Brown Tumors: The Hidden Face of Primary and Renal Hyperparathyroidism Amid Real-Life Settings
Abstract
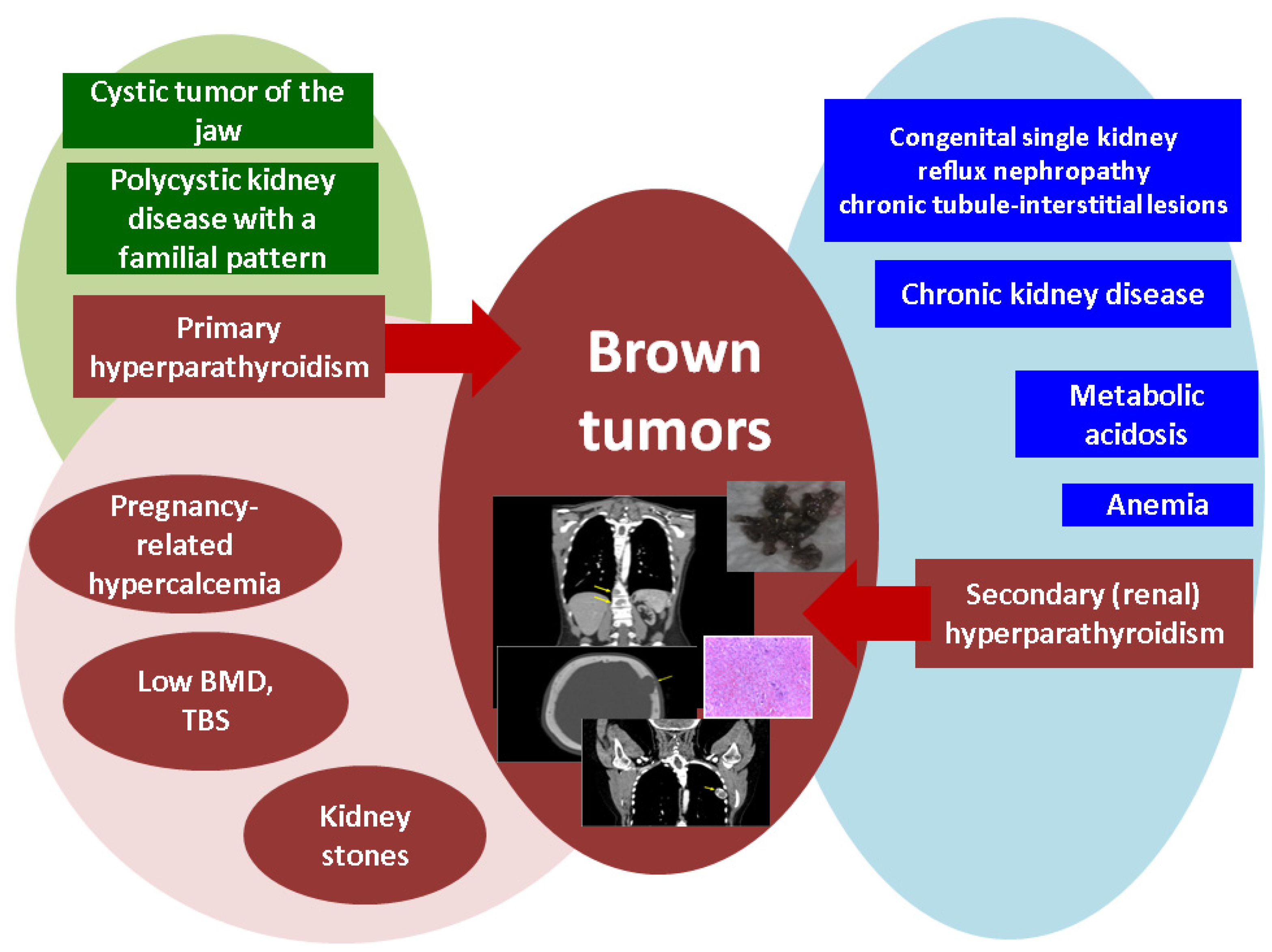
1. Introduction
Objective
2. Brown Tumors in Primary Hyperparathyroidism
2.1. Admission
2.2. Medical Background
2.3. Blood Panel: Hormonal and Biochemistry Assays
2.4. Imagery Assessments
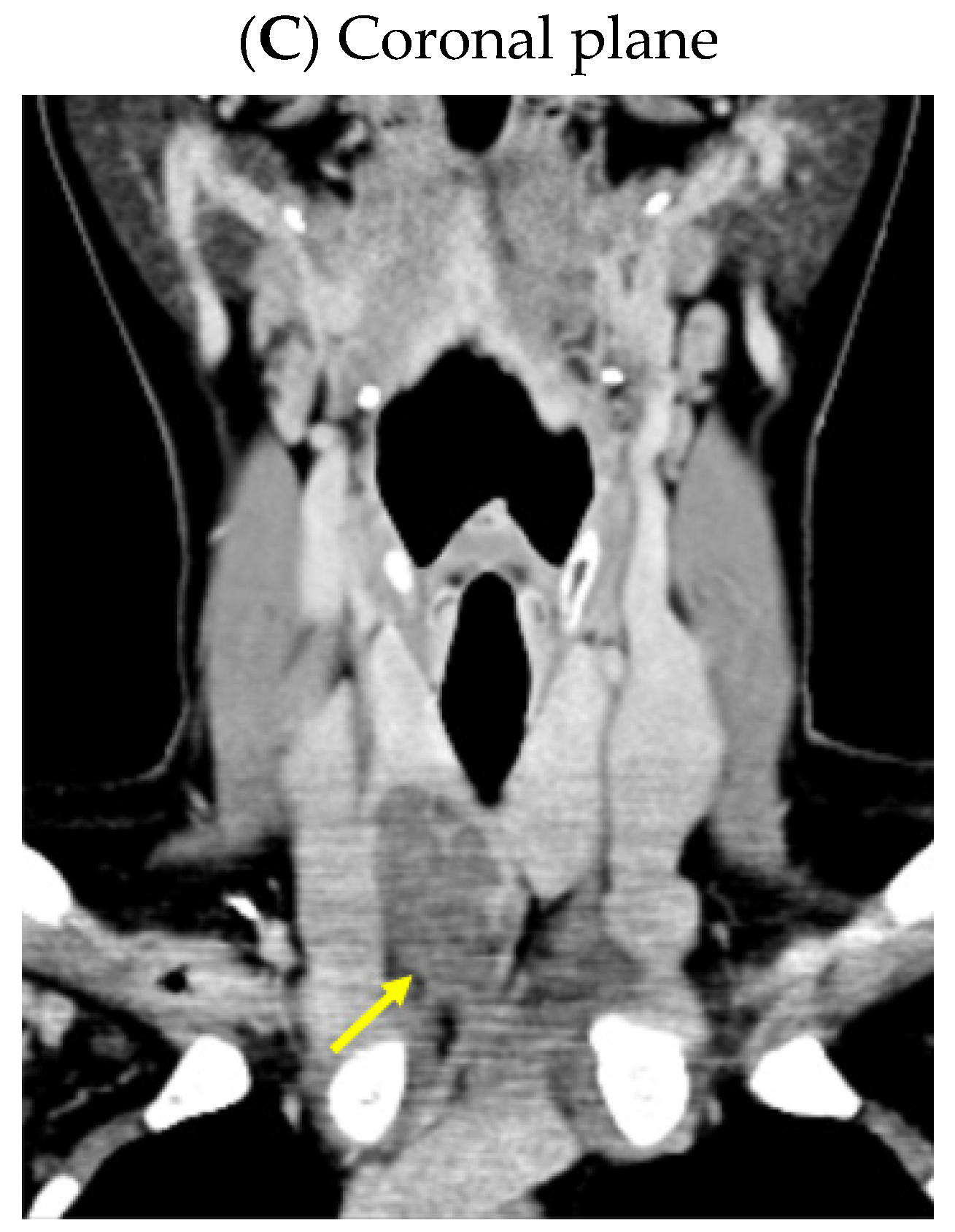
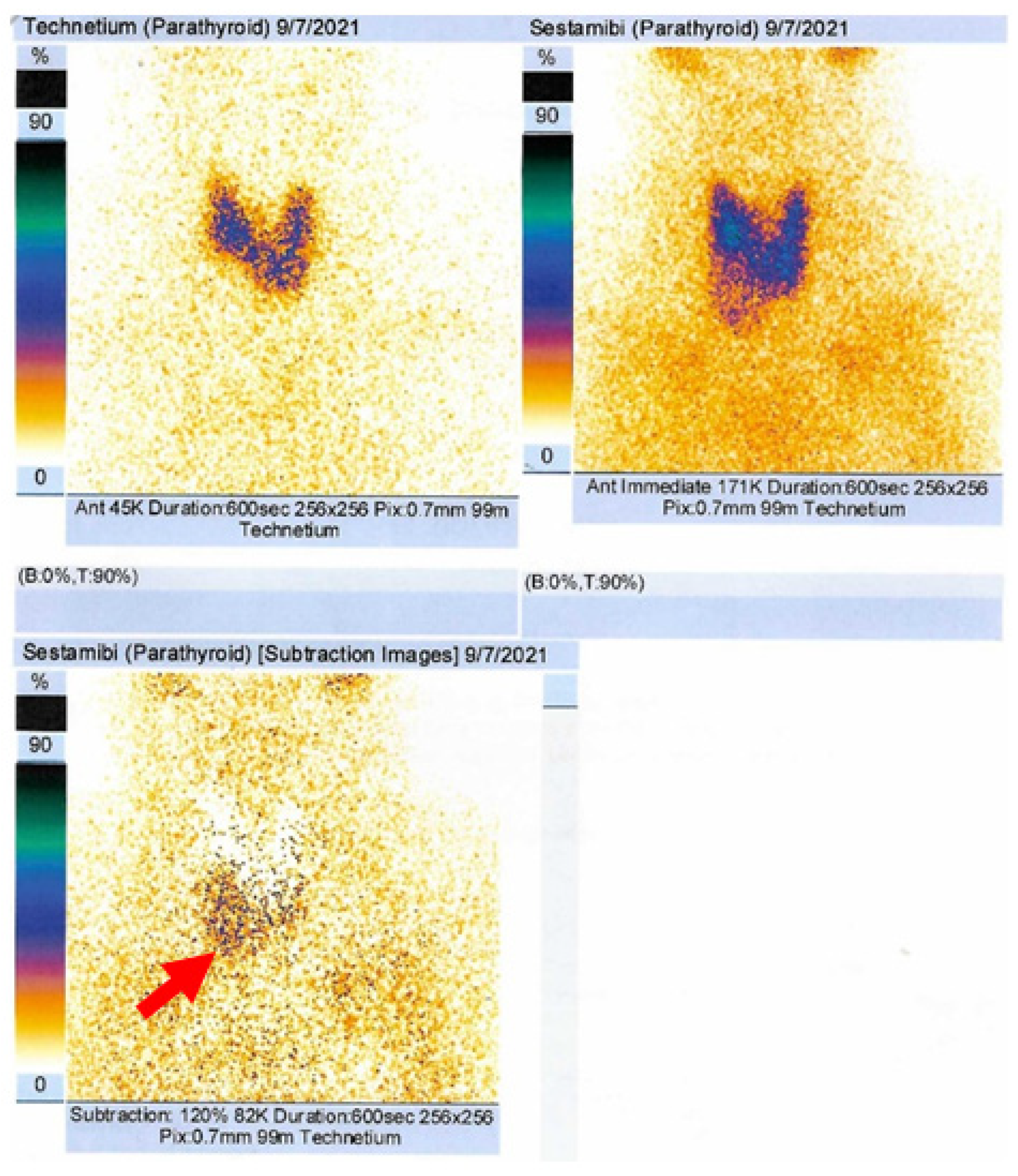
2.4.1. Confirmation of the Parathyroid Tumor
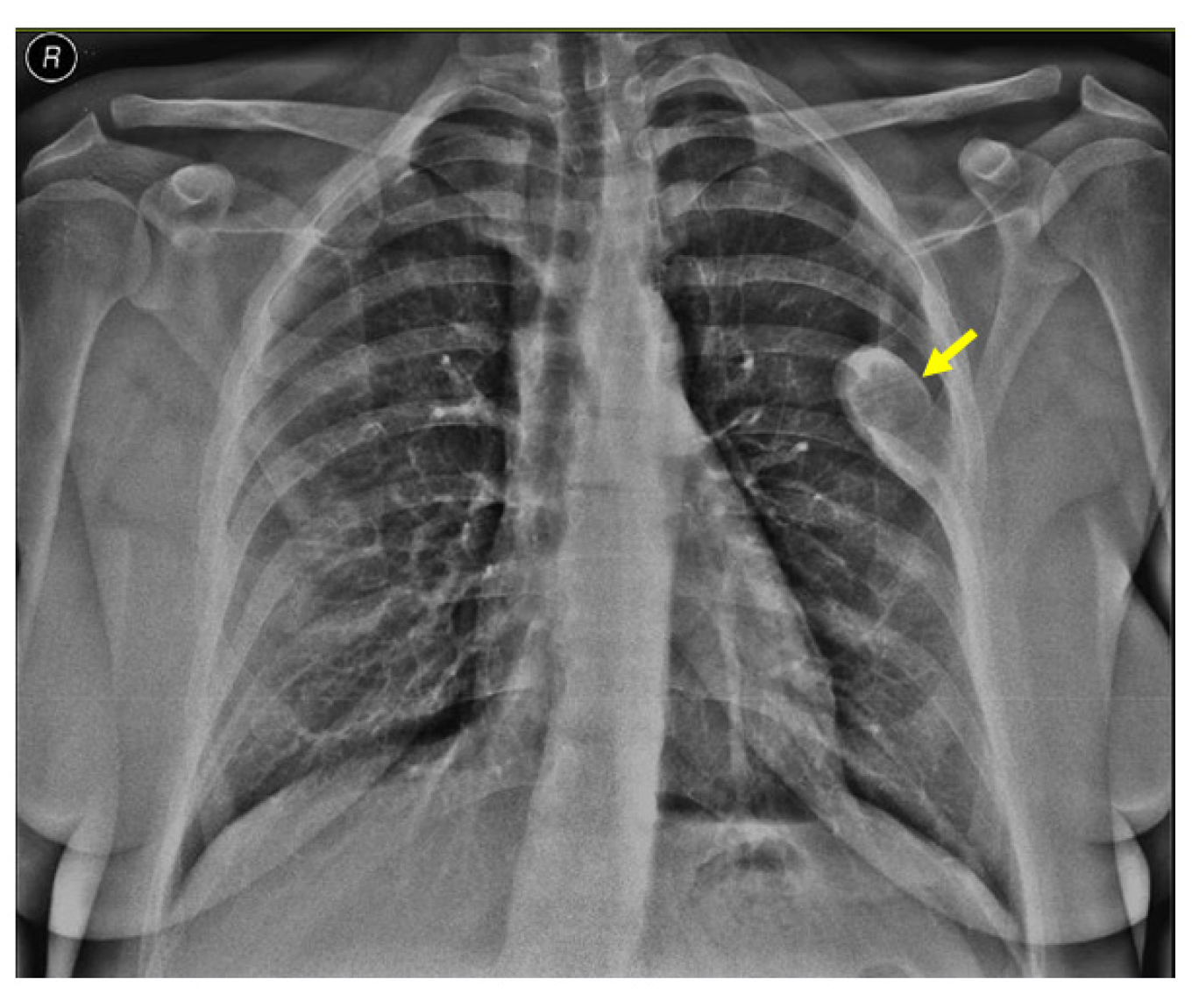
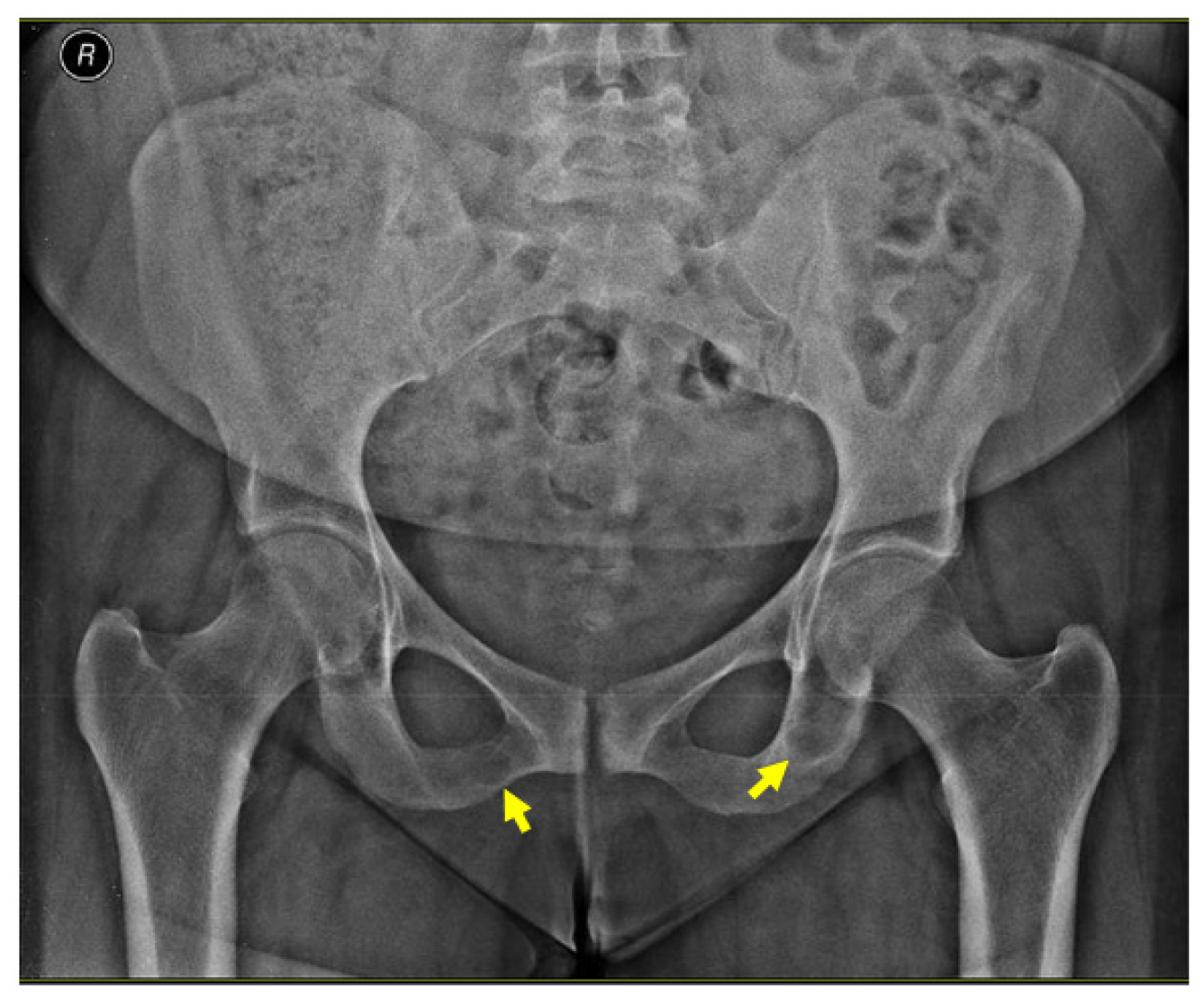
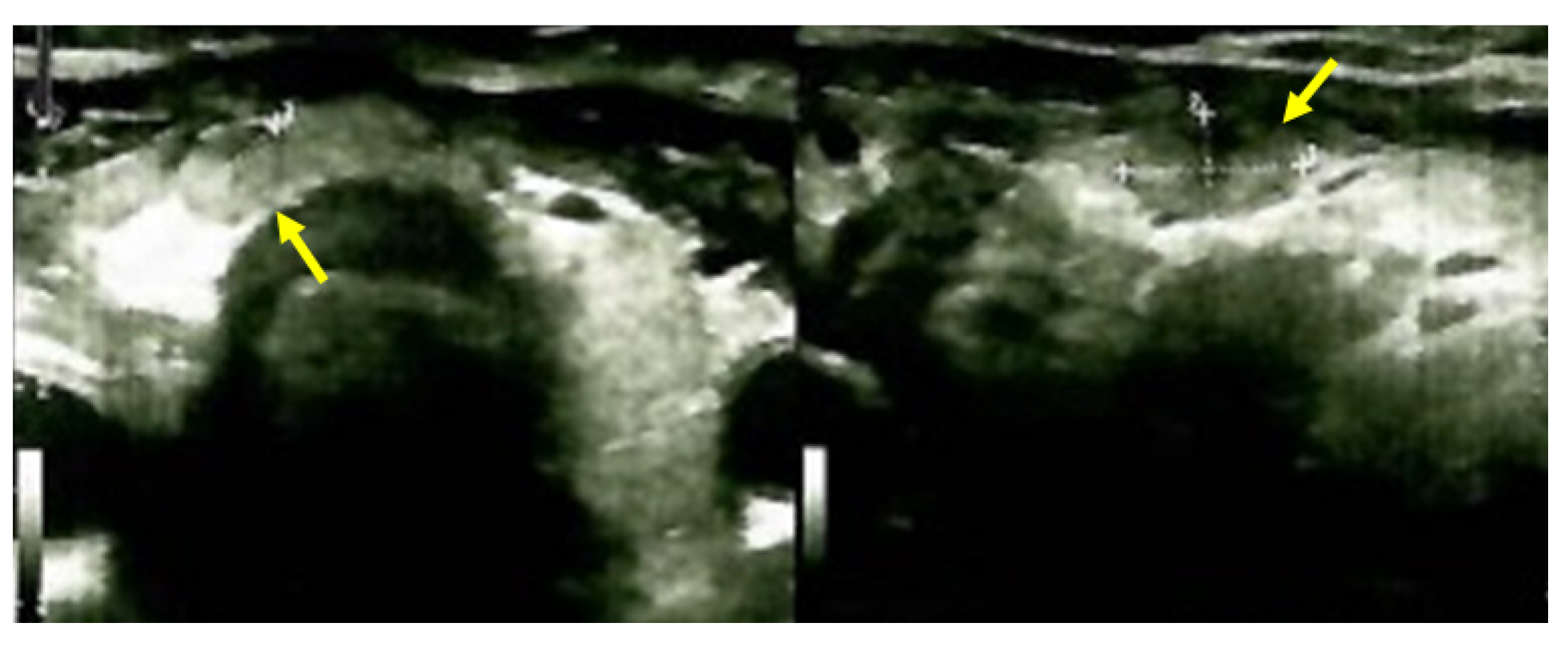
2.4.2. Identification of the Brown Tumors
2.5. Other Investigations
2.6. Management
2.7. Outcome
3. Brown Tumors in Renal Hyperparathyroidism
3.1. Admission
3.2. Medical History
3.3. Investigations amid Newly Detected Seizures during Dialysis
3.4. Management
3.5. Outcome
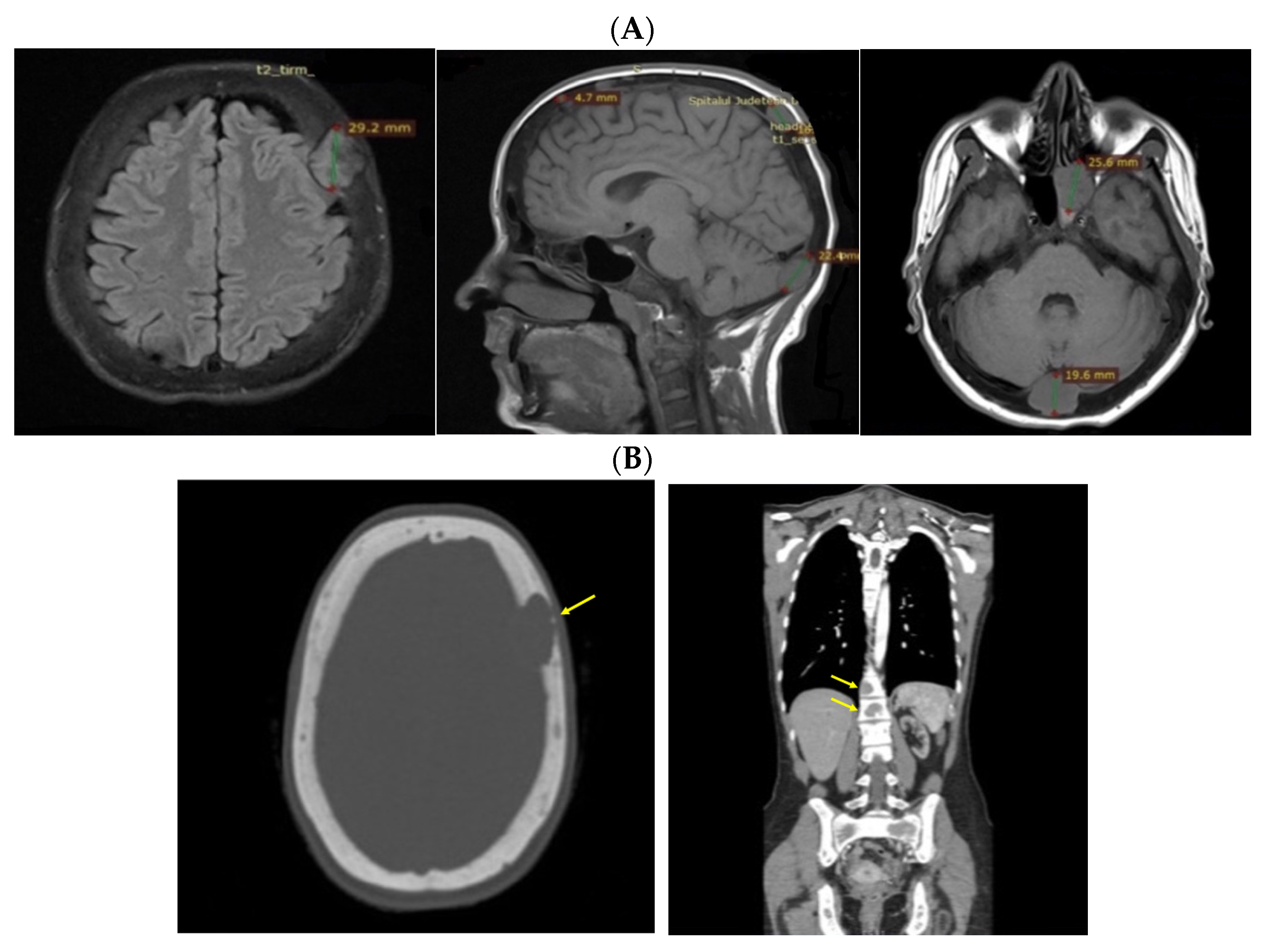
3.6. Approach of the Skull Tumors
4. Discussion
4.1. Poor Compliance/Adherence to Recommendations and Severe Parathyroid Conditions
4.2. Brown Tumors and Highly Elevated PTH
4.3. Bone Turnover Markers: A Friendly Tool for Practitioners
4.4. Imagery Assessment of Brown Tumors
4.5. Decision of Biopsy and Resection for Brown Tumors: A Personalized Matter
