Expert Consensus on the Use of Diphenhydramine for Short-Term Insomnia: Efficacy, Safety, and Clinical Applications
Abstract
1. Introduction
1.1. Pathophysiological and Clinical Aspects of Insomnia
1.2. Treatment of Insomnia
2. Materials and Methods
2.1. Selection of Consensus Committee Members and Topics Being Assessed
2.2. Literature Research
2.3. Consensus Workflow and Methods to Achieve Consensus
- Chapter 1: Evaluation of the use of diphenhydramine for insomnia: efficacy, safety, convenience, and cost of diphenhydramine in the management of insomnia:
- 1.
- Diphenhydramine is an effective medication for the management of short-term insomnia.
- 2.
- Diphenhydramine is a safe medication for the management of short-term insomnia.
- 3.
- If diphenhydramine were available in the Colombian market, do you consider that this medication could be an accessible option for managing short-term insomnia?
- 4.
- Diphenhydramine is a convenient medication for most patients with acute insomnia, regardless of their comorbidities or clinical situations, and therefore, has the potential to be marketed as an over-the-counter medication for managing short-term insomnia.
- Chapter 2: type(s) of insomnia where diphenhydramine could be used:
- 5.
- Diphenhydramine is a useful medication for short-term insomnia (less than 3 months in duration).
- 6.
- Diphenhydramine is a useful medication for chronic insomnia (more than 3 months in duration).
- Chapter 3: use of diphenhydramine as a hypnotic/sedative by age group:
- 7.
- Diphenhydramine is an effective and safe medication for children and adolescents (7 to 17 years old) for managing short-term insomnia.
- 8.
- Diphenhydramine is an effective and safe medication for young adults (18 to 65 years old) for managing short-term insomnia.
- 9.
- Diphenhydramine is an effective and safe medication for elderly individuals (65 years and older) for managing short-term insomnia.
- Chapter 4: duration of diphenhydramine treatment for managing insomnia:
- 10.
- The maximum recommended duration for using diphenhydramine as a hypnotic/sedative for short-term insomnia should be around four weeks.
- Chapter 5: evidence and levels of evidence on the use of diphenhydramine for managing short-term insomnia:
- 11.
- There is a sufficient body of clinical evidence to recommend the use of diphenhydramine in patients with short-term insomnia.
- 12.
- There is a sufficient level of clinical evidence to recommend the use of diphenhydramine in patients with short-term insomnia.
3. Results and Discussion
3.1. Diphenhydramine Pharmacodynamics and Efficacy in Insomnia
3.2. Pharmacokinetics of Diphenhydramine
3.3. Toxic Effects of Diphenhydramine
3.4. Consensus Results
- For question 1, “Diphenhydramine is an effective medication for the management of acute insomnia”, the panel of experts unanimously agreed, giving a rating of 5/5 with an interquartile range of 0 (100% agreement). This indicates complete agreement and consensus on the premise. This unanimous consensus highlights a shared confidence in diphenhydramine’s efficacy in managing acute insomnia.
- For question 2, “Diphenhydramine is a safe medication for the management of short-term insomnia”, 80% of the experts agreed, demonstrating strong agreement with this premise. The interquartile range was 0.5, reflecting a consensus. Frequency analysis revealed that one out of five experts were neutral, four out of six partially agreed, and one out of six fully agreed. These findings suggest a consensus regarding the safety of diphenhydramine for short-term use, although the neutral stance of one expert and partial agreements indicate a need for the further exploration of specific safety concerns.
- For question 3, “If diphenhydramine were available in the Colombian market, do you consider this medication could be an accessible option for managing short-term insomnia?”, the panel showed full agreement (100%) on this statement, with a median value of five and an interquartile range of zero. These results indicate a unanimous consensus among the experts, affirming that diphenhydramine is perceived as an accessible option for managing short-term insomnia if made available in the Colombian market. This agreement reflects the experts’ confidence in its potential affordability and practicality for patients.
