A Mixed-Rate Strategy on a Bilaterally-Synchronized Cochlear Implant Processor Offering the Opportunity to Provide Both Speech Understanding and Interaural Time Difference Cues
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
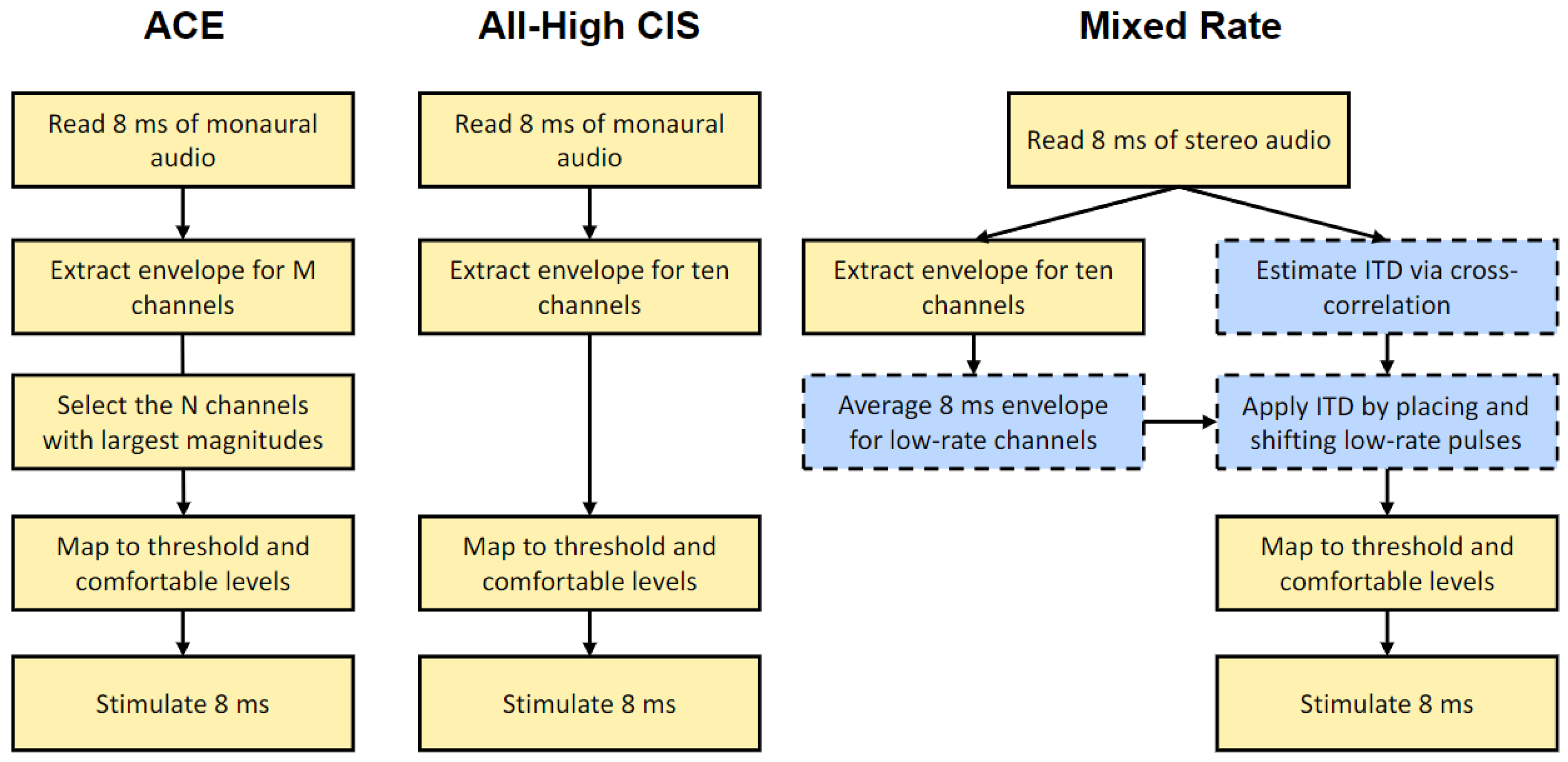
2.3. Processing Strategies
- Buffering. Input audio is stereo and at a sampling frequency of 16,000 Hz. On the CCi-MOBILE, incoming audio is buffered as 8 ms frames. The buffered frames are processed as overlapping blocks of 128 samples, with a hop size of 1 ms and 7 ms of overlap in each block.
- Windowing. A 128-point Hann window is applied to each block. The Hann window is calculated as:w(n) = 0.5 − 0.5 cos(πn/2), for n = 0 to 127
- Fast Fourier Transform (FFT). A 128-point FFT is then applied to each block. Only the first 65 bins are retained, discarding bins for negative frequencies.
- Magnitude estimation. The complex values in the transformed frame are multiplied by their complex conjugates to estimate the power in each frequency bin. A frequency-weighted scaling is applied based on how many channels are active. The frequency bins are consolidated into ten frequency channels (see Table 2 for frequency allocations). The square root of each entry in this matrix is then calculated to provide an estimate of the channel energy.