4.6. Other Aspects of Primary/Secondary Hyperparathyroidism Complicated with Brown Tumors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| CT | Computed tomography |
| DXA | Dual-energy X-ray absorptiometry |
| HDP | Hydroxymethylene diphosphonate |
| 25OHD | 25-hydroxyvitamin D |
| FT4 | Free levothyroxine |
| IL | Interleukine |
| M-CSF | Macrophage colony-stimulating factor |
| MRI | Magnetic resonance imagery |
| NFATc1 | Nuclear factor of activated T cells 1 |
| PTH | Parathormone |
| P1NP | Procollagen type 1 N-terminal propeptide |
| PET/CT | Positronic emission tomography/computed tomography |
| RANKL | Receptor activator of nuclear factor kappa-B |
| RAAS | Renin-angiotensin-aldosterone system |
| TSH | Thyroid-stimulating hormone |
| TBS | Trabecular bone score |
| Tc | Technetium |
References
- Miwa, S.; Tanaka, T.; Aiba, H.; Yamada, S.; Otsuka, T.; Tsuchiya, H. Multiple Bone Cysts Caused by Hyperparathyroidism: A Case Report and Review of the Literature. Cancer Diagn. Progn. 2023, 3, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari, M.R.; Moharamzad, Y. Brown tumor of the spine in patients with primary hyperparathyroidism. Spine 2014, 39, E1073–E1079. [Google Scholar] [CrossRef] [PubMed]