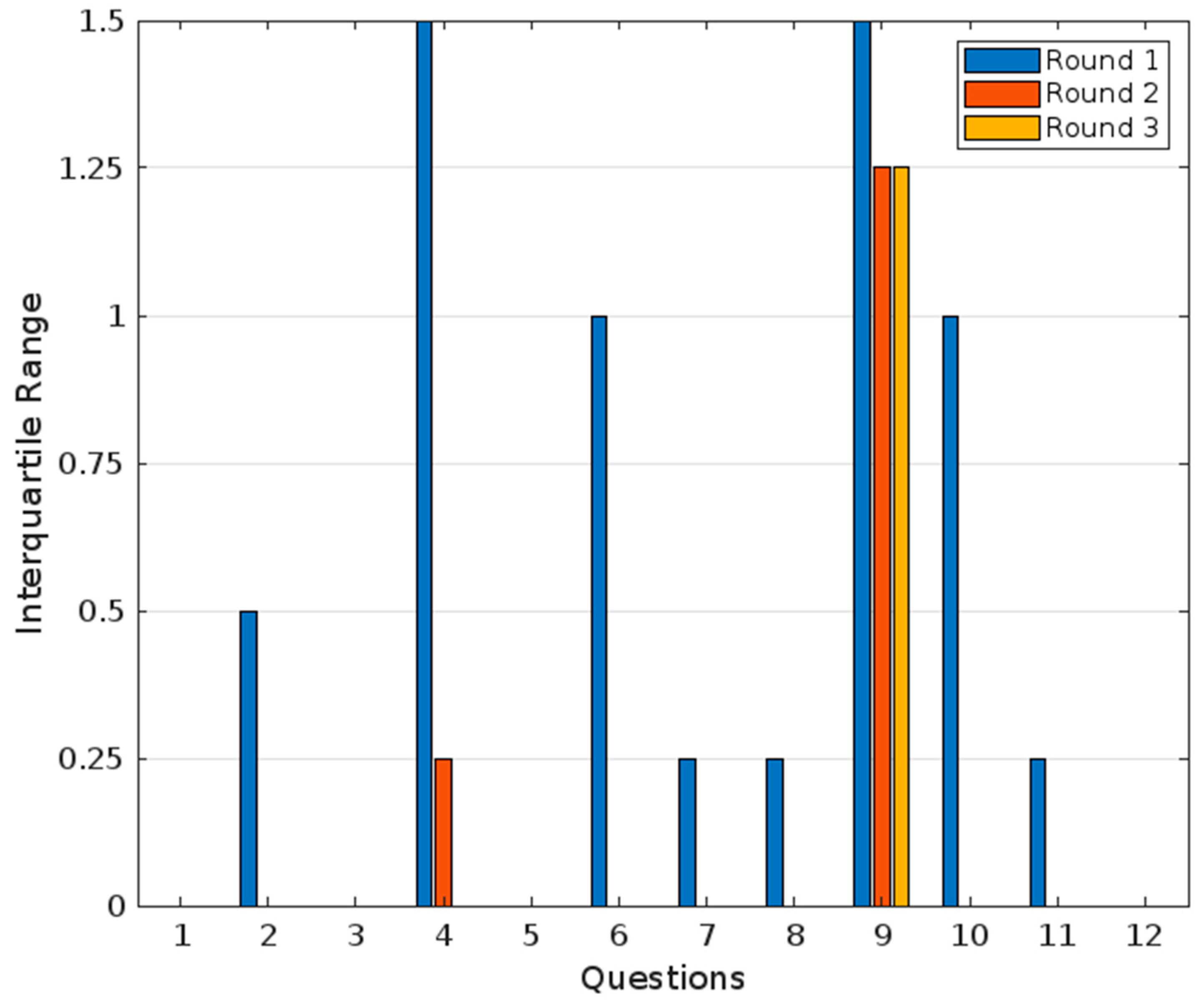
- For question 4, “Diphenhydramine is a convenient medication for most patients with short-term insomnia, regardless of their comorbidities or clinical situations, and therefore has the potential to be marketed as an over-the-counter medication for managing short-term insomnia”, 80% of experts agreed, showing strong agreement, but not a unanimous consensus. The median value was 4, with an interquartile range of 1.5, indicating slight variability in responses. Frequency analysis revealed that one out of six experts partially disagreed, two out of six partially agreed, and two out of six fully agreed. This variability suggests differing perspectives on the convenience of diphenhydramine, particularly regarding its suitability for patients with comorbidities or diverse clinical situations. The partial disagreement and variability highlight that while there is a general agreement, additional research or clarification may be necessary to address specific concerns.
- For question 5, “Diphenhydramine is a useful medication for short-term insomnia (less than 3 months in duration)”, the median value was five, and the interquartile range was zero, reflecting a unanimous agreement and consensus (100% agreement). Frequency analysis revealed that all five experts rated this statement with a five, further affirming the unanimity of the consensus.
