/L, n (%) | |||
| <84.6 | ref | ||
| 84.6–165.9 | 1.28 | 0.71–2.32 | 0.41 |
| ≥166 | 1.87 | 1.05–3.35 | 0.03 |
| Hypercalcemia | 1.14 | 0.64–2.05 | 0.65 |
| Hyperphosphatemia | 0.99 | 0.57–1.73 | 0.97 |
| Age ≥65 years | 1.68 | 1.00–2.81 | 0.04 |
| Male | 1.31 | 0.85–2.01 | 0.22 |
| HT | 0.68 | 0.41–1.13 | 0.13 |
| DM | 1.57 | 0.99–2.49 | 0.056 |
| Medication | |||
| ACEI | 1.33 | 0.69–2.58 | 0.39 |
| ARB | 0.57 | 0.32–1.00 | 0.051 |
| SGLT2i | 0.2 | 0.03–1.44 | 0.11 |
| Statin | 0.44 | 0.27–0.73 | 0.002 |
| Joint Model | Coefficients (95% CI) | p Value |
|---|---|---|
| Longitudinal submodel | ||
| Intercept | 232.75 (217.17 to 248.32) | <0.001 |
| Time | 0.16 (0.12 to 0.19) | <0.001 |
| Survival submodel | ||
| Time-varying PTH | 0.001 (0.0001 to 0.002) | 0.022 |
| Hypercalcemia | −0.04 (0.63 to 0.55) | 0.91 |
| Hyperphosphatemia | 0.04 (−0.52 to 0.59) | 0.9 |
| Age ≥65 years | 0.54 (0.02 to 1.07) | 0.04 |
| Male | −0.33 (−0.76 to 0.10) | 0.14 |
| HT | −0.39 (−0.90 to 0.12) | 0.13 |
| DM | 0.51 (0.05 to 0.97) | 0.03 |
| Medication | ||
| ACEI | 0.24 (−0.41 to 0.90) | 0.47 |
| ARB | −0.57 (−1.13 to −0.01) | 0.046 |
| SGLT2i | −1.71 (−3.70 to 0.29) | 0.09 |
| Statin | −0.82 (−1.3 to −0.31) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anumas, S.; Tantiyavarong, P.; Pattharanitima, P. Parathyroid Hormone Levels as an Independent Predictor of Ischemic Heart Disease in Stage 3–5 Non-Dialysis Chronic Kidney Disease: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 3311. https://doi.org/10.3390/jcm14103311
Anumas S, Tantiyavarong P, Pattharanitima P. Parathyroid Hormone Levels as an Independent Predictor of Ischemic Heart Disease in Stage 3–5 Non-Dialysis Chronic Kidney Disease: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(10):3311. https://doi.org/10.3390/jcm14103311
Chicago/Turabian StyleAnumas, Suthiya, Pichaya Tantiyavarong, and Pattharawin Pattharanitima. 2025. "Parathyroid Hormone Levels as an Independent Predictor of Ischemic Heart Disease in Stage 3–5 Non-Dialysis Chronic Kidney Disease: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 10: 3311. https://doi.org/10.3390/jcm14103311
APA StyleAnumas, S., Tantiyavarong, P., & Pattharanitima, P. (2025). Parathyroid Hormone Levels as an Independent Predictor of Ischemic Heart Disease in Stage 3–5 Non-Dialysis Chronic Kidney Disease: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(10), 3311. https://doi.org/10.3390/jcm14103311







