Revascularization Strategy in Myocardial Infarction with Multivessel Disease
Abstract
1. Introduction
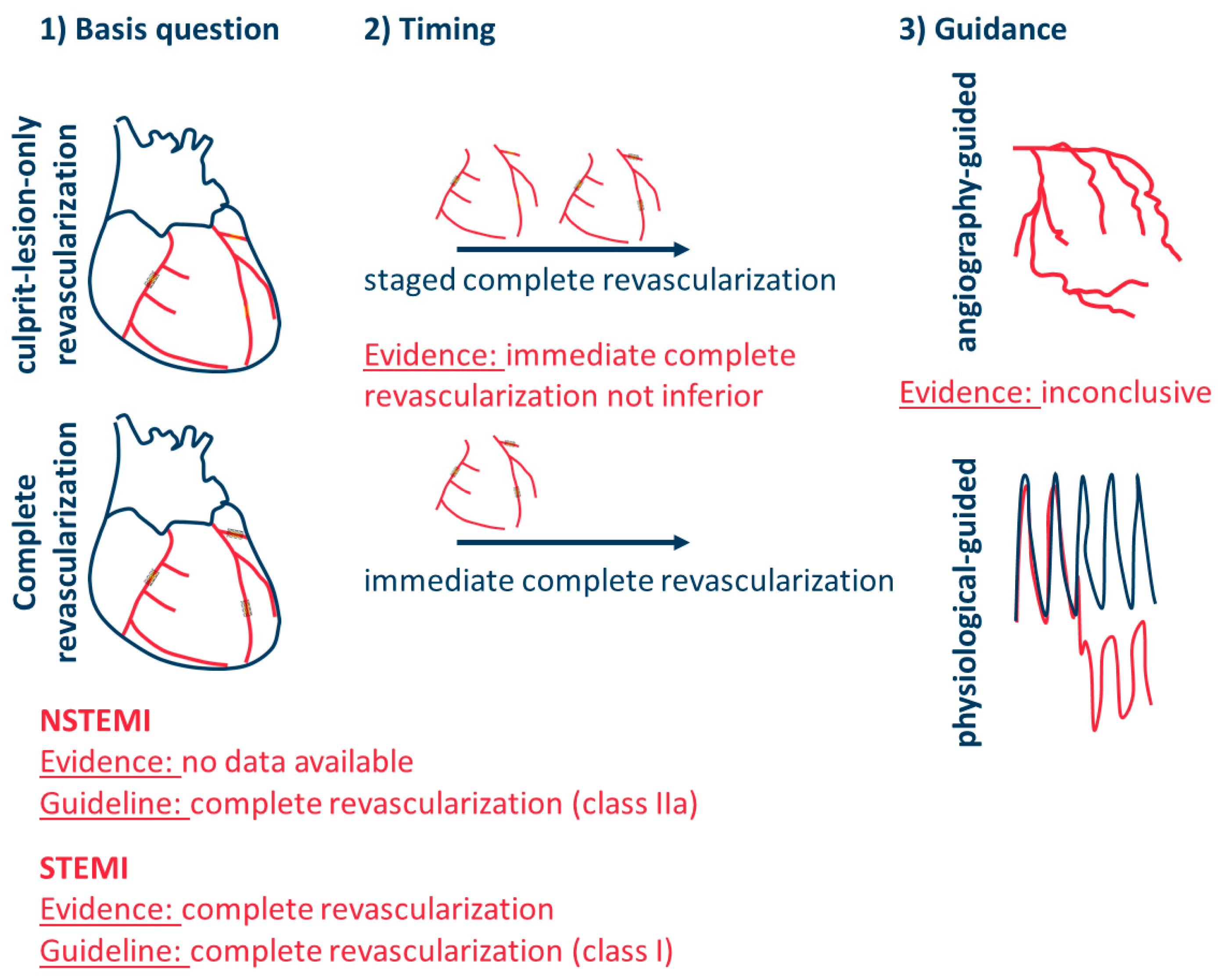
- (1)
- Should relevant non-culprit lesions be revascularized in addition to the culprit lesion (basic question)?