- For question 6, “Diphenhydramine is a useful medication for chronic insomnia (more than 3 months in duration)”, the median value was 1.6, and the interquartile range was 1, reflecting total disagreement and consensus (0% agreement) within the panel. Frequency analysis showed that two out of five experts rated it as one, and three out of five rated it as two. This result indicates that the panel does not recommend diphenhydramine for chronic insomnia.
- For question 7, “Diphenhydramine is an effective and safe medication for children aged 7 and older”, the panel showed a median value of 4.2 and an interquartile range of 0.25, reflecting unanimous agreement and consensus (100% agreement). Frequency analysis revealed that four out five experts rated it as four, and one out of five rated it as five. This indicates a consistent and strong level of agreement with the statement.
- For question 8, “Diphenhydramine is an effective and safe medication for young adults (18 to 65 years) for managing short-term insomnia”, the median value was 4.8, and the interquartile range was 0.25, reflecting a unanimous agreement and consensus (100% agreement). Frequency analysis showed that one out of five experts rated it as four, while four out of five rated it as five, demonstrating a high level of agreement with slight variability.
- For question 9, “Diphenhydramine is an effective and safe medication for elderly individuals (65 years and older) for managing short-term insomnia”, the median value was 3, and the interquartile range was 1.5, reflecting no agreement and no consensus (20% agreement). Frequency analysis indicated that two out of five experts rated it as two, two out of five as three, and one out of five as five. This wide distribution of ratings underscores the lack of consensus and varying perspectives on this statement in the first round.
- For question 10, “The maximum recommended duration for using diphenhydramine as a hypnotic/sedative for short-term insomnia should be around four weeks”, the median value was 4.6, and the interquartile range was 1, showing a strong agreement and tight consensus (100% agreement). Frequency analysis revealed that two out five experts rated it as four, while three out of five rated it as five. This indicates a shared belief in limiting the duration of diphenhydramine use, with a small degree of variability.
- For question 11, “There is a sufficient body of clinical evidence to recommend the use of diphenhydramine in patients with short-term insomnia”, the panel’s answers showed a median value of 4.8 and an interquartile range of 0.25, reflecting a strong agreement and consensus (100% agreement). Frequency analysis showed that one out of five experts partially agreed and four out of five strongly agreed with the statement.
- For question 12, “There is a sufficient level of clinical evidence to recommend the use of diphenhydramine in patients with short-term insomnia”, the median value was four, and the interquartile range was zero, reflecting a partial agreement and strong consensus (100% agreement). Frequency analysis revealed that all experts rated this statement as a four, emphasizing a unified agreement.
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perez, M.N.; Salas, R.M.E. Insomnia. Contin. Lifelong Learn. Neurol. 2020, 26, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Wittchen, H.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Berglund, P.A.; Coulouvrat, C.; Hajak, G.; Roth, T.; Shahly, V.; Shillington, A.C.; Stephenson, J.J.; Walsh, J.K. Insomnia and the Performance of US Workers: Results from the America Insomnia Survey. Sleep 2011, 34, 1161–1171. [Google Scholar] [CrossRef]
- Bouscoulet, L.T.; Vázquez-García, J.C.; Muiño, A.; Márquez, M.; López, M.V.; de Oca, M.M.; Talamo, C.; Valdivia, G.; Pertuze, J.; Menezes, A.M.; et al. Prevalence of Sleep Related Symptoms in Four Latin American Cities. J. Clin. Sleep Med. 2008, 04, 579–585. [Google Scholar] [CrossRef]