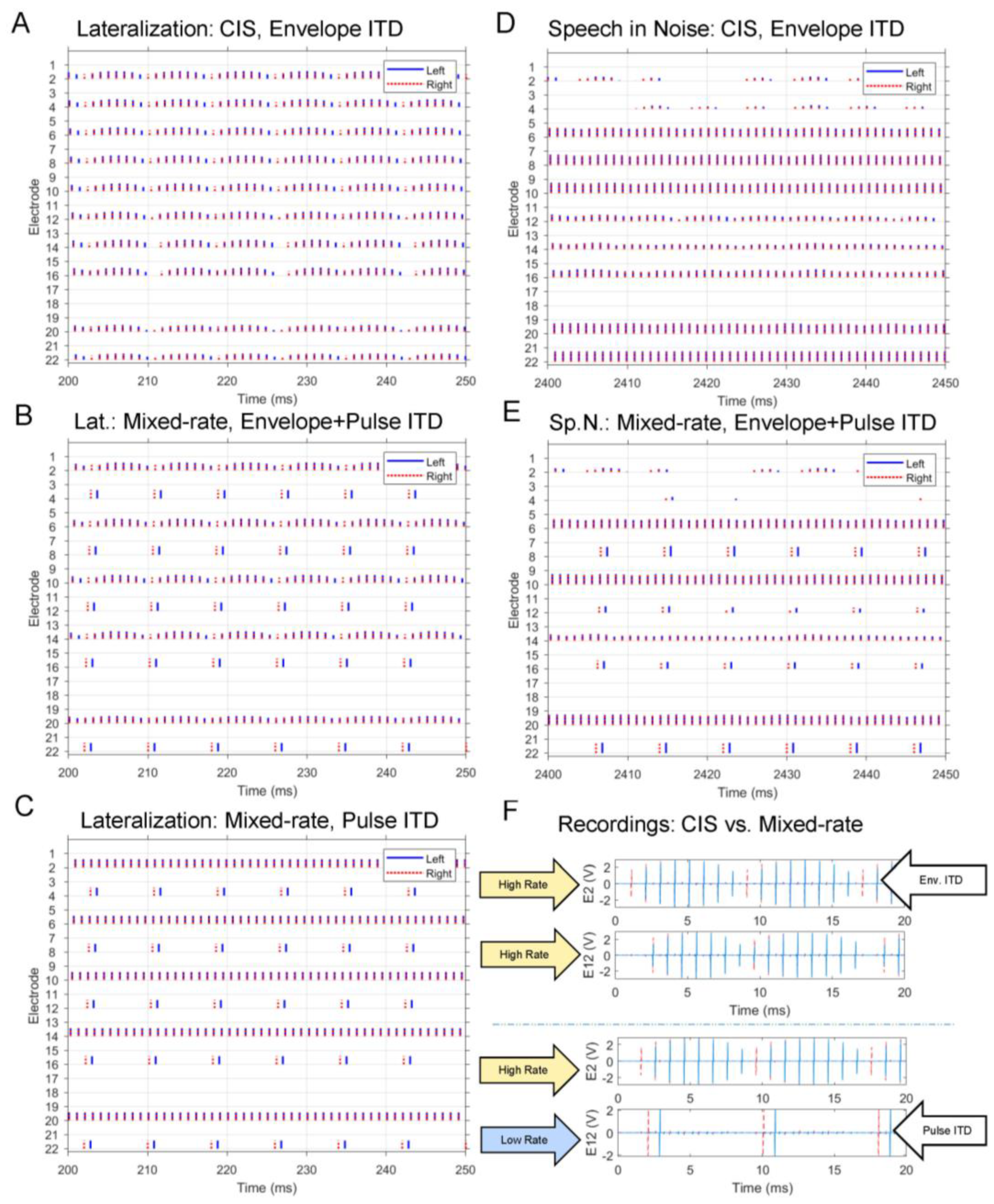
- ITD estimation. The delay that maximized the cross-correlation of left and right buffered frames from Step 1 above is used as an estimate of the ITD in the input signal at that particular point in time. The delay is rounded up to the nearest multiple of 100 µs, the reciprocal of the overall stimulation rate of 10,000 pps across all channels.
- ITD encoding. ITDs are encoded once per 8 ms frame on each channel designated for low-rate stimulation. Depending on the delay estimated in the ITD estimation step, either the left or right pulses are delayed to encode the ITD. Because Cochlear devices can only stimulate one electrode at a time in each ear, any high-rate pulses that overlap in time with the low-rate pulses are removed. If there is an ITD of 0 μs, pulses of the two implants will be simultaneously scheduled in the low-rate channels. The amplitude of the low-rate pulses is the average energy over the entire 8 ms frame for that channel.
- Dynamic range compression. The amplitude values are then normalized between an arbitrary base level and saturation level. The normalized values are then compressed using a logarithmic function and transformed to the proper current levels for each channel based on the Threshold (T) and Comfortable (C) levels in each patient’s clinical MAPs.
