Tumour-Induced Osteomalacia—A Long Way to the Diagnosis Facilitated by [68Ga]Ga-DOTATATE PET/CT
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Biochemical Parameters
2.4. [68Ga]Ga-DOTATATE PET/CT Imaging
2.5. Image Analysis
3. Results
3.1. Patient Characteristics
3.2. Other Imaging
3.3. Somatostatin Receptor Imaging
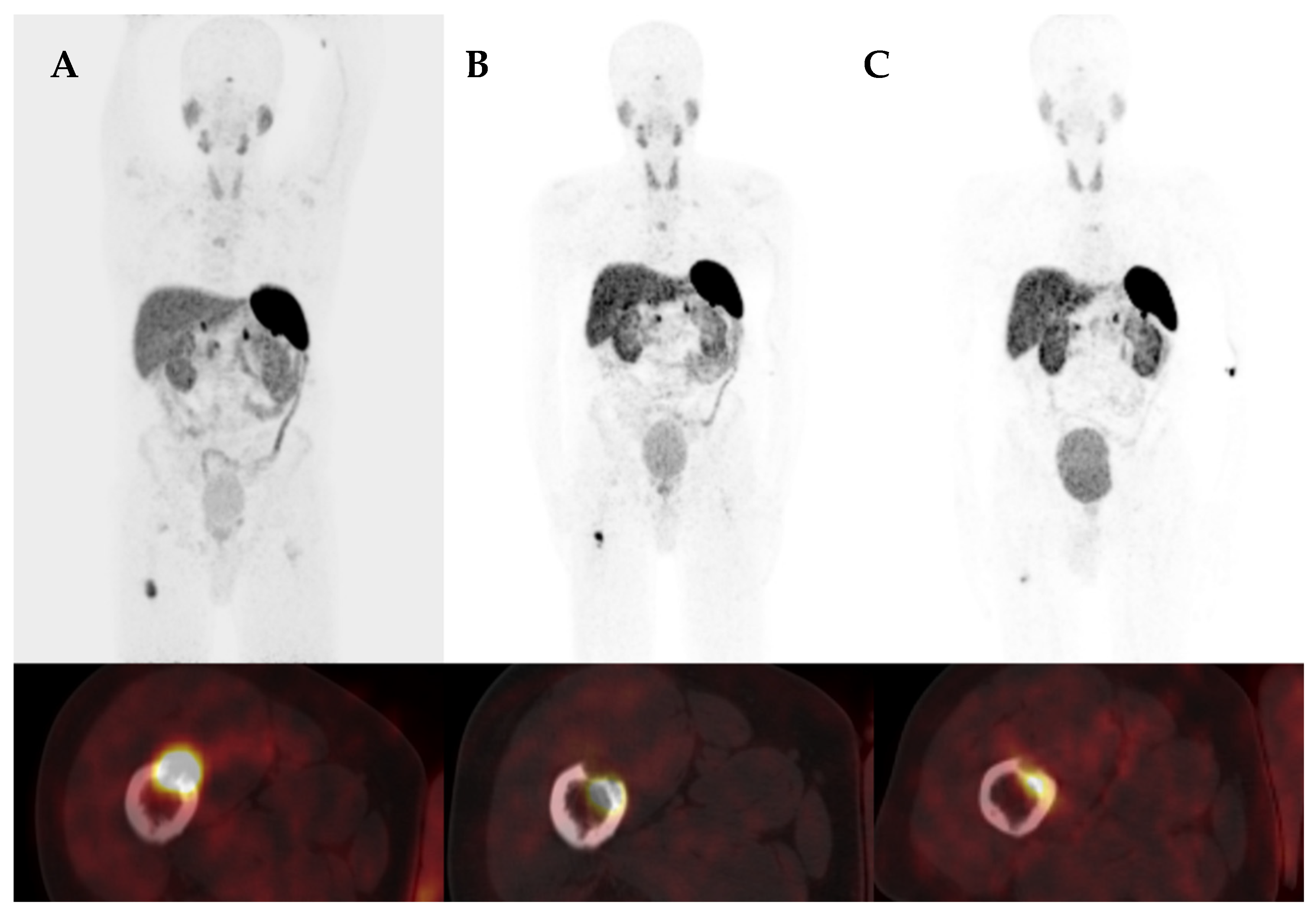
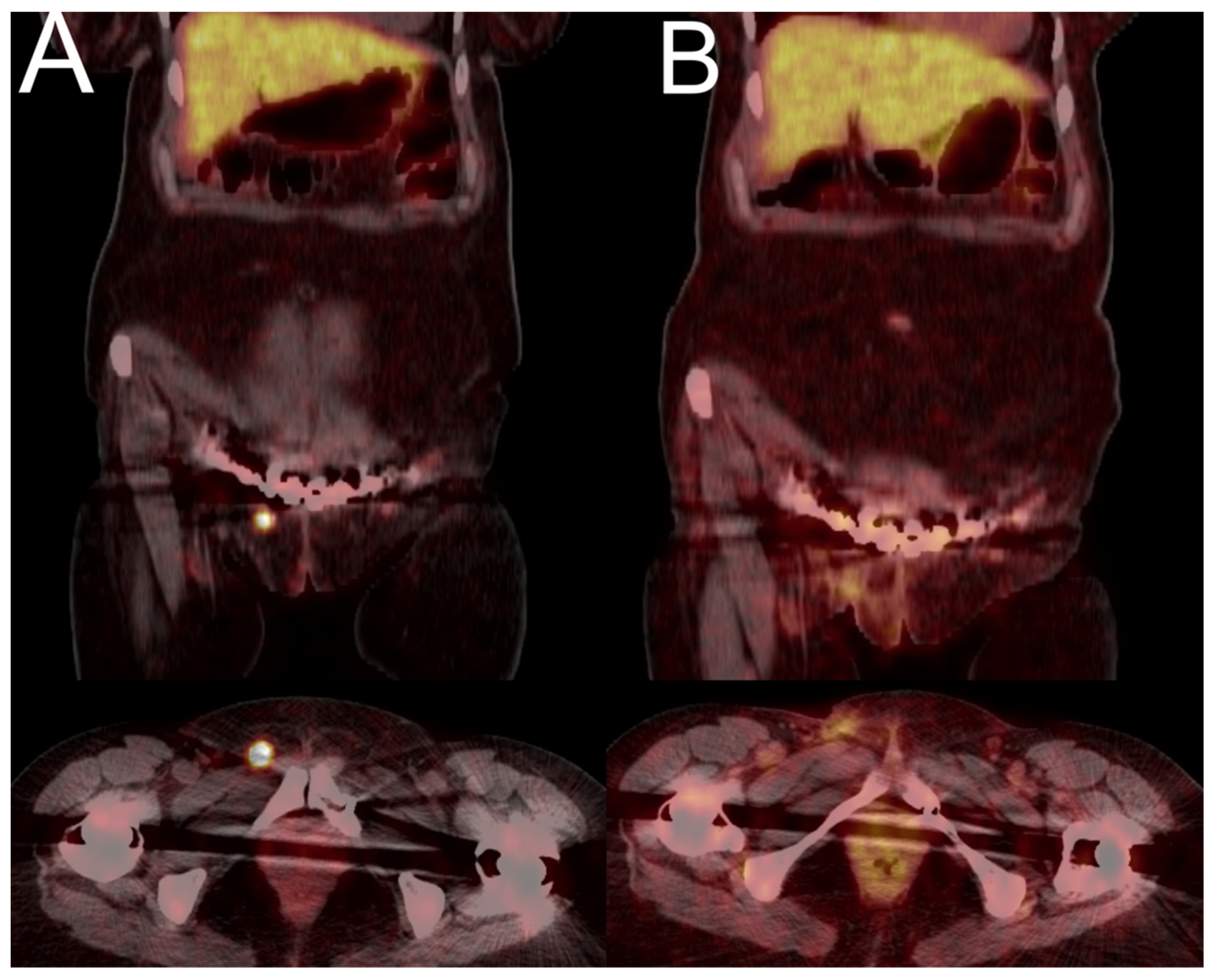
3.4. Case Presentation
3.4.1. Case 1
3.4.2. Case 2
3.4.3. Case 3
3.4.4. Case 4
3.4.5. Case 5
3.4.6. Case 6

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florenzano, P.; Hartley, I.R.; Jimenez, M.; Roszko, K.; Gafni, R.I.; Collins, M.T. Tumor-induced osteomalacia. Calcif. Tissue Int. 2021, 108, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L. Phosphaturic mesenchymal tumors: A review and update. Semin. Diagn. Pathol. 2019, 36, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Salusky, I.B. Evaluation of hypophosphatemia: Lessons from patients with genetic disorders. Am. J. Kidney Dis. 2012, 59, 152–159. [Google Scholar] [CrossRef]
- Katoh, M. FGF (Fibroblast Growth Factor). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, Y.; Wang, O.; Li, M.; Xing, X.; Huo, L.; Li, F.; Yu, W.; Zhong, D.-R.; Jin, J.; et al. The diagnostic dilemma of tumor induced osteomalacia: A retrospective analysis of 144 cases. Endocr. J. 2017, 64, 675–683. [Google Scholar] [CrossRef]
- Colazo, J.M.; DeCorte, J.A.; Gillaspie, E.A.; Folpe, A.L.; Dahir, K.M. Hiding in plain sight: Gene panel and genetic markers reveal 26-year undiagnosed tumor-induced osteomalacia of the rib concurrently misdiagnosed as X-linked hypophosphatemia. Bone Rep. 2021, 14, 100744. [Google Scholar] [CrossRef]
- Yang, M.; Doshi, K.B.; Roarke, M.C.; Nguyen, B.D. Molecular imaging in diagnosis of tumor-induced osteomalacia. Curr. Probl. Diagn. Radiol. 2019, 48, 379–386. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, G.; Cheng, W. Performance of 68Ga-DOTA-SST PET/CT, octreoscan SPECT/CT and 18F-FDG PET/CT in the detection of culprit tumors causing osteomalacia: A meta-analysis. Nucl. Med. Commun. 2020, 41, 370–376. [Google Scholar] [CrossRef] [PubMed]