- Monterrosa-Castro, A.; Marrugo-Flórez, M.; Romero-Pérez, I.; Chedraui, P.; Fernández-Alonso, A.M.; Pérez-López, F.R. Prevalence of insomnia and related factors in a large mid-aged female Colombian sample. Maturitas 2013, 74, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.Y.; Chan, J.W.Y.; Li, S.X.; Wing, Y.K. Non-pharmacological Approaches for Management of Insomnia. Neurotherapeutics 2021, 18, 32–43. [Google Scholar] [CrossRef]
- Madari, S.; Golebiowski, R.; Mansukhani, M.P.; Kolla, B.P. Pharmacological Management of Insomnia. Neurotherapeutics 2021, 18, 44–52. [Google Scholar] [CrossRef]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The Pathophysiology of Insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef]
- Dopheide, J.A. Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am. J. Manag. Care 2020, 26 (Suppl. 4), S76–S84. [Google Scholar]
- Kutscher, S.; Juang, C. Insomnia. Contin. Lifelong Learn. Neurol. 2023, 29, 1167–1187. [Google Scholar] [CrossRef]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatr. Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Sateia, M.J. International Classification of Sleep Disorders-Third Edition. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef]
- Riemann, D.; Espie, C.A.; Altena, E.; Arnardottir, E.S.; Baglioni, C.; Bassetti, C.L.A.; Bastien, C.; Berzina, N.; Bjorvatn, B.; Dikeos, D.; et al. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J. Sleep Res. 2023, 32, e14035. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Regen, W.; Teghen, A.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Riemann, D. Sleep changes in the disorder of insomnia: A meta-analysis of polysomnographic studies. Sleep Med. Rev. 2014, 18, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2018, 14, 1231–1237. [Google Scholar] [CrossRef]
- Hickman, R.; D’Oliveira, T.C.; Davies, A.; Shergill, S. Monitoring Daily Sleep, Mood, and Affect Using Digital Technologies and Wearables: A Systematic Review. Sensors 2024, 24, 4701. [Google Scholar] [CrossRef]
- Perlis, M.L.; Posner, D.; Riemann, D.; Bastien, C.H.; Teel, J.; Thase, M. Insomnia. Lancet 2022, 400, 1047–1060. [Google Scholar] [CrossRef]
- Keeney, S.; Hasson, F.; McKenna, H. The Delphi Technique in Nursing and Health Research; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Church, M.; Church, D. Pharmacology of antihistamines. Indian J. Dermatol. 2013, 58, 219–224. [Google Scholar] [CrossRef]
- Sicari, V.; Zabbo, C.P. Diphenhydramine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Panula, P. Histamine receptors, agonists, and antagonists in health and disease. Handb. Clin. Neurol. 2021, 180, 377–387. [Google Scholar]
- Wang, D.; Guo, Q.; Wu, Z.; Li, M.; He, B.; Du, Y.; Zhang, K.; Tao, Y. Molecular mechanism of antihistamines recognition and regulation of the histamine H1 receptor. Nat. Commun. 2024, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the Nervous System. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A.; Youmbi-Balderer, G. Effect of diphenhydramine on subjective sleep parameters and on motor activity during bedtime. Int. J. Clin. Pharmacol. 1988, 26, 392–396. [Google Scholar]
- Borowy, C.S.; Mukherji, P. Antihistamine Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Carruthers, S.G.; Shoeman, D.W.; Hignite, C.E.; Azarnoff, D.L. Correlation between plasma diphenhydramine level and sedative and antihistamine effects. Clin. Pharmacol. Ther. 1978, 23, 375–382. [Google Scholar] [CrossRef]
- Roth, T.; Roehrs, T.; Koshorek, G.; Sicklesteel, J.; Zorick, F. Sedative effects of antihistamines. J. Allergy Clin. Immunol. 1987, 80, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; Lewis, E.D.; Crowley, D.C.; Langston, J.; Evans, M. A randomized, double-blind, placebo-controlled, cross-over pilot study to investigate the efficacy of Rest-ZZZ formula in healthy participants with occasional sleeplessness. Sleep Biol. Rhythm. 2023, 21, 59–68. [Google Scholar] [CrossRef]