2.4. Stimulus
2.5. Experimental Procedure
2.6. Analysis
3. Results
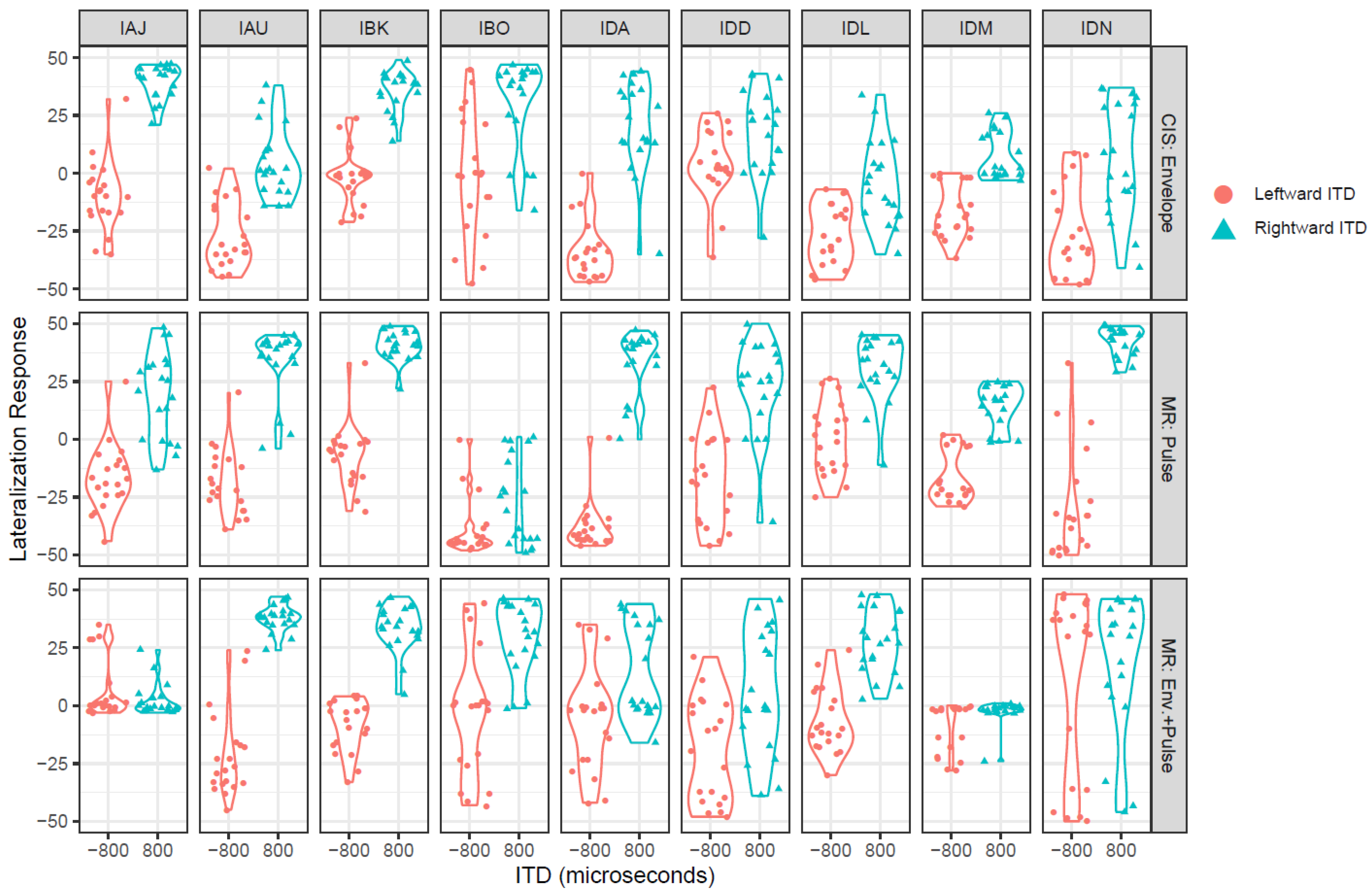
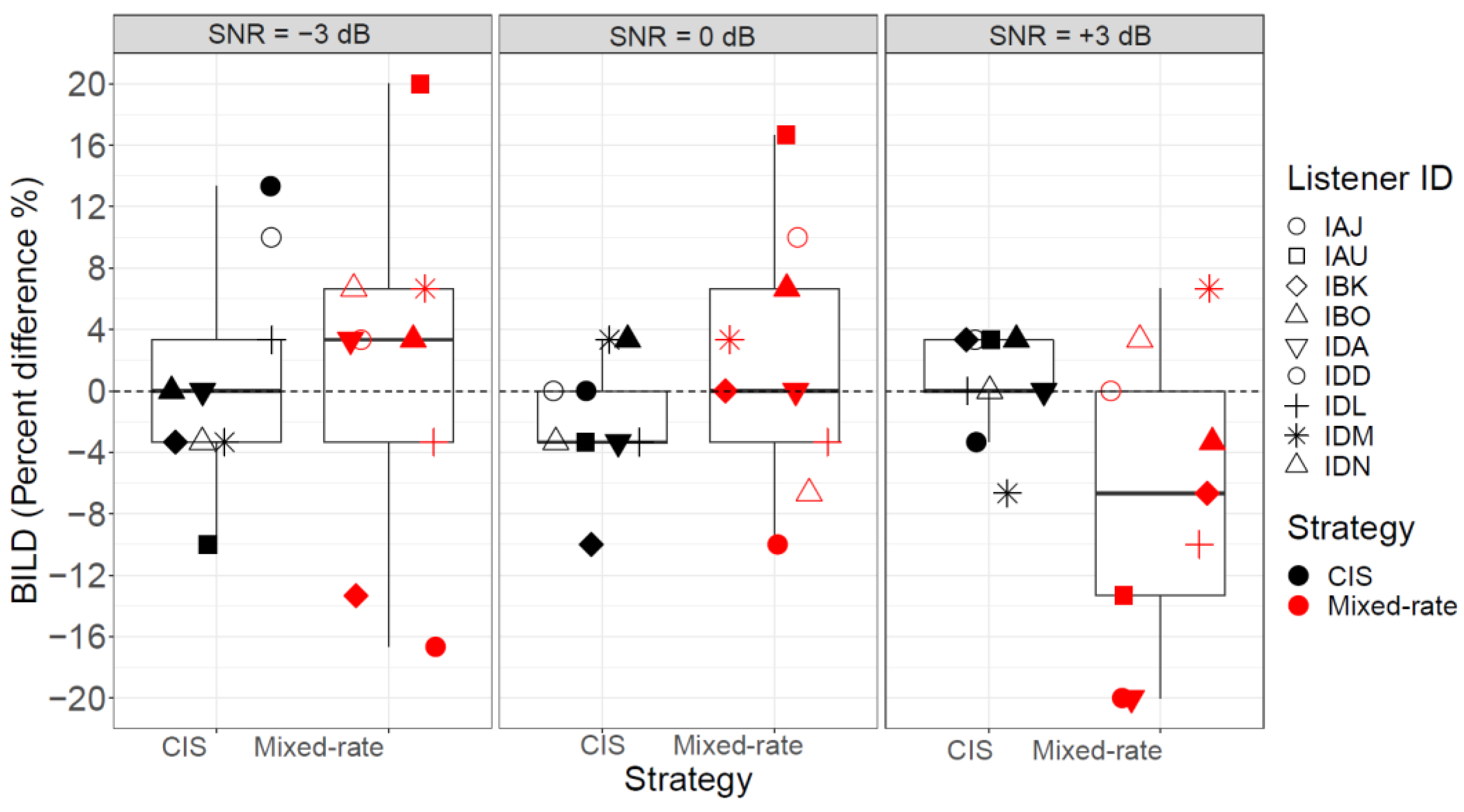
3.1. Lateralization
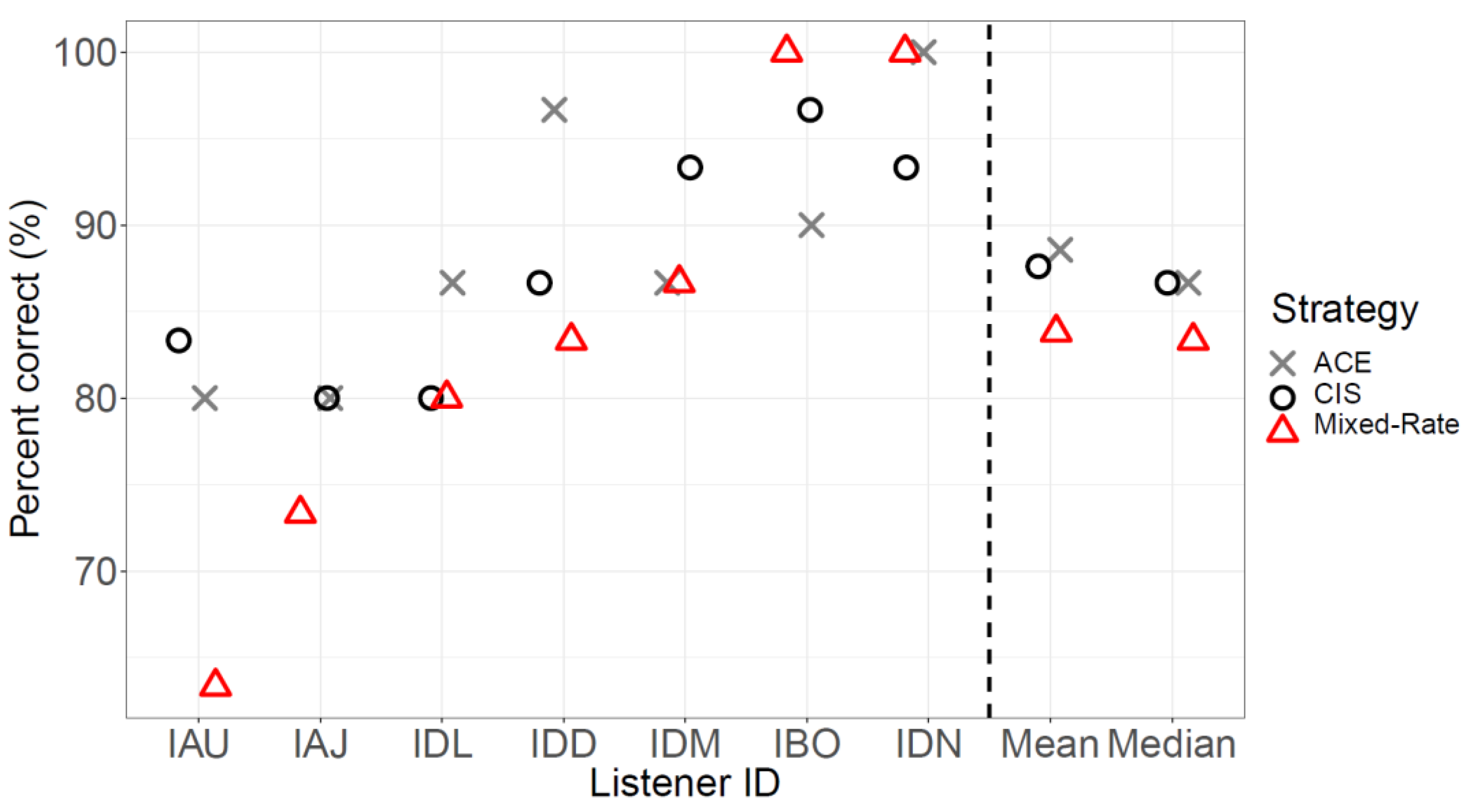
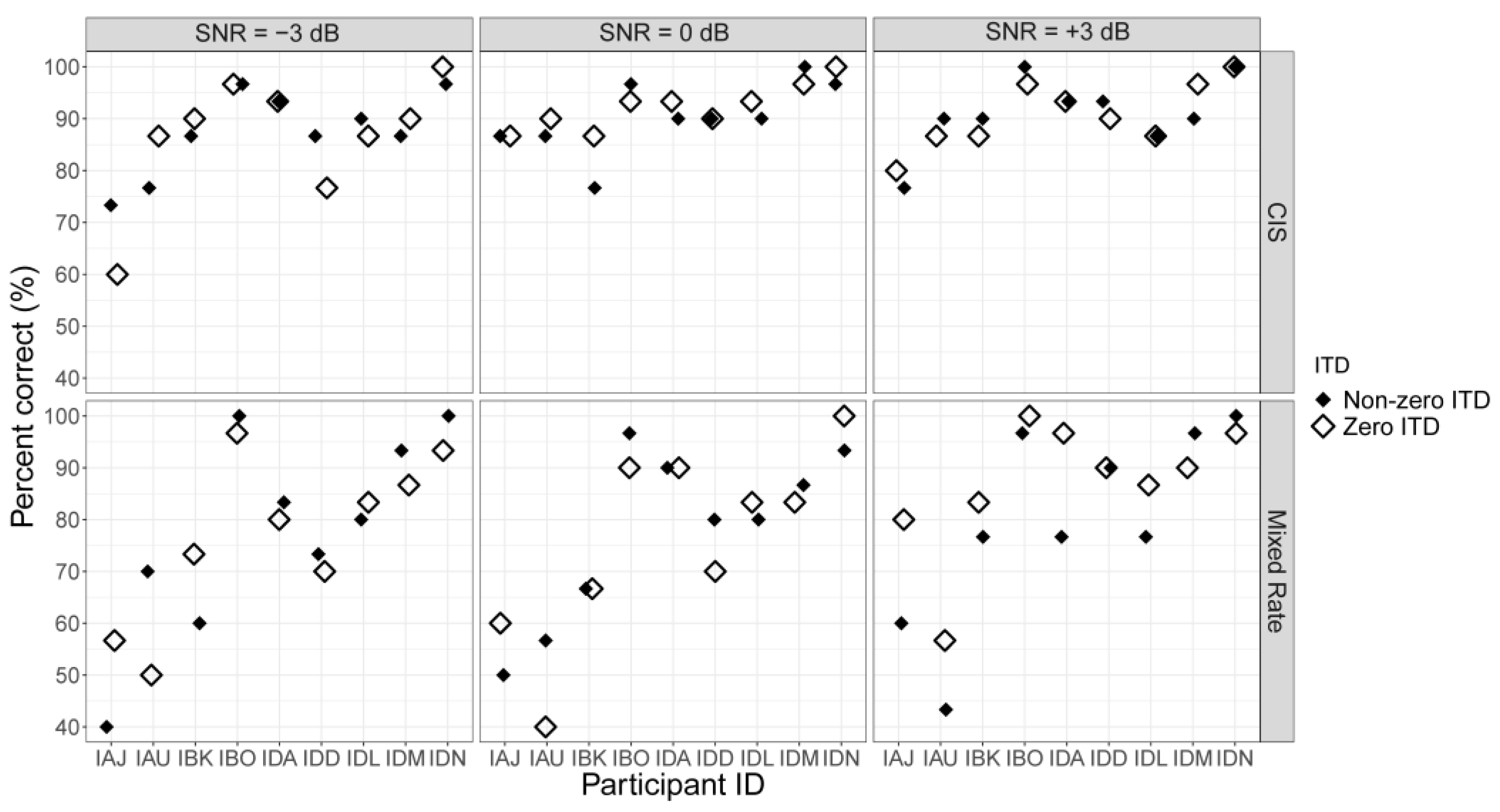
3.2. Speech Testing
3.3. Relationship between Lateralization and Speech Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, B.R.; Wyss, J.; Manrique, M. Worldwide Trends in Bilateral Cochlear Implantation. Laryngoscope 2010, 120, S17–S44. [Google Scholar] [CrossRef]
- van Hoesel, R.J.M. Exploring the Benefits of Bilateral Cochlear Implants. Audiol. Neurotol. 2004, 9, 234–246. [Google Scholar] [CrossRef]
- Veekmans, K.; Ressel, L.; Mueller, J.; Vischer, M.; Brockmeier, S.J. Comparison of Music Perception in Bilateral and Unilateral Cochlear Implant Users and Normal-Hearing Subjects. Audiol. Neurotol. 2009, 14, 315–326. [Google Scholar] [CrossRef]
- McRackan, T.R.; Fabie, J.E.; Bhenswala, P.N.; Nguyen, S.A.; Dubno, J.R. General Health Quality of Life Instruments Underestimate the Impact of Bilateral Cochlear Implantation. Otol. Neurotol. 2019, 40, 745–753. [Google Scholar] [CrossRef]
- Dunn, C.C.; Noble, W.; Tyler, R.S.; Kordus, M.; Gantz, B.J.; Ji, H. Bilateral and Unilateral Cochlear Implant Users Compared on Speech Perception in Noise. Ear Hear. 2010, 31, 296–298. [Google Scholar] [CrossRef]
- Seeber, B.U.; Fastl, H. Localization Cues with Bilateral Cochlear Implants. J. Acoust. Soc. Am. 2008, 123, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Laback, B.; Egger, K.; Majdak, P. Perception and Coding of Interaural Time Differences with Bilateral Cochlear Implants. Hear. Res. 2015, 322, 138–150. [Google Scholar] [CrossRef]
- van Hoesel, R.J.M.; Tyler, R.S. Speech Perception, Localization, and Lateralization with Bilateral Cochlear Implants. J. Acoust. Soc. Am. 2003, 113, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Wightman, F.L.; Kistler, D.J. The Dominant Role of Low-frequency Interaural Time Differences in Sound Localization. J. Acoust. Soc. Am. 1992, 91, 1648–1661. [Google Scholar] [CrossRef]
