Popliteal Venous Aneurysms: A Systematic Review of Treatment Strategies and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Literature Search
2.2. Study Selection
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Quality Assessment
3.2. Study Selection
3.3. Demographics
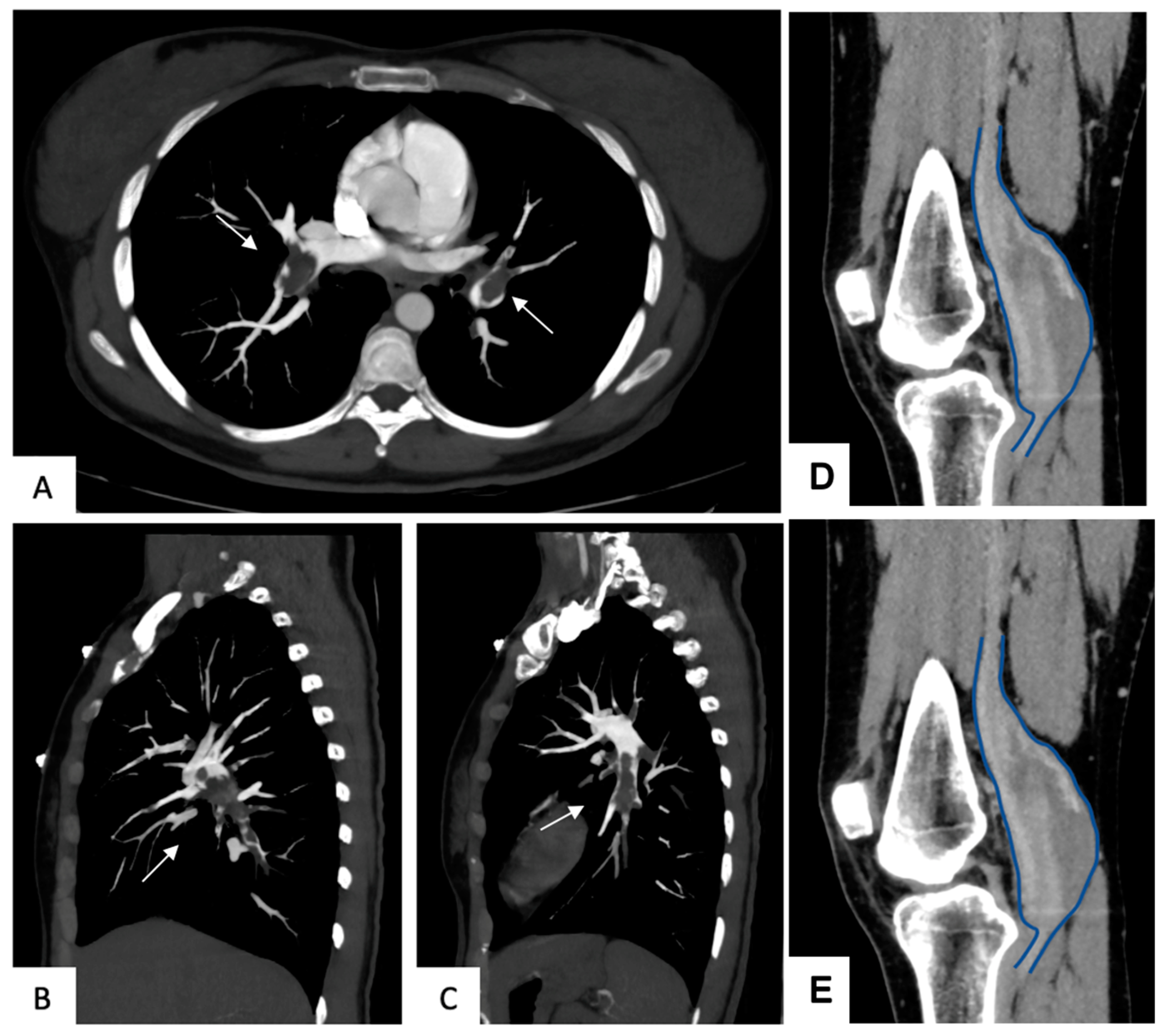
3.4. Aneurysms Characteristics
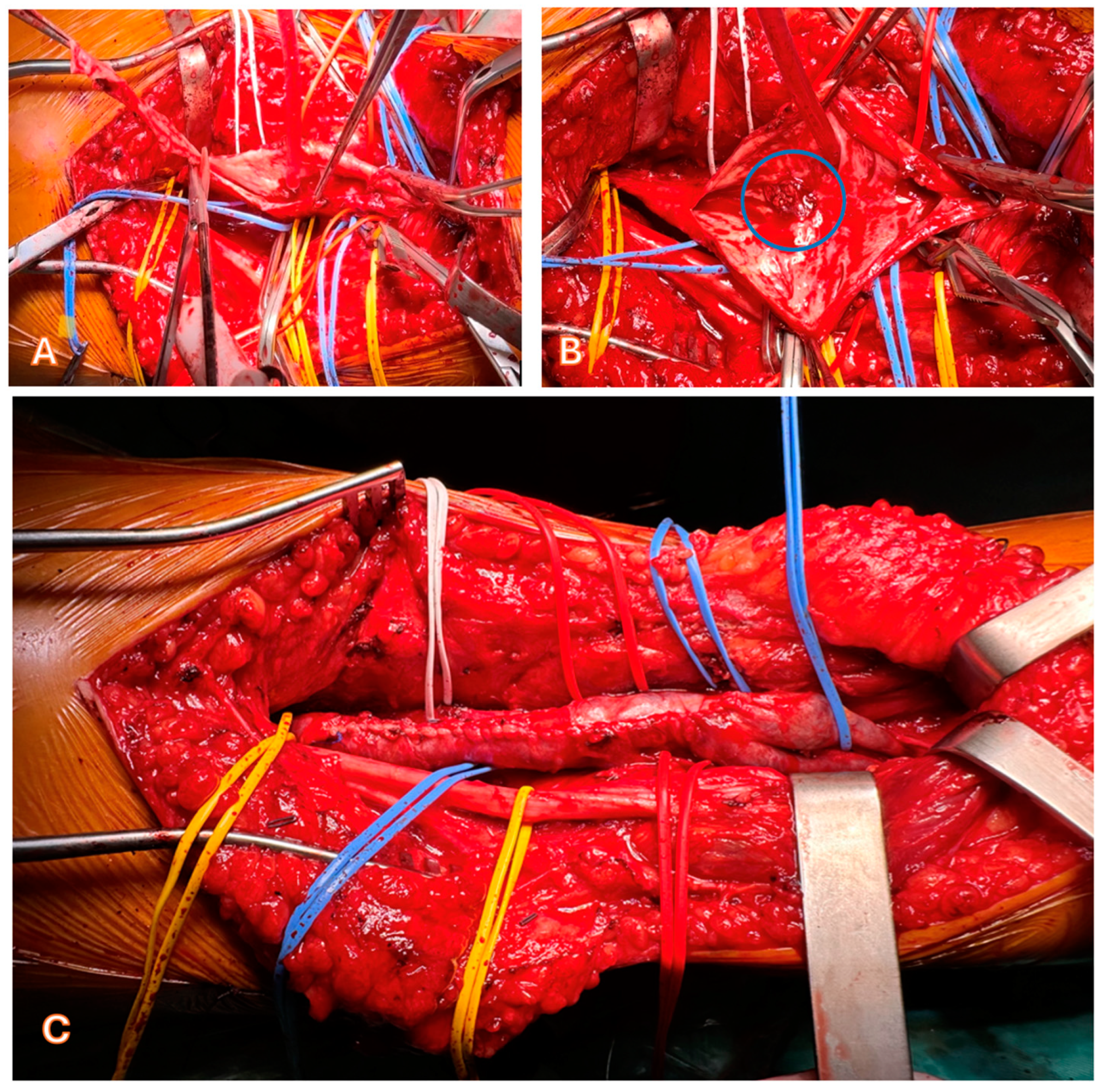
3.5. Treatment Strategies
3.6. Outcomes
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sessa, C.; Nicolini, P.; Perrin, M.; Farah, I.; Magne, J.L.; Guidicelli, H. Management of symptomatic and asymptomatic popliteal venous aneurysms: A retrospective analysis of 25 patients and review of the literature. J. Vasc. Surg. 2000, 32, 902–912. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, D.T.; Lohr, J.M.; Martin, K.D.; Welling, R.E.; Sampson, M.G. Bilateral Popliteal Vein Aneurysms. Ann. Vasc. Surg. 1993, 7, 282–286. [Google Scholar] [CrossRef]
- Noppeney, T.; Kopp, R.; Pfister, K.; Schierling, W.; Noppeney, J.; Cucuruz, B. Treatment of popliteal vein aneurysms. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 535–542. [Google Scholar] [CrossRef]
- Bergqvist, D.; Björck, M.; Ljungman, C. Popliteal Venous Aneurysm—A Systematic Review. World J. Surg. 2006, 30, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinicalquestion: A key to evidence-based decisions. ACP J. Club 1995, 123, A12-3. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA2020 explanation elaboration: Updated guidance exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Quality Assessment of Controlled Interventionstudies. 2020. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 16 July 2020).