- Roehrs, T.; Zwyghuizen-Doorenbos, A.; Roth, T. Sedative Effects and Plasma Concentrations Following Single Doses of Triazolam, Diphenhydramine, Ethanol and Placebo. Sleep 1993, 16, 301–305. [Google Scholar] [CrossRef]
- Rickels, K.; Morris, R.J.; Newman, H.; Rosenfeld, H.; Schiller, H.; Weinstock, R. Diphenhydramine in Insomniac Family Practice Patients: A Double-Blind Study. J. Clin. Pharmacol. 1983, 23, 234–242. [Google Scholar] [CrossRef]
- Morin, C.M.; Koetter, U.; Bastien, C.; Ware, J.C.; Wooten, V. Valerian-Hops Combination and Diphenhydramine for Treating Insomnia: A Randomized Placebo-Controlled Clinical Trial. Sleep 2005, 28, 1465–1471. [Google Scholar] [CrossRef]
- Schweitzer, P.K.; Muehlbach, M.J.; Welsh, J.K. Sleepiness and performance during three-day administration of cetirizine or diphenhydramine. J. Allergy Clin. Immunol. 1994, 94, 716–724. [Google Scholar] [CrossRef]
- Teutsch, G.; Mahler, D.L.; Brown, C.R.; Forrest, W.H.; James, K.E.; Brown, B.W. Hypnotic efficacy of diphenhydramine, methapyrilene, and pentobarbital. Clin. Pharmacol. Ther. 1975, 17, 195–201. [Google Scholar] [CrossRef]
- Glass, J.R.; Sproule, B.A.; Herrmann, N.; Busto, U.E. Effects of 2-Week Treatment with Temazepam and Diphenhydramine in Elderly Insomniacs. J. Clin. Psychopharmacol. 2008, 28, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.B.; Meuleman, J.R.; Nelson, R.C.; Clark, R.L. Evaluation of Temazepam and Diphenhydramine as Hypnotics in a Nursing-Home Population. Drug Intell. Clin. Pharm. 1987, 21, 716–720. [Google Scholar] [CrossRef]
- Russo, R.M.; Gururaj, V.J.; Allen, J.E. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J. Clin. Pharmacol. 1976, 16, 284–288. [Google Scholar] [CrossRef]
- Sunshine, A.; Zighelboim, I.; Laska, E. Hypnotic Activity of Diphenhydramine, Methapyrilene, and Placebo. J. Clin. Pharmacol. 1978, 18, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Kurihara, M. Clinical Evaluation of Diphenhydramine Hydrochloride for the Treatment of Insomnia in Psychiatric Patients: A Double-Blind Study. J. Clin. Pharmacol. 1990, 30, 1041–1048. [Google Scholar] [CrossRef]
- Richardson, G.S.; Roehrs, T.A.; Rosenthal, L.; Koshorek, G.; Roth, T. Tolerance to Daytime Sedative Effects of H1 Antihistamines. J. Clin. Psychopharmacol. 2002, 22, 511–515. [Google Scholar] [CrossRef]
- Simons, K.J.; Watson, W.T.A.; Martin, T.J.; Chen, X.Y.; Simons, F.E.R. Diphenhydramine: Pharmacokinetics and Pharmacodynamics in Elderly Adults, Young Adults, and Children. J. Clin. Pharmacol. 1990, 30, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Kaplan, S.; Muschler, K.; Hoyte, C.; Brent, J. The role of QRS complex prolongation in predicting severe toxicity in single-xenobiotic overdose. Clin. Toxicol. 2024, 62, 32–38. [Google Scholar] [CrossRef]
- DrugBank Online. n.a. 2025. Diphenhydramine. Available online: https://go.drugbank.com/salts/DBSALT000056 (accessed on 15 December 2024).
- DynaMed Editorial Team. Diphenhydramine. Available online: https://www.dynamed.com/drug-monograph/diphenhydramine (accessed on 15 December 2024).
- Blackstone, N.G.; Olson, A.; Ainapurapu, B. Physostigmine in Anticholinergic Poisoning: An Old Antidote With Resurgence. Cureus 2020, 12, e11739. [Google Scholar] [CrossRef]
- Nemanich, A.; Liebelt, E.; Sabbatini, A.K. Increased rates of diphenhydramine overdose, abuse, and misuse in the United States, 2005–2016. Clin. Toxicol. 2021, 59, 1002–1008. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA advierte sobre problemas graves con altas dosis del medicamento para alergias difenhidramina (Benadryl). 2020. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-serious-problems-high-doses-allergy-medicine-diphenhydramine-benadryl (accessed on 15 December 2024).