- Loizou, P.C.; Poroy, O.; Dorman, M. The Effect of Parametric Variations of Cochlear Implant Processors on Speech Understanding. J. Acoust. Soc. Am. 2000, 108, 790–802. [Google Scholar] [CrossRef]
- Loizou, P.C.; Dorman, M.; Tu, Z. On the Number of Channels Needed to Understand Speech. J. Acoust. Soc. Am. 1999, 106, 2097–2103. [Google Scholar] [CrossRef]
- Vandali, A.E.; Whitford, L.A.; Plant, K.L.; Clark, G.M. Speech Perception as a Function of Electrical Stimulation Rate: Using the Nucleus 24 Cochlear Implant System. Ear Hear. 2000, 21, 608–624. [Google Scholar] [CrossRef]
- Gray, W.O.; Mayo, P.G.; Goupell, M.J.; Brown, A.D. Transmission of Binaural Cues by Bilateral Cochlear Implants: Examining the Impacts of Bilaterally Independent Spectral Peak-Picking, Pulse Timing, and Compression. Trends Hear. 2021, 25, 23312165211030412. [Google Scholar] [CrossRef]
- Churchill, T.H.; Kan, A.; Goupell, M.J.; Litovsky, R.Y. Spatial Hearing Benefits Demonstrated with Presentation of Acoustic Temporal Fine Structure Cues in Bilateral Cochlear Implant Listenersa). J. Acoust. Soc. Am. 2014, 136, 1246–1256. [Google Scholar] [CrossRef]
- Thakkar, T.; Kan, A.; Jones, H.G.; Litovsky, R.Y. Mixed Stimulation Rates to Improve Sensitivity of Interaural Timing Differences in Bilateral Cochlear Implant Listeners. J. Acoust. Soc. Am. 2018, 143, 1428. [Google Scholar] [CrossRef]
- Thakkar, T.; Anderson, S.R.; Kan, A.; Litovsky, R.Y. Evaluating the Impact of Age, Acoustic Exposure, and Electrical Stimulation on Binaural Sensitivity in Adult Bilateral Cochlear Implant Patients. Brain Sci. 2020, 10, 406. [Google Scholar] [CrossRef]
- Egger, K.; Majdak, P.; Laback, B. Channel Interaction and Current Level Affect Across-Electrode Integration of Interaural Time Differences in Bilateral Cochlear-Implant Listeners. J. Assoc. Res. Otolaryngol. 2016, 17, 55–67. [Google Scholar] [CrossRef][Green Version]
- Anderson, S.R.; Easter, K.; Goupell, M.J. Effects of Rate and Age in Processing Interaural Time and Level Differences in Normal-Hearing and Bilateral Cochlear-Implant Listenersa). J. Acoust. Soc. Am. 2019, 146, 3232–3254. [Google Scholar] [CrossRef]