- (2)
- What is the optimal timing for complete revascularization: either immediate complete revascularization or staged complete revascularization?
- (3)
- If staged revascularization is performed, should it be performed either during the index hospital stay or at some interval within a defined time window as part of elective readmission?
- (4)
- How should complete revascularization be guided: angiographically, based on physiological parameters indicating hemodynamic relevance (e.g., FFR, RFR, …), or based on morphological characteristics identifying vulnerable plaques (e.g., OCT)?
- (5)
- Are there differences in these strategies between patients with STEMI, NSTEMI, and those with or without cardiogenic shock?
2. Multivessel PCI in STEMI
3. Theoretical Harms and Benefits of Culprit Lesion-Only and Complete Revascularization
4. Multivessel PCI in NSTEMI
5. Timing of Complete Revascularization
| Trial | Year | n | Population | Comparison | Indication for Complete Revascularization 1 | Primary Endpoint and Result |
|---|---|---|---|---|---|---|
| Complete revascularization vs. culprit lesion-only revascularization | ||||||
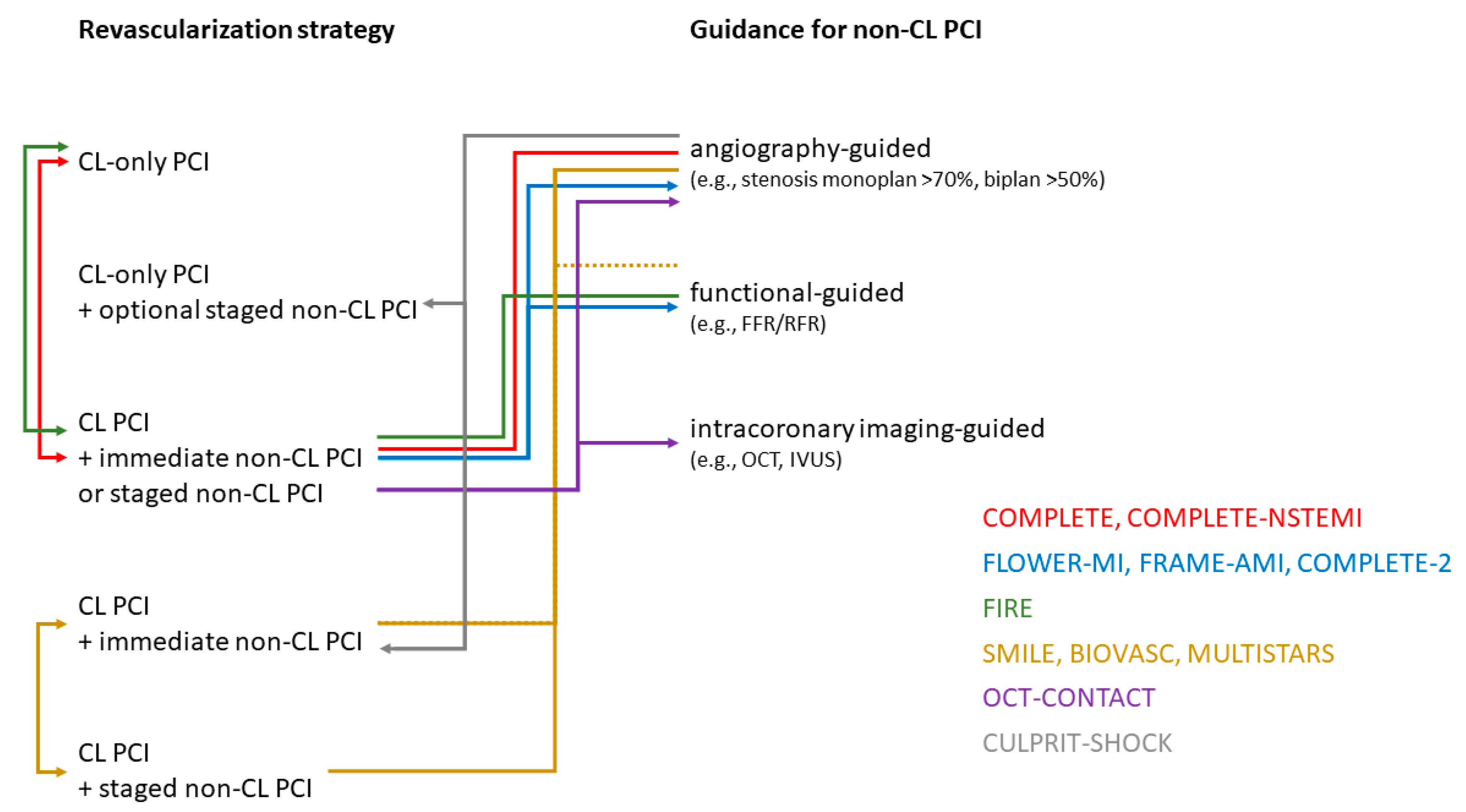
| PRAMI [29] | 2013 | 465 | STEMI | COR vs. immediate CompR | stenosis > 50% | CV death, MI, refractory angina within a mean FU of 23 months HR 0.35; 95% CI 0.21–0.58 p < 0.001 |
| CvLPRIT [30] | 2014 | 296 | STEMI | COR vs. immediate/staged CompR | stenosis > 70% monoplan or > 50% biplan | death, MI, HHF, IDR @1 year HR 0.45; 95% CI 0.24–0.84 p = 0.009 |
| DANAMI-3-PRIMULTI [13] | 2015 | 627 | STEMI | COR vs. staged (index hospitalization) CompR | FFR ≤ 0.80 or stenosis > 90% | death, MI, IDR within a median FU of 287 months HR 0.56, 95% CI 0.38–0.83 p = 0·004 |
| Compare-Acute [31] | 2017 | 885 | STEMI | COR vs. immediate CompR | FFR ≤ 0.80 | death, MI, revascularization, cerebrovascular events @1year HR 0.35; 95% CI 0.22–0.55 p < 0.001 |
| COMPLETE [32] | 2019 | 4041 | STEMI | COR vs. staged (index hospitalization or with 45 days) CompR | stenosis > 70% or FFR ≤ 0.80 in stenosis 50–70% | CV death or MI within a median FU of 3 years HR 0.74, 95% CI 0.60–0.91 p = 0.004 |
| FIRE [22] | 2023 | 1445 | Age ≥ 75, STEMI (35%), NSTEMI (65%) | COR vs. single-stage or multi-stage physiology-guided CompR | FFR ≤ 0.80 or iwFR ≤ 0.89 or cFFR ≤ 0.85 or QFR ≤ 0.80 | death, MI, stroke, or IDR @1y HR 0.73, 95% 0.57–0.93 p = 0.01 |