- Wallum, M.; Vakkalanka, J.P.; Krispin, S.; McCabe, D.J. Risk of mortality among adolescents and young adults following hospitalization from an intentional overdose. Am. J. Emerg. Med. 2025, 88, 140–144. [Google Scholar] [CrossRef] [PubMed]
- 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.; Fraser, T.G.; Reggin, J.D.; Simons, K.J. Comparison of the central nervous system effects produced by six H1-receptor antagonists. Clin. Exp. Allergy 1996, 26, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Katayose, Y.; Aritake, S.; Kitamura, S.; Enomoto, M.; Hida, A.; Takahashi, K.; Mishima, K. Carryover effect on next-day sleepiness and psychomotor performance of nighttime administered antihistaminic drugs: A randomized controlled trial. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 428–436. [Google Scholar] [CrossRef]
- Kay, G.G.; Schwartz, H.I.; Wingertzahn, M.A.; Jayawardena, S.; Rosenberg, R.P. Next-day residual effects of gabapentin, diphenhydramine, and triazolam on simulated driving performance in healthy volunteers: A phase 3, randomized, double-blind, placebo-controlled, crossover trial. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 217–226. [Google Scholar] [CrossRef]
- Erbe, S.; Bschor, T. Diphenhydramin-Abhängigkeit und -Entzug. Psychiatr. Prax. 2013, 40, 248–251. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Miuli, A.; Mosca, A.; Santovito, M.C.; Corkery, J.M.; Guirguis, A.; Pettorruso, M.; Di Giannantonio, M.; Martinotti, G. Focus on Over-the-Counter Drugs’ Misuse: A Systematic Review on Antihistamines, Cough Medicines, and Decongestants. Front. Psychiatry 2021, 12, 657397. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Guirguis, A. An Insight into Z-Drug Abuse and Dependence: An Examination of Reports to the European Medicines Agency Database of Suspected Adverse Drug Reactions. Int. J. Neuropsychopharmacol. 2019, 22, 270–277. [Google Scholar] [CrossRef]
- Brandt, J.; Leong, C. Benzodiazepines and Z-Drugs: An Updated Review of Major Adverse Outcomes Reported on in Epidemiologic Research. Drugs RD 2017, 17, 493–507. [Google Scholar] [CrossRef]
- Diphenhydramine Disease Interactions. Available online: https://www.drugs.com/disease-interactions/diphenhydramine.html?utm_source=chatgpt.com (accessed on 15 December 2024).
- Gray, S.L.; Anderson, M.L.; Dublin, S.; Hanlon, J.T.; Hubbard, R.; Walker, R.; Yu, O.; Crane, P.K.; Larson, E.B. Cumulative Use of Strong Anticholinergics and Incident Dementia. JAMA Intern. Med. 2015, 175, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Harvard Health Publishing Staff. Common Anticholinergic Drugs Like Benadryl Linked to Increased Dementia Risk. 2025. Available online: https://www.health.harvard.edu/blog/common-anticholinergic-drugs-like-benadryl-linked-to-increased-dementia-risk-20150128812 (accessed on 15 December 2024).
- Organization of Teratology Information Specialists (OTIS). Diphenhydramine. In MotherToBaby Fact Sheet; Organization of Teratology Information Specialists (OTIS): Brentwood, TN, USA, 1994. [Google Scholar]
- U.S. Food and Drug Administration. Over-the-Counter (OTC) Monograph M010: Nighttime Sleep-Aid Drug Products for Over-the-Counter Human Use. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/omuf/OTCMonograph_M010-NighttimeSleepAidDrugProductsforOTCHumanUse_09202021.pdf (accessed on 15 December 2024).
| Drug | Pharmacological Action/Group | Dose | T max | Vd | t1/2 | Metabolism/Elimination | Indication | Use in Special Populations (Caution/Avoid) |
|---|---|---|---|---|---|---|---|---|
| Diphenhydramine | H1RA | 12.5–50 mg | 2–3 h | 3.3–6.8 L/kg | 2.4–9.3 h | First-pass; CYP450 isoenzymes/urine | Insomnia, allergies, nausea | Chronic liver disease, QT prolongation |
| Hydroxyzine | H1RA | 50–100 mg | 2 h | 16.0 ± 3.0 L/kg | 14–25 h | Liver; CYP3A4, CYP3A5/urine | Anxiety, pruritus, insomnia, allergies | Elderly, renal, and hepatic impairment |