- van Hoesel, R.J.M. Sensitivity to Binaural Timing in Bilateral Cochlear Implant Users. J. Acoust. Soc. Am. 2007, 121, 2192–2206. [Google Scholar] [CrossRef]
- Hochmair, I.; Nopp, P.; Jolly, C.; Schmidt, M.; Schößer, H.; Garnham, C.; Anderson, I. MED-EL Cochlear Implants: State of the Art and a Glimpse into the Future. Trends Amplif. 2006, 10, 201–219. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Hochmair, I. Thirty Years of Translational Research Behind MED-EL. Acta Otolaryngol. 2021, 141, 106–134. [Google Scholar] [CrossRef]
- Zirn, S.; Arndt, S.; Aschendorff, A.; Laszig, R.; Wesarg, T. Perception of Interaural Phase Differences with Envelope and Fine Structure Coding Strategies in Bilateral Cochlear Implant Users. Trends Hear. 2016, 20, 1–12. [Google Scholar] [CrossRef]
- Feddersen, W.E.; Sandel, T.T.; Teas, D.C.; Jeffress, L.A. Localization of High-Frequency Tones. J. Acoust. Soc. Am. 1957, 29, 988–991. [Google Scholar] [CrossRef]
- Williges, B.; Jürgens, T.; Hu, H.; Dietz, M. Coherent Coding of Enhanced Interaural Cues Improves Sound Localization in Noise With Bilateral Cochlear Implants. Trends Hear. 2018, 22, 2331216518781746. [Google Scholar] [CrossRef]
- Thakkar, T.; Kan, A.; Litovsky, R.Y. Lateralization of Interaural Time Differences with Mixed Rates of Stimulation in Bilateral Cochlear Implant Listeners. J. Acoust. Soc. Am. 2023, 153, 1912–1923. [Google Scholar] [CrossRef]
- Dennison, S.R.; Thakkar, T.; Kan, A.; Litovsky, R.Y. Lateralization of Binaural Envelope Cues Measured with a Mobile Cochlear-Implant Research Processora. J. Acoust. Soc. Am. 2023, 153, 3543–3558. [Google Scholar] [CrossRef]
- Ghosh, R.; Ali, H.; Hansen, J.H.L. CCi-MOBILE: A Portable Real Time Speech Processing Platform for Cochlear Implant and Hearing Research. IEEE Trans. Biomed. Eng. 2021, 69, 1251–1263. [Google Scholar] [CrossRef]
- Wilson, B.S.; Finley, C.C.; Lawson, D.T.; Wolford, R.D.; Zerbi, M. Design and Evaluation of a Continuous Interleaved Sampling (CIS) Processing Strategy for Multichannel Cochlear Implants. J. Rehabil. Res. Dev. 1993, 30, 110–116. [Google Scholar]