- El-Maouche, D.; Sadowski, S.M.; Papadakis, G.Z.; Guthrie, L.; Cottle-Delisle, C.; Merkel, R.; Millo, C.; Chen, C.C.; Kebebew, E.; Collins, M.T. 68Ga-DOTATATE for Tumor Localization in Tumor-Induced Osteomalacia. J. Clin. Endocrinol. Metab. 2016, 101, 3575–3581. [Google Scholar] [CrossRef]
- Hou, G.; Zhang, Y.; Liu, Y.; Wang, P.; Xia, W.; Xing, X.; Huo, L.; Li, F.; Jing, H. Head-to-Head Comparison of 68Ga-DOTA-TATE and 68Ga-DOTA-JR11 PET/CT in Patients With Tumor-Induced Osteomalacia: A Prospective Study. Front. Oncol. 2022, 12, 811209. [Google Scholar] [CrossRef]
- Walton, R.; Bijvoet, O. Nomogram for derivation of renal threshold phosphate concentration. Lancet 1975, 306, 309–310. [Google Scholar] [CrossRef]
- Kunikowska, J.; Lewington, V.; Krolicki, L. Optimizing Somatostatin Receptor Imaging in Patients with Neuroendocrine Tumors: The Impact of 99mTc-HYNICTOC SPECT/SPECT/CT Versus 68Ga-DOTATATE PET/CT upon Clinical Management. Clin. Nucl. Med. 2017, 42, 905–911. [Google Scholar] [CrossRef]
- Zalewska, E.; Płoszaj-Neć, M.; Kaniuka-Jakubowska, S.; Orzechowska-Pawiłojć, A.; Śledziński, M.; Piskunowicz, M.; Biernat, W.; Sworczak, K. Oncogenic osteomalacia—Detection of the tumour site upon physical examination. Endokrynol. Pol. 2022, 73, 792–793. [Google Scholar] [CrossRef]
- Molendowska, A.; Jakubowska, J.; Jędrzejuk, D.; Szymczak, J. Tumour-induced osteomalacia (TIO). Endokrynol. Pol. 2022, 73, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Kaniuka-Jakubowska, S.; Biernat, W.; Lewczuk, A.; Świątkowska-Stodulska, R.; Sworczak, K. Oncogenic osteomalacia should be considered in hypophosphatemia, bone pain and pathological fractures. Endokrynol. Pol. 2012, 63, 234–238. [Google Scholar] [PubMed]
- Papierska, L.; Ćwikła, J.B.; Misiorowski, W.; Rabijewski, M.; Sikora, K.; Wanyura, H. Unusual case of phosphaturic mesenchymal tumor. Pol. Arch. Intern. Med. 2013, 123, 255–256. [Google Scholar] [CrossRef]