- Sarda, P.; Gurram, P.; Ramakrishnan, K.; Narayanan, V.; Chandran, S.; Vinayachandran, D. Diagnostic dilemma in maxillofacial pathologies: A case series. J. Med. Case Rep. 2024, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.S.; Purohit, S.; Harsh, A.; Vangala, N. A complex case of brown tumors as initial manifestation of primary hyperparathyroidism in a young female. J. Oral Maxillofac. Pathol. 2019, 23, 477. [Google Scholar] [CrossRef]
- Diao, Z.; Zhang, J.; Zhao, J.; Sun, W.; Pu, Z. Brown tumor due to primary hyperparathyroidism in a familial case: A case report. BMC Endocr. Disord. 2023, 23, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Huang, Y.; Luo, J.; Ye, Y. Misdiagnosis of brown tumour caused by primary hyperparathyroidism: A case report with literature review. BMC Endocr. Disord. 2022, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Misiorowski, W.; Czajka-Oraniec, I.; Kochman, M.; Zgliczyński, W.; Bilezikian, J.P. Osteitis fibrosa cystica-a forgotten radiological feature of primary hyperparathyroidism. Endocrine 2017, 58, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Weng, S.F.; Yang, T.T.; Lee, Y.W.; Liu, J.H.; Hsieh, Y.S. Unusual body weight loss due to primary hyperparathyroidism: A case study with literature review. Heliyon 2024, 10, e28333. [Google Scholar] [CrossRef] [PubMed]