- Levitt, H.; Rabiner, L.R. Binaural Release from Masking for Speech and Gain in Intelligibility. J. Acoust. Soc. Am. 1967, 42, 601–608. [Google Scholar] [CrossRef]
- Litovsky, R.Y.; Goupell, M.J.; Kan, A.; Landsberger, D.M. Use of Research Interfaces for Psychophysical Studies with Cochlear-Implant Users. Trends Hear. 2017, 21, 2331216517736464. [Google Scholar] [CrossRef]
- Wilson, B.S.; Dorman, M.F. Cochlear Implants: A Remarkable Past and a Brilliant Future. Hear. Res. 2008, 242, 3–21. [Google Scholar] [CrossRef]
- Wouters, J.; McDermott, H.J.; Francart, T. Sound Coding in Cochlear Implants: From Electric Pulses to Hearing. IEEE Signal Process. Mag. 2015, 32, 67–80. [Google Scholar] [CrossRef]
- Fitzgerald, M.B.; Kan, A.; Goupell, M.J. Bilateral Loudness Balancing and Distorted Spatial Perception in Recipients of Bilateral Cochlear Implants. Ear Hear. 2015, 36, e225–e236. [Google Scholar] [CrossRef]
- Baumgärtel, R.M.; Dietz, M. Extent of Sound Image Lateralization: Influence of Measurement Method. Acta Acust. United Acust. 2018, 104, 748–752. [Google Scholar] [CrossRef]
- Baumgärtel, R.M.; Hu, H.; Kollmeier, B.; Dietz, M. Extent of Lateralization at Large Interaural Time Differences in Simulated Electric Hearing and Bilateral Cochlear Implant Users. J. Acoust. Soc. Am. 2017, 141, 2338–2352. [Google Scholar] [CrossRef]
- Noel, V.A.; Eddington, D.K. Sensitivity of Bilateral Cochlear Implant Users to Fine-Structure and Envelope Interaural Time Differences. J. Acoust. Soc. Am. 2013, 133, 2314–2328. [Google Scholar] [CrossRef]
- Smith, Z.M.; Delgutte, B. Sensitivity of Inferior Colliculus Neurons to Interaural Time Differences in the Envelope versus the Fine Structure with Bilateral Cochlear Implants. J. Neurophysiol. 2008, 99, 2390–2407. [Google Scholar] [CrossRef]
- Smith, Z.M.; Delgutte, B. Sensitivity to Interaural Time Differences in the Inferior Colliculus with Bilateral Cochlear Implants. J. Neurosci. 2007, 27, 6740–6750. [Google Scholar] [CrossRef]