| COMPLETE-NSTEMI | recruiting | 3390 | NSTEMI | COR vs. single-stage or multi-stage CompR | stenosis > 70% monoplan or > 50% biplan | CV death or MI within the FU period of min. 1 to max. 3 years |
| Timing of complete revascularization | ||||||
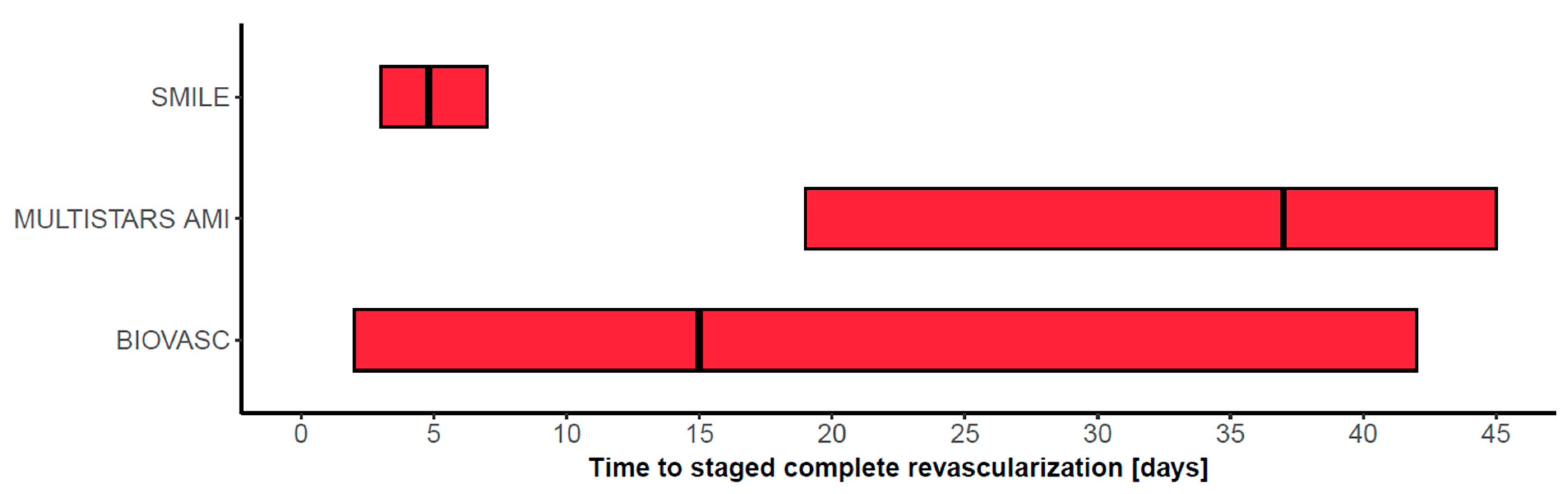
| SMILE [24] | 2016 | 584 | NSTEMI | single-stage vs. multi-stage (index hospitalization) CompR | Angio, FFR optional | death, MI, rehospitalization for unstable angina, RR, or stroke @1y HR 0.55, 95% CI 0.36–0.83 p = 0.004 |
| BIOVASC [26] | 2023 | 1525 | STEMI (40%), NSTEMI (52%), UA (8%) | single-stage vs. multi-stage (index hospitalization or within 6 weeks) CompR | stenosis > 70% or FFR ≤ 0.80 | death, MI, IDR, or cerebrovascular events @1y HR 0.78, 95% CI 0.55–1.11 p = 0.0011 for non-inferiority |
| MULTISTARS [25] | 2023 | 840 | STEMI | single-stage vs. multi-stage (within 19 to 45 days) CompR | stenosis ≥ 70% FFR optional | death, MI, stroke, IDR, or HHF @1y HR 0.52, 95% CI 0.38–0.72 p < 0.001 |
| Guidance of complete revascularization | ||||||
| FLOWER-MI [9] | 2021 | 1171 | STEMI | FFR-guided CompR vs. angio-guided CompR | FFR ≤ 0.80) vs. stenosis ≥ 50% (staged ~96%) | death, MI, or RR @1y HR 1.32, 95% CI 0.78–2.23 p = 0.31 |
| FRAME-AMI [15] | 2023 | 562 | STEMI (47%) and NSTEMI (53%) | FFR-guided CompR vs. angio-guided CompR | FFR ≤ 0.80) vs. stenosis > 50% (staged ~40%) | death, MI, and RR @ median FU of 3.5 years HR 0.43, 95% CI 0.25–0.75 p = 0.003 |
| COMPLETE-2 | recruiting | 5100 | STEMI and NSTEMI | physiology-guided CompR vs. angio-guided CompR | RFR ≤ 0.89) or FFR ≤ 0.80 vs. stenosis ≥ 50% | CV death, MI, or IDR |
6. Summary
7. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIOVASC | Percutaneous complete revascularization strategies using sirolimus-eluting biodegradable polymer-coated stents in patients presenting with acute coronary syndrome and multivessel disease |
| COMPARE-ACUTE | Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction |
| COMPLETE | Complete vs. Culprit-Only Revascularization Strategies to Treat Multivessel Disease after Early PCI for STEMI |
| COMPLETE-2 | Physiology-guided vs. Angiography-guided Non-culprit Lesion Complete Revascularization for Acute MI & Multivessel Disease |
| COMPLETE-NSTEMI | Complete Revascularization Versus Culprit Lesion Only PCI in NSTEMI |
| CvLPRIT | Complete vs. Lesion-only Primary PCI trial |
| DANAMI-3-PRIMULTI | Complete revascularization vs. treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease |
| FIRE | Functional Assessment in Elderly MI Patients with Multivessel Disease |