- Sommer, A.E.; Golden, B.P.; Peterson, J.; Knoten, C.A.; O’Hara, L.; O’Leary, K.J. Hospitalized patients’ knowledge of care: A systematic review. J. Gen. Intern. Med. 2018, 33, 2210–2229. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Fernandez, N.; Lopez-Espada, C.; Martinez-Gamez, F.J.; Galan-Zafra, M.; Sanchez-Maestre, M.L.; Herrero-Martinez, E.; Mata-Campos, J.E. Popliteal venous aneurysms: Results of surgical treatment. Ann. Vasc. Surg. 2013, 27, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.K.; Fleming, M.D.; Gloviczki, P.; Stone, W.; Kalra, M.; Oderich, G.S.; Duncan, A.A.; De Martino, R.R.; Bower, T.C. Surgical treatment of popliteal venous aneurysms. Ann. Vasc. Surg. 2015, 29, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, X.; Sheng, H.; Huang, W.; Zhu, Y. Our experience of symptomatic and asymptomatic popliteal venous aneurysm. J. Vasc. Surg. Cases Innov. Tech. 2017, 4, 1–4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azevedo Mendes, D.; Machado, R.; Veiga, C.; Almeida, R. Institutional Experience with Venous Aneurysms—Insights on the Natural History and Outcomes of Surgical Treatment. Port. J. Card. Thorac. Vasc. Surg. 2023, 30, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Woo, K.; Wakefield, T.W.; Beaulieu, R.J.; Khashram, M.; De Caridi, G.; Benedetto, F.; Shalhub, S.; El-Ghazali, A.; Silpe, J.E.; et al. Contemporary management and outcomes of peripheral venous aneurysms: A multi-institutional study. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beaulieu, R.J.; Boniakowski, A.M.; Coleman, D.M.; Vemuri, C.; Obi, A.T.; Wakefield, T.W. Closed plication is a safe and effective method for treating popliteal vein aneurysm. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, C.W.; Oklu, R.; Watkins, M.T.; Donaldson, M.C.; Abtahian, F.; Schainfeld, R.M.; Jaff, M.R.; Weinberg, I. Popliteal venous aneurysms: Characteristics, management strategies, and clinical outcomes—A modern single-center series. Ann. Vasc. Surg. 2014, 28, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Nasr, W.; Babbitt, R.; Eslami, M.H. Popliteal Vein Aneurysm: A Case Report and Review of Literature. Vasc. Endovasc. Surg. 2008, 41, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, S.C.; Comerota, A.J.; Katz, M.L.; Wolk, J.H.; Goldman, B.I.; White, J.V. Popliteal venous aneurysm: Report of two cases and review of the world literature. J. Vasc. Surg. 1993, 18, 708–715. [Google Scholar] [CrossRef] [PubMed]

| PICO ELEMENTS | Keywords | Search Terms |
|---|---|---|
| P (Patient or Population) | Patients with popliteal vein aneurysm | Popliteal venous aneurysms; lower limb venous aneurysms; venous aneurysms. |
| I (Intervention) | Aneurism resection and reconstruction | Aneurysmorraphy; Lateral venorraphy; plication; aneurysm resection; vein graft. |
| C (Comparison) | Conservative management, anticoagulant therapy | Anticoaulant therapy; compression therapy. |
| O (Outcomes) | Deep vein thrombosis, patency of the vascular recontruction, pulmonary embolism | Pulmonary embolism; Deep vein thrombosis; recurrence; survival. |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total Yes | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sessa [1] | yes | yes | NR | Yes | NR | Yes | Yes | NA | Yes | No | Yes | NR | Yes | NR | 8 yes | fair |
| Noppeney [3] | Yes | yes | CD | yes | No | yes | Yes | Yes | yes | Yes | yes | NR | NR | NR | 9 yes | fair |
| Fernandez [10] | yes | yes | NR | yes | Yes | NR | yes | Yes | NA | Yes | NR | Yes | NR | NR | 8 yes | fair |
| Johnstone [11] | yes | yes | NR | yes | NR | yes | yes | NA | yes | No | Yes | NR | NA | NR | 7 yes | fair |
| Zhao [12] | yes | yes | NR | yes | Yes | Yes | NR | Yes | Yes | NA | NA | NA | NA | NA | 7 yes | good |