- Cleary, M.; Bernstein, J.G.W.; Stakhovskaya, O.A.; Noble, J.; Kolberg, E.; Jensen, K.K.; Hoa, M.; Kim, H.J.; Goupell, M.J. The Relationship Between Interaural Insertion-Depth Differences, Scalar Location, and Interaural Time-Difference Processing in Adult Bilateral Cochlear-Implant Listeners. Trends Hear. 2022, 26, 23312165221129164. [Google Scholar] [CrossRef]
- Smith, Z.M.; Kan, A.; Jones, H.G.; Buhr-Lawler, M.; Godar, S.P.; Litovsky, R.Y. Hearing Better with Interaural Time Differences and Bilateral Cochlear Implants. J. Acoust. Soc. Am. 2014, 135, 2190–2191. [Google Scholar] [CrossRef]
- Aronoff, J.M.; Yoon, Y.; Freed, D.J.; Vermiglio, A.J.; Pal, I.; Soli, S.D. The Use of Interaural Time and Level Difference Cues by Bilateral Cochlear Implant Users. J. Acoust. Soc. Am. 2010, 127, EL87–EL92. [Google Scholar] [CrossRef] [PubMed]
- Klingel, M.; Laback, B. Reweighting of Binaural Localization Cues in Bilateral Cochlear-Implant Listeners. J. Assoc. Res. Otolaryngol. 2022, 23, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Gajecki, T.; Nogueira, W. Enhancement of Interaural Level Differences for Bilateral Cochlear Implant Users. Hear. Res. 2021, 409, 108313. [Google Scholar] [CrossRef] [PubMed]

| Participant ID | Age | Years Bilaterally Implanted | Preferred Ear | Bilateral CNC Word Score in Sound Field | Best ITD JND at 100 pps (µs) |
|---|---|---|---|---|---|
| IAJ | 76 | 18 | Right | 70% | 240 |
| IAU | 72 | 15 | Left | 53% | 269 |
| IBK | 82 | 13 | Left | 90% | 58 |
| IBO | 57 | 14 | Right | 84% | 100 |
| IDA | 55 | 8 | Left | 84% | 438 |
| IDD | 23 | 8 | Right | 78% | 610 |
| IDL | 67 | 4 | Right | 74% | 303 |
| IDM | 44 | 9 | Left | 74% | 287 |
| IDN | 20 | 9 | Right | 80% | N/A |
| Analysis Channel | Electrode Array Index | Analysis Channel Low-Freq. (Hz) | Analysis Channel High-Freq. (Hz) | Rate of Stimulation, CIS (pps) | Rate of Stimulation, Mixed-Rate (pps) |
|---|---|---|---|---|---|
| 1 | 22 | 188 | 438 | 1000 | 125 |
| 2 | 20 | 438 | 688 | 1000 | 1000 |
| 3 | 16 | 688 | 1063 | 1000 | 125 |
| 4 | 14 | 1063 | 1438 | 1000 | 1000 |
| 5 | 12 | 1438 | 1938 | 1000 | 125 |