| FLOWER-MI | Flow Evaluation to Guide Revascularization in Multivessel ST-Elevation Myocardial Infarction |
| FORZA | Fractional Flow Reserve vs. Optical Coherence Tomography to Guide Revascularization of Intermediate Coronary Stenoses |
| FRAME-AMI | Fractional Flow Reserve vs. Angiography-Guided Strategy for Management of Non-Infarction Related Artery Stenosis in Patients with Acute Myocardial Infarction |
| MULTISTARS AMI | Multivessel Immediate vs. Staged Revascularization in Acute Myocardial Infarction |
| OCT-CONTACT | OCT Guided vs. COmplete Pci in patieNts With sT-Segment Elevation myocArdial infarCtion and mulTivessel Disease |
| PRAMI | Preventive Angioplasty in Acute Myocardial Infarction |
| SMILE | Impact of Different Treatment in Multivessel Non-ST Elevation Myocardial Infarction Patients: One Stage vs. Multistaged Percutaneous Coronary Intervention |
| VULNERABLE | Treatment of Functionally Non-significant Vulnerable Plaques in Patients With Multivessel ST-elevation Myocardial Infarction |
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Di Vito, L.; Gramegna, M.; Refaat, H.; Scalone, G.; Leone, A.M.; Trani, C.; Burzotta, F.; Porto, I.; et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur. Heart J. 2015, 36, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Brener, S.J.; Milford-Beland, S.; Roe, M.T.; Bhatt, D.L.; Weintraub, W.S.; Brindis, R.G.; American College of Cardiology-National Cardiovascular Data Registry. Culprit-only or multivessel revascularization in patients with acute coronary syndromes: An American College of Cardiology National Cardiovascular Database Registry report. Am. Heart J. 2008, 155, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Clare, R.M.; Schulte, P.J.; Pieper, K.S.; Shaw, L.K.; Califf, R.M.; Ohman, E.M.; Van de Werf, F.; Hirji, S.; Harrington, R.A.; et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA 2014, 312, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Koganti, S.; Jain, A.K.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Kalra, S.S.; Dalby, M.C.; O’Mahony, C.; Malik, I.S.; et al. Complete Versus Culprit-Only Lesion Intervention in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 72, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; Lopez-Sendon, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Puymirat, E.; Cayla, G.; Simon, T.; Steg, P.G.; Montalescot, G.; Durand-Zaleski, I.; le Bras, A.; Gallet, R.; Khalife, K.; Morelle, J.F.; et al. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N. Engl. J. Med. 2021, 385, 297–308. [Google Scholar] [CrossRef]