- Brociek-Piłczyńska, A.; Brodowska-Kania, D.; Szczygielski, K.; Lorent, M.; Zieliński, G.; Kowalewski, P.; Jurkiewicz, D. A rare combination of tumor-induced osteomalacia caused by sinonasal glomangiopericytoma and coexisting parathyroid adenoma: Case report and literature review. BMC Endocr. Disord. 2022, 22, 31. [Google Scholar] [CrossRef]
- Bosman, A.; Palermo, A.; Vanderhulst, J.; De Beur, S.M.J.; Fukumoto, S.; Minisola, S.; Xia, W.; Body, J.-J.; Zillikens, M.C. Tumor-Induced Osteomalacia: A Systematic Clinical Review of 895 Cases. Calcif. Tissue Int. 2022, 111, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Bartoli, F.; Coletto, L.; Manara, M.; Marini, E.; Daolio, P.; Parafioriti, A.; Armiraglio, E.; Zucchi, F.; Sinigaglia, L.; et al. Tumor induced osteomalacia: A single center experience on 17 patients. Bone 2021, 152, 116077. [Google Scholar] [CrossRef] [PubMed]
- Filipová, L.; Zikán, V.; Krsek, M.; Netuka, D.; Michal, M.; Lazúrová, I. Tumor induced osteomalacia–A long way toward correct diagnosis and management. Bone Rep. 2022, 16, 101180. [Google Scholar] [CrossRef] [PubMed]
- Luthra, K.; Gauthaman, D.; Lele, V. A Pictorial Essay of Somatostatin Receptor Imaging in Tumor-Induced Osteomalacia: A Single Institutional Experience. Indian J. Nucl. Med. 2022, 37, 83–90. [Google Scholar] [CrossRef]
- Breer, S.; Brunkhorst, T.; Beil, F.T.; Peldschus, K.; Heiland, M.; Klutmann, S.; Barvencik, F.; Zustin, J.; Gratz, K.-F.; Amling, M. 68Ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111In-octreotide SPECT/CT. Bone 2014, 64, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Parghane, R.V.; Basu, S. False-positive68ga-DOTATATE PET/CT findings in hereditary hypophosphatemia–osteomalacia mimicking culprit lesions of tumor-induced osteomalacia. J. Nucl. Med. Technol. 2023, 51, 73–74. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age [years] | 44 | 55 | 58 | 42 | 45 | 66 |
| Gender | M | M | F | F | F | M |
| Delay of diagnosis [months] | 42 | 70 | 60 | 65 | 45 | 3 |
| Serum phosphate N: 0.87–1.45 mmol/L | 0.47 | 0.30 | 0.51 | 0.57 | 0.65 | 0.45 |
| Urine phoshate N: 12.9–40.2 mmol/L | 56.4 | 13.26 | 21.96 | 18.72 | 66 | >40 |