- Vanitcharoenkul, E.; Singsampun, N.; Unnanuntana, A.; Sirinvaravong, S. Osteitis Fibrosa Cystica and pathological fractures-the classic but neglected skeletal manifestation of primary hyperparathyroidism: A case report. BMC Musculoskelet. Disord. 2021, 22, 443. [Google Scholar] [CrossRef]
- Minisola, S.; Gianotti, L.; Bhadada, S.; Silverberg, S.J. Classical complications of primary hyperparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 791–803. [Google Scholar] [CrossRef]
- Castellano, E.; Attanasio, R.; Boriano, A.; Borretta, V.; Gennaro, M.; Latina, A.; Borretta, G. Radiologic Manifestation of Bone Involvement in Primary Hyperparathyroidism: Prevalence and Clinical Significance in a Southern European Series. Endocr. Pract. 2020, 26, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.W. Clinical aspects of primary hyperparathyroidism: Clinical manifestations, diagnosis, and therapy. Wien Med. Wochenschr. 2013, 163, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Sun, P.; Li, J.; Yang, Z.; Li, X.; Li, Z.; Yan, J.; Li, K.; Wang, L. Skeletal lesions in primary hyperparathyroidism. Am. J. Med. Sci. 2015, 349, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Cosme, I.; Nobre, E.; Travessa, A.; Santos, C.; Rocha, J.; Presa, D.; Barbosa, A.P. Ectopic Intrathyroidal Parathyroid Adenoma Presenting with Osteoporotic Fractures in a Young Man: A Case Report. Cureus 2023, 15, e47461. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Lopes, J.; Caridade, S. Secondary Hyperparathyroidism Presenting as a Brown Tumor: A Case Report and Review of the Literature. Cureus 2023, 15, e33820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, H.; Tan, H.; Song, R.; Zhang, Y.; Zhao, L. Brown tumor of the cervical spine with primary hyperparathyroidism: A case report and literature review. Medicine 2023, 102, e32768. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, R.; Magesh, K.T.; Vivek, N.; Saravanan, C. Maxillary brown tumor due to secondary hyperparathyroidism in a Hemodialysis patient: A case report and literature review. J. Oral Maxillofac. Pathol. 2021, 25, 527–532. [Google Scholar] [CrossRef]
- Kim, S.J.; Shoback, D.M. Sporadic Primary Hyperparathyroidism. Endocrinol. Metab. Clin. N. Am. 2021, 50, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Hakkou, F.; Benjelloun, L.; Hallab, L.; Chbicheb, S. Brown tumor of the jaw as a rare manifestation of hyperparathyroidism: Two case reports and literature review. Int. J. Surg. Case Rep. 2023, 111, 108823. [Google Scholar] [CrossRef]
- Fedhila, M.; Belkacem Chebil, R.; Marmouch, H.; Terchalla, S.; Ayachi, S.; Oueslati, Y.; Oualha, L.; Douki, N.; Khochtali, H. Brown Tumors of the Jaws: A Retrospective Study. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231210143. [Google Scholar] [CrossRef]
- Majic Tengg, A.; Cigrovski Berkovic, M.; Zajc, I.; Salaric, I.; Müller, D.; Markota, I. Expect the unexpected: Brown tumor of the mandible as the first manifestation of primary hyperparathyroidism. World J. Clin. Cases 2024, 12, 1200–1204. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Tatani, I.; Kourea, H.P.; Kokkalis, Z.T.; Panagopoulos, K.; Megas, P. Osteolytic lesions (brown tumors) of primary hyperparathyroidism misdiagnosed as multifocal giant cell tumor of the distal ulna and radius: A case report. J. Med. Case Rep. 2018, 12, 176. [Google Scholar] [CrossRef]
- Guimarães, L.M.; Valeriano, A.T.; Rebelo Pontes, H.A.; Gomez, R.S.; Gomes, C.C. Manifestations of hyperparathyroidism in the jaws: Concepts, mechanisms, and clinical aspects. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 547–555. [Google Scholar] [CrossRef]
- Turner, R.T.; Iwaniec, U.T.; Marley, K.; Sibonga, J.D. The role of mast cells in parathyroid bone disease. J. Bone Miner. Res. 2010, 25, 1637–1649. [Google Scholar] [CrossRef]