- Denormandie, P.; Simon, T.; Cayla, G.; Steg, P.G.; Montalescot, G.; Durand-Zaleski, I.; le Bras, A.; le Breton, H.; Valy, Y.; Schiele, F.; et al. Compared Outcomes of ST-Segment-Elevation Myocardial Infarction Patients with Multivessel Disease Treated with Primary Percutaneous Coronary Intervention and Preserved Fractional Flow Reserve of Nonculprit Lesions Treated Conservatively and of Those with Low Fractional Flow Reserve Managed Invasively: Insights from the FLOWER-MI Trial. Circ. Cardiovasc. Interv. 2021, 14, e011314. [Google Scholar] [CrossRef]
- van der Hoeven, N.W.; Janssens, G.N.; de Waard, G.A.; Everaars, H.; Broyd, C.J.; Beijnink, C.W.H.; van de Ven, P.M.; Nijveldt, R.; Cook, C.M.; Petraco, R.; et al. Temporal Changes in Coronary Hyperemic and Resting Hemodynamic Indices in Nonculprit Vessels of Patients with ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2019, 4, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Echeverri, N.; Mehta, S.R.; Wang, J.; Lavi, S.; Schampaert, E.; Cantor, W.J.; Bainey, K.R.; Welsh, R.C.; Kassam, S.; Mehran, R.; et al. Nonculprit Lesion Plaque Morphology in Patients with ST-Segment-Elevation Myocardial Infarction: Results from the COMPLETE Trial Optical Coherence Tomography Substudys. Circ. Cardiovasc. Interv. 2020, 13, e008768. [Google Scholar] [CrossRef]
- Engstrom, T.; Kelbaek, H.; Helqvist, S.; Hofsten, D.E.; Klovgaard, L.; Holmvang, L.; Jorgensen, E.; Pedersen, F.; Saunamaki, K.; Clemmensen, P.; et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Leone, A.M.; Aurigemma, C.; Zambrano, A.; Zimbardo, G.; Arioti, M.; Vergallo, R.; De Maria, G.L.; Cerracchio, E.; Romagnoli, E.; et al. Fractional Flow Reserve or Optical Coherence Tomography to Guide Management of Angiographically Intermediate Coronary Stenosis: A Single-Center Trial. JACC Cardiovasc. Interv. 2020, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Kim, H.K.; Park, K.H.; Choo, E.H.; Kim, C.J.; Lee, S.H.; Kim, M.C.; Hong, Y.J.; Ahn, S.G.; Doh, J.H.; et al. Fractional flow reserve versus angiography-guided strategy in acute myocardial infarction with multivessel disease: A randomized trial. Eur. Heart J. 2023, 44, 473–484. [Google Scholar] [CrossRef]