| TRP N: 85–95% | Not assessed | 57 | 58 | 63 | 70 | Not assessed |
| PTH N: 15–65 pg/mL | 75 | 28 | 92 | 50 | 45 | 57 |
| Serum calcium N: 2.12–2.55 mmol/L | 2.13 | 2.12 | 2.33 | 2.32 | 2.2 | 2.37 |
| Alkaline phosphatase N: 38–126 U/L | 165 | 447 | 245 | 294 | 237 | 588 |
| FGF23 N: <100 RU/mL | 423 | 248 | 406 | 686 | Not assessed | 318 |
| Densitometry | DXA spine T-score—3.6 DXA hip T-score total—3.3, neck—3.3 | DXA hip: Tsc neck—6.0 total—5.8 DXA of spine was not assessed due to fractures | DXA spine T-score—3.5 DXA hip was not assessed due to bilateral endoprosthesis | DXA spine T-score—3.7 DXA hip T-score— neck—4.2; total—4.3 | Osteoporosis: details are not accessible | DXA spine T-score:—0.8 DXA T-score for 33% of the distal forearm:—0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunikowska, J.; Andryszak, N.; Skowrońska-Jóźwiak, E.; Pełka, K.; Zygmunt, A.; Lewiński, A.; Ruchała, M.; Czepczyński, R. Tumour-Induced Osteomalacia—A Long Way to the Diagnosis Facilitated by [68Ga]Ga-DOTATATE PET/CT. J. Clin. Med. 2024, 13, 1817. https://doi.org/10.3390/jcm13061817
Kunikowska J, Andryszak N, Skowrońska-Jóźwiak E, Pełka K, Zygmunt A, Lewiński A, Ruchała M, Czepczyński R. Tumour-Induced Osteomalacia—A Long Way to the Diagnosis Facilitated by [68Ga]Ga-DOTATATE PET/CT. Journal of Clinical Medicine. 2024; 13(6):1817. https://doi.org/10.3390/jcm13061817
Chicago/Turabian StyleKunikowska, Jolanta, Natalia Andryszak, Elżbieta Skowrońska-Jóźwiak, Kacper Pełka, Arkadiusz Zygmunt, Andrzej Lewiński, Marek Ruchała, and Rafał Czepczyński. 2024. "Tumour-Induced Osteomalacia—A Long Way to the Diagnosis Facilitated by [68Ga]Ga-DOTATATE PET/CT" Journal of Clinical Medicine 13, no. 6: 1817. https://doi.org/10.3390/jcm13061817
APA StyleKunikowska, J., Andryszak, N., Skowrońska-Jóźwiak, E., Pełka, K., Zygmunt, A., Lewiński, A., Ruchała, M., & Czepczyński, R. (2024). Tumour-Induced Osteomalacia—A Long Way to the Diagnosis Facilitated by [68Ga]Ga-DOTATATE PET/CT. Journal of Clinical Medicine, 13(6), 1817. https://doi.org/10.3390/jcm13061817