| Quetiapine | D2/5-HT2A RA | 25–100 mg | 1.5 h | 10 ± 4 L/kg | 6–7 h | Liver; CYP3A4, CYP2D6/urine and feces | Psychiatric disorders, insomnia (low dose) | Young and elderly, QT prolongation |
| Levomepromazine | D2/H1/MRA | 5–25 mg | 1–2 h (est.) | 16 L/kg (est.) | ~20 h | Extensive first-pass; liver | Amnesia, nausea and vomiting, psychiatric disorders, insomnia (low doses) | Elderly |
| Temazepam | GABA-A PAM | 7.5–30 mg | 2–3 h | 1.3–1.5 L/kg | 3.5–18 h | Liver, conjugation/urine | Insomnia | Pregnancy (caution) |
| Triazolam | GABA-A PAM | 0.125–0.5 mg | 1–2 h (est.) | ~1 L/kg (est.) | 1.5–5.5 h | Liver, conjugation/urine | Insomnia | Elderly |
| Eszopiclone | GABA-A AG | 1–3 mg | 1 h | 89.9 L | 6.1 h | Liver, CYP3A, CYP2C8, CYP2E1/urine | Insomnia | Elderly |
| Zaleplon | GABABZ | 5–20 mg/day | 1 h | 1.4 L/kg | 1 h | Aldehyde oxidase | Insomnia | Hepatic impairment |
| Zolpidem | GABA-A SA | 5–10 mg | 1.6 h | 0.54–0.68 L/kg | 2.5 h | Liver, CYP3A4, CYP1A2, CYP2C9/urine | Insomnia | Elderly, hepatic impairment |
| Amitriptyline | SERT/NETI | 10–100 mg | 2–12 h | 1221 ± 280 L | 24.65 ± 6.31 h | Liver, CYP2C19, CYP3A4, CYP2D6/urine | MDD, neuropathic pain, migraine, insomnia | Pregnancy, breastfeeding, QT prolongation |
| Trazodone | SERTI/5-HT2A RA | 25–200 mg | 8 h | 0.84 ± 0.16 L/kg | 7.3 ± 0.8 h | Liver, CYP3A4/urine | MDD, insomnia, anxiety | QT prolongation |
| Gabapentin | VGCC AI | 100–600 mg | 2–3 h | 58 ± 6 L | 5–7 h | Unchanged | Antiseizure, neuropathic pain, insomnia | Renal impairment |
| Melatonin | MT1/MT2 AG | 1–5 mg | Variable | ~1.2–1.5 L/kg (est.) | 35–50 min | Liver, various | Insomnia, circadian rhythm disorders | Elderly, pregnancy (caution) |
| Lemborexant | OX1R/OX2RA | 5–10 mg | 1–3 h | 1970 L | 17–19 h | Liver, CYP3A4 | Insomnia | Narcolepsy |
| Daridorexant | OX1R/OX2RA | 25–50 mg | 1.3 h | 31 L | 8 h | Liver, CYP3A4 | Insomnia | Narcolepsy |
| Suvorexant | OX1R/OX2RA | 10 mg | 2 h | 49 L | 12 h | Liver, CYP3A4, CYP2C19 | Delirium Prophylaxis, Insomnia | Narcolepsy, hepatic impairment |
| Reference | Population | Design | Doses | Main Findings | Safety |
|---|---|---|---|---|---|
| Teutsch et al. (1975) [34] | More than 100 elderly patients in VA hospitals | Comparative with placebo | 50 mg and 150 mg | It was not significantly different from pentobarbital for control of insomnia | No significant differences in adverse effects |
| Russo et al. (1976) [37] | 50 children with sleep disorders | Placebo controlled | 10 mg/kg | Significantly reduced sleep latency and night awakenings | Significantly reduced sleep latency and night awakenings |
| Carruthers et al. (1978) [27] | 6 healthy volunteers | Double blind, crossover | 50 mg | Positive correlation between plasma concentration and sedative effects | No specific adverse effects are detailed |
| Sunshine et al. (1978) [38] | 1295 postpartum women with insomnia | Double-blind controlled study | 12.5, 25, and 50 mg | Effective hypnotics in comparison to placebo | No significant adverse events reported |
| Rickels et al. (1983) [31] | 111 patients with mild to moderate insomnia | Double blind, crossover | 50 mg | Improved several sleep parameters, patients reported feeling more rested | More side effects reported with diphenhydramine |
| Stewart et al. (1987) [36] | 17 nursing home residents with insomnia | Double blind, crossover | 50 mg | Shorter sleep latency and longer sleep duration than temazepam | Worse performance on neurological tests compared to placebo |
| Roth et al. (1987) [28] | 16 healthy adults | Crossover | 50 mg (3 times a day) | No significant difference compared to loratadine | Daytime sedation and decreased performance |
| Borbély et al. (1988) [25] | 10 young and healthy adults | Double blind, crossover | 50 mg and 75 mg | No significant differences in subjective sleep parameters compared to placebo | Diphenhydramine did not cause deterioration in psychomotor performance or rebound insomnia |