- Seung, J.; Choo, E.H.; Kim, C.J.; Kim, H.K.; Park, K.H.; Lee, S.H.; Kim, M.C.; Hong, Y.J.; Ahn, S.G.; Doh, J.H.; et al. Angiographic Severity of the Non-culprit Lesion and the Efficacy of Fractional Flow Reserve-guided Complete Revascularization in AMI Patients: FRAMI-AMI Substudy. Circ. Cardiovasc. Interv. 2024, 17, e013611. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wang, J.; Wood, D.A.; Spertus, J.A.; Cohen, D.J.; Mehran, R.; Storey, R.F.; Steg, P.G.; Pinilla-Echeverri, N.; Sheth, T.; et al. Complete Revascularization vs Culprit Lesion-Only Percutaneous Coronary Intervention for Angina-Related Quality of Life in Patients with ST-Segment Elevation Myocardial Infarction: Results from the COMPLETE Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1091–1099. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef]
- Dumpies, O.; Jobs, A.; Obradovic, D.; van Wiechen, M.; Hartung, P.; Rotta Detto Loria, J.; Wilde, J.; Majunke, N.; Kiefer, P.; Noack, T.; et al. Comparison of plug-based versus suture-based vascular closure for large-bore arterial access: A collaborative meta-analysis of observational and randomized studies. Clin. Res. Cardiol. 2023, 112, 614–625. [Google Scholar] [CrossRef]
- Siebert, V.R.; Borgaonkar, S.; Jia, X.; Nguyen, H.L.; Birnbaum, Y.; Lakkis, N.M.; Alam, M. Meta-analysis Comparing Multivessel Versus Culprit Coronary Arterial Revascularization for Patients with Non-ST-Segment Elevation Acute Coronary Syndromes. Am. J. Cardiol. 2019, 124, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, S.; Guiducci, V.; Escaned, J.; Moreno, R.; Lanzilotti, V.; Santarelli, A.; Cerrato, E.; Sacchetta, G.; Jurado-Roman, A.; Menozzi, A.; et al. Complete or Culprit-Only PCI in Older Patients with Myocardial Infarction. N. Engl. J. Med. 2023, 389, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.A.; Cairns, J.A.; Wang, J.; Mehran, R.; Storey, R.F.; Nguyen, H.; Meeks, B.; Kunadian, V.; Tanguay, J.F.; Kim, H.H.; et al. Timing of Staged Nonculprit Artery Revascularization in Patients with ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J. Am. Coll. Cardiol. 2019, 74, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Sardella, G.; Lucisano, L.; Garbo, R.; Pennacchi, M.; Cavallo, E.; Stio, R.E.; Calcagno, S.; Ugo, F.; Boccuzzi, G.; Fedele, F.; et al. Single-Staged Compared with Multi-Staged PCI in Multivessel NSTEMI Patients: The SMILE Trial. J. Am. Coll. Cardiol. 