- Pechalova, P.F.; Poriazova, E.G. Brown tumor at the jaw in patients with secondary hyperparathyroidism due to chronic renal failure. Acta Medica 2013, 56, 83–86. [Google Scholar] [CrossRef]
- Silva, M.T.; Cedraz, J.S.; Pontes, C.G.; Trento, C.L.; Brasileiro, B.F.; Piva, M.R.; Pereira, F.A. Brown tumor: Clinical findings of secondary hyperparathyroidism in patients with renal osteodystrophy. Gen. Dent. 2017, 65, 70–74. [Google Scholar]
- Shavlokhova, V.; Goeppert, B.; Gaida, M.M.; Saravi, B.; Weichel, F.; Vollmer, A.; Vollmer, M.; Freudlsperger, C.; Mertens, C.; Hoffmann, J. Mandibular Brown Tumor as a Result of Secondary Hyperparathyroidism: A Case Report with 5 Years Follow-Up and Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 7370. [Google Scholar] [CrossRef]
- Bransky, N.; Iyer, N.R.; Cannon, S.M.; Tyan, A.H.; Mylavarapu, P.; Orosco, R.; Hom, D.B.; Moazzam, A.A. Three Rare Concurrent Complications of Tertiary Hyperparathyroidism: Maxillary Brown Tumor, Uremic Leontiasis Ossea, and Hungry Bone Syndrome. J. Bone Metab. 2020, 27, 217–226. [Google Scholar] [CrossRef]
- Staruschenko, A.; Alexander, R.T.; Caplan, M.J.; Ilatovskaya, D.V. Calcium signalling and transport in the kidney. Nat. Rev. Nephrol. 2024. [Google Scholar] [CrossRef]
- Zechner, C.; Rhee, E.P. Phosphate sensing in health and disease. Curr. Opin. Nephrol. Hypertens 2024. [Google Scholar] [CrossRef]