| Mendes [13] | yes | yes | NR | yes | NR | yes | yes | NA | yes | NR | yes | NR | NR | NR | 7 yes | fair |
| Patel [14] | yes | yes | CD | yes | NR | yes | yes | No | yes | No | yes | NA | CD | NR | 7 yes | fair |
| Beaulieu [15] | yes | yes | NR | yes | NR | yes | NA | NA | yes | NA | yes | NR | NR | NR | 6 yes | poor |
| Donaldson [16] | yes | yes | NR | yes | Yes | NR | yes | Yes | NA | Yes | NR | Yes | NR | NR | 9 yes | fair |
| N | Study | Year | N. Patients | Mean Age | M | W | Conservative Treatment | Surgical Treatment | Follow Up (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sessa [1] | 2000 | 25 | 59 | 5 | 20 | 0 | 25 | 63 |
| 2 | Noppeney [3] | 2019 | 39 | 57.4 | 6 | 33 | 10 | 29 | 57.9 |
| 3 | Fernandez [10] | 2013 | 4 | 61 | 3 | 1 | 0 | 4 | 66 |
| 8 | Johnstone [11] | 2015 | 8 | 38.6 | 5 | 3 | 0 | 8 | 26 |
| 9 | Zhao [12] | 2017 | 7 | 55.8 | 5 | 2 | 4 | 3 | 24 |
| 6 | Mendes [13] | 2023 | 19 | 57.7 | UN | UN | 7 | 12 | 32 |
| 4 | Patel [14] | 2022 | 40 | 54 | UN | UN | 16 | 24 | 27 |
| 5 | Beaulieu [15] | 2020 | 10 | 52.9 | 5 | 5 | 0 | 10 | 32.2 |
| 7 | Donaldson [16] | 2014 | 21 | 58 | 12 | 9 | 17 | 4 | 38 |
| Total (n 173) | % | |
|---|---|---|
| Sex | ||
| Male | 41 | 23.7 |
| Female | 73 | 42.2 |
| Not reported | 59 | 34.1 |
| Age | 56 (range 18–86 year) | |
| Size of the PVA | ||
| Conservative treatment | 20.7 mm | |
| Surgical treatment | 26.3 mm | |
| Comorbidities | ||
| Hypertension | 30 | 17.3 |
| Heart disease | 6 | 3.5 |
| Diabetes | 3 | 1.7 |
| Chronic vein insufficiency | 78 | 45 |
| Previous varicose vein surgery | 7 | 4 |
| Previous knee trauma | 8 | 4.6 |
| Deep venous thrombosis | 11 | 6.4 |
| Clinical presentation | ||
| Asymptomatic | 20 | 11.5 |
| Pulmonary embolism | 21 | 12.1 |
| Edema or swelling | 27 | 15.6 |
| Lower limb pain | 31 | 17.9 |
| Discomfort of the limb | 4 | 2.3 |
| Popliteal mass | 1 | 0.6 |
| Surgical treatment | ||
| Aneurysmectomy with lateral venorrhaphy | 73 | 61.3 |
| Plication | 19 | 16 |
| Ligation | 6 | 5 |
| Resection with end-to-end anastomosis | 4 | 3.4 |
| Patchplasty using the GSV | 7 | 5.9 |
| Venous bypass | 9 | 7.6 |
| Vein transposition | 1 | 0.8 |
| Total (n 173) | % | |
|---|---|---|
| 30-day mortality | 0 | 0 |
| Long-term mortality | 3 | 5.5 |
| Clinical Success | 173 | 100 |
| Post-operative complications | 15 | 13 |
| Wound infections | 4 | 3.5 |
| Hematoma | 7 | 6 |
| Nerve injury | 4 | 3.5 |
| Patency rate | 172 | 99.4 |
| Recurrence rate | 12 | 10.4 |
| Data are reported as n (%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghese, O.; Pascucci, D.; Peluso, N.; Sposato, F.; Marzullo, A.; Donati, T.; Rascio, L.; Tshomba, Y. Popliteal Venous Aneurysms: A Systematic Review of Treatment Strategies and Outcomes. J. Clin. Med. 2025, 14, 3296. https://doi.org/10.3390/jcm14103296
Borghese O, Pascucci D, Peluso N, Sposato F, Marzullo A, Donati T, Rascio L, Tshomba Y. Popliteal Venous Aneurysms: A Systematic Review of Treatment Strategies and Outcomes. Journal of Clinical Medicine. 2025; 14(10):3296. https://doi.org/10.3390/jcm14103296
Chicago/Turabian StyleBorghese, Ottavia, Domenico Pascucci, Nicolò Peluso, Francesco Sposato, Antonino Marzullo, Tommaso Donati, Laura Rascio, and Yamume Tshomba. 2025. "Popliteal Venous Aneurysms: A Systematic Review of Treatment Strategies and Outcomes" Journal of Clinical Medicine 14, no. 10: 3296. https://doi.org/10.3390/jcm14103296
APA StyleBorghese, O., Pascucci, D., Peluso, N., Sposato, F., Marzullo, A., Donati, T., Rascio, L., & Tshomba, Y. (2025). Popliteal Venous Aneurysms: A Systematic Review of Treatment Strategies and Outcomes. Journal of Clinical Medicine, 14(10), 3296. https://doi.org/10.3390/jcm14103296