2016, 67, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Stahli, B.E.; Varbella, F.; Linke, A.; Schwarz, B.; Felix, S.B.; Seiffert, M.; Kesterke, R.; Nordbeck, P.; Witzenbichler, B.; Lang, I.M.; et al. Timing of Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2023, 389, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Diletti, R.; den Dekker, W.K.; Bennett, J.; Schotborgh, C.E.; van der Schaaf, R.; Sabate, M.; Moreno, R.; Ameloot, K.; van Bommel, R.; Forlani, D.; et al. Immediate versus staged complete revascularisation in patients presenting with acute coronary syndrome and multivessel coronary disease (BIOVASC): A prospective, open-label, non-inferiority, randomised trial. Lancet 2023, 401, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Group, E.S.C.S.D. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Jobs, A.; Mehta, S.R.; Montalescot, G.; Vicaut, E.; Van’t Hof, A.W.J.; Badings, E.A.; Neumann, F.J.; Kastrati, A.; Sciahbasi, A.; Reuter, P.G.; et al. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: A meta-analysis of randomised trials. Lancet 2017, 390, 737–746. [Google Scholar] [CrossRef]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G.; Investigators, P. Randomized trial of preventive angioplasty in myocardial infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: The CvLPRIT trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Faro, D.C.; Laudani, C.; Agnello, F.G.; Ammirabile, N.; Finocchiaro, S.; Legnazzi, M.; Mauro, M.S.; Mazzone, P.M.; Occhipinti, G.; Rochira, C.; et al. Complete Percutaneous Coronary Revascularization in Acute Coronary Syndromes with Multivessel Coronary Disease: A Systematic Review. JACC Cardiovasc. Interv. 2023, 16, 2347–2364. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jobs, A.; Desch, S.; Freund, A.; Feistritzer, H.-J.; Thiele, H. Revascularization Strategy in Myocardial Infarction with Multivessel Disease. J. Clin. Med. 2024, 13, 1918. https://doi.org/10.3390/jcm13071918
Jobs A, Desch S, Freund A, Feistritzer H-J, Thiele H. Revascularization Strategy in Myocardial Infarction with Multivessel Disease. Journal of Clinical Medicine. 2024; 13(7):1918. https://doi.org/10.3390/jcm13071918
Chicago/Turabian StyleJobs, Alexander, Steffen Desch, Anne Freund, Hans-Josef Feistritzer, and Holger Thiele. 2024. "Revascularization Strategy in Myocardial Infarction with Multivessel Disease" Journal of Clinical Medicine 13, no. 7: 1918. https://doi.org/10.3390/jcm13071918
APA StyleJobs, A., Desch, S., Freund, A., Feistritzer, H.-J., & Thiele, H. (2024). Revascularization Strategy in Myocardial Infarction with Multivessel Disease. Journal of Clinical Medicine, 13(7), 1918. https://doi.org/10.3390/jcm13071918