| 6 | 10 | 1938 | 2563 | 1000 | 1000 |
| 7 | 8 | 2563 | 3438 | 1000 | 125 |
| 8 | 6 | 3438 | 4563 | 1000 | 1000 |
| 9 | 4 | 4563 | 6063 | 1000 | 125 |
| 10 | 2 | 6063 | 7938 | 1000 | 1000 |
| Condition | Strategy | Amplitude Modulation on High-Rate Channels | ITD Provided |
|---|---|---|---|
| 1 | All-high CIS | 125 Hz | Envelope |
| 2 | Mixed-rate | 125 Hz | Envelope + Pulse |
| 3 | Mixed-rate | 0 Hz | Pulse |
| Strategy | MAP | ITDs | Condition |
|---|---|---|---|
| ACE | Clinical | 0 μs | Quiet |
| CIS | Ten-channel | 0, +800 μs | Quiet, −3, 0, +3 dB |
| Mixed-rate | Ten-channel | 0, +800 μs | Quiet, −3, 0, +3 dB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennison, S.R.; Thakkar, T.; Kan, A.; Svirsky, M.A.; Azadpour, M.; Litovsky, R.Y. A Mixed-Rate Strategy on a Bilaterally-Synchronized Cochlear Implant Processor Offering the Opportunity to Provide Both Speech Understanding and Interaural Time Difference Cues. J. Clin. Med. 2024, 13, 1917. https://doi.org/10.3390/jcm13071917
Dennison SR, Thakkar T, Kan A, Svirsky MA, Azadpour M, Litovsky RY. A Mixed-Rate Strategy on a Bilaterally-Synchronized Cochlear Implant Processor Offering the Opportunity to Provide Both Speech Understanding and Interaural Time Difference Cues. Journal of Clinical Medicine. 2024; 13(7):1917. https://doi.org/10.3390/jcm13071917
Chicago/Turabian StyleDennison, Stephen R., Tanvi Thakkar, Alan Kan, Mario A. Svirsky, Mahan Azadpour, and Ruth Y. Litovsky. 2024. "A Mixed-Rate Strategy on a Bilaterally-Synchronized Cochlear Implant Processor Offering the Opportunity to Provide Both Speech Understanding and Interaural Time Difference Cues" Journal of Clinical Medicine 13, no. 7: 1917. https://doi.org/10.3390/jcm13071917
APA StyleDennison, S. R., Thakkar, T., Kan, A., Svirsky, M. A., Azadpour, M., & Litovsky, R. Y. (2024). A Mixed-Rate Strategy on a Bilaterally-Synchronized Cochlear Implant Processor Offering the Opportunity to Provide Both Speech Understanding and Interaural Time Difference Cues. Journal of Clinical Medicine, 13(7), 1917. https://doi.org/10.3390/jcm13071917