| Kudo and Kurihara (1990) [39] | 144 psychiatric patients aged 15 to 82 years old with insomnia | Randomized, Double blind | 12.5, 25, and 50 mg | Diphenhydramine was effective in improving sleep quality in psychiatric patients | Well tolerated, no serious side effects during the trial |
| Roehrs et al. (1993) [30] | 12 young and healthy men | Double blind, Latin square | 50 mg | Significant sedative effects for 6.5 h, similar to triazolam | Residual sedation for ethanol but not for diphenhydramine and triazolam |
| Schweitzer et al. (1994) [33] | 12 atopic subjects | Double blind, crossover | 50 mg (3 times a day) | Decreased alertness and performance on day 1, tolerance developed by day 3 | Central nervous system depression only on the first day |
| Richardson et al. (2002) [40] | 15 healthy volunteers aged 18–50 years | Randomized, double-blind, crossover | 50 mg (2 times a day) | Increased drowsiness on day 1, tolerance developed by day 4 | Performance decline reversed on day 4 |
| Morin et al. (2005) [32] | 184 patients with mild insomnia | Multicenter, randomized, placebo-controlled | 50 mg (2 times a day) | Improvements in subjective sleep parameters, increased sleep efficiency in the first 14 days | There were no significant residual effects or serious adverse events. |
| Glass et al. (2008) [35] | 25 elderly with insomnia | Latin Square Desing | 50 mg | Improvement only in the number of awakenings compared to placebo; no better than temazepam | Similar number of adverse events, one fall reported with temazepam |
| Moulin et al. (2022) [29] | 27 healthy adult participants | Randomized, double-blind, placebo-controlled, crossover | 50 mg for 7 days | Improvement in sleep debt, natural supplement showed better efficacy in sleep parameters | No serious adverse effects |
| Parameter | Children (8.9 ± 1.7 y.o.) | Young Adults (31.5 ± 10.4 y.o.) | Elderly (69.4 ± 4.3 y.o.) |
|---|---|---|---|
| Weight (kg) | 31.6 ± 6.8 | 70.3 ± 9.9 | 71.0 ± 11.4 |
| Dose (mg) | 39.5 ± 8.4 | 87.9 ± 12.4 | 86.0 ± 7.3 |
| Cmax (ng/mL) | 81.8 ± 30.2 | 133.2 ± 37.6 | 188.4 ± 54.5 |
| Tmax (h) | 1.3 ± 0.5 | 1.7 ± 1.0 | 1.7 ± 0.8 |
| t½ (h) | 5.4 ± 1.8 | 9.2 ± 2.5 | 13.5 ± 4.2 |
| Cl (mL/min/kg) | 49.2 ± 22.8 | 23.3 ± 9.4 | 11.7 ± 3.1 |
| Vdss (L/kg) | 17.9 ± 5.9 | 14.6 ± 4.0 | 10.2 ± 3.0 |
| Vd (L/kg) | 21.7 ± 6.6 | 17.4 ± 4.8 | 13.6 ± 6.3 |
| AUC (ng/mL/h) | 475 ± 137 | 1031 ± 437 | 1902 ± 572 |
| MRT (h) | 6.4 ± 1.6 | 11.3 ± 3.1 | 14.8 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza-Salamanca, D.F.; Venegas, M.; Parejo, K.; Amado, S.; Echeverry, J.; Calderón-Ospina, C.A. Expert Consensus on the Use of Diphenhydramine for Short-Term Insomnia: Efficacy, Safety, and Clinical Applications. J. Clin. Med. 2025, 14, 3297. https://doi.org/10.3390/jcm14103297
Ariza-Salamanca DF, Venegas M, Parejo K, Amado S, Echeverry J, Calderón-Ospina CA. Expert Consensus on the Use of Diphenhydramine for Short-Term Insomnia: Efficacy, Safety, and Clinical Applications. Journal of Clinical Medicine. 2025; 14(10):3297. https://doi.org/10.3390/jcm14103297
Chicago/Turabian StyleAriza-Salamanca, Daniel Felipe, Marco Venegas, Karem Parejo, Steve Amado, Jorge Echeverry, and Carlos Alberto Calderón-Ospina. 2025. "Expert Consensus on the Use of Diphenhydramine for Short-Term Insomnia: Efficacy, Safety, and Clinical Applications" Journal of Clinical Medicine 14, no. 10: 3297. https://doi.org/10.3390/jcm14103297
APA StyleAriza-Salamanca, D. F., Venegas, M., Parejo, K., Amado, S., Echeverry, J., & Calderón-Ospina, C. A. (2025). Expert Consensus on the Use of Diphenhydramine for Short-Term Insomnia: Efficacy, Safety, and Clinical Applications. Journal of Clinical Medicine, 14(10), 3297. https://doi.org/10.3390/jcm14103297