- Simic, P. Bone and bone derived factors in kidney disease. Front. Physiol. 2024, 15, 1356069. [Google Scholar] [CrossRef]
- Fernández-Villabrille, S.; Martín-Carro, B.; Martín-Vírgala, J.; Rodríguez-Santamaria, M.D.M.; Baena-Huerta, F.; Muñoz-Castañeda, J.R.; Fernández-Martín, J.L.; Alonso-Montes, C.; Naves-Díaz, M.; Carrillo-López, N.; et al. Novel Biomarkers of Bone Metabolism. Nutrients 2024, 16, 605. [Google Scholar] [CrossRef]
- Pazianas, M.; Miller, P.D. Osteoporosis and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Back to Basics. Am. J. Kidney Dis. 2021, 78, 582–589. [Google Scholar] [CrossRef]
- Santoso, D.; Thaha, M.; Empitu, M.A.; Kadariswantiningsih, I.N.; Suryantoro, S.D.; Haryati, M.R.; Hertanto, D.M.; Pramudya, D.; Bintoro, S.U.Y.; Nasronudin, N.; et al. Brown Tumour in Chronic Kidney Disease: Revisiting an Old Disease with a New Perspective. Cancers 2023, 15, 4107. [Google Scholar] [CrossRef]
- Yamada, S.; Nakano, T. Role of Chronic Kidney Disease (CKD)-Mineral and Bone Disorder (MBD) in the Pathogenesis of Cardiovascular Disease in CKD. J. Atheroscler. Thromb 2023, 30, 835–850. [Google Scholar] [CrossRef]
- Fusaro, M.; Pereira, L.; Bover, J. Current and Emerging Markers and Tools Used in the Diagnosis and Management of Chronic Kidney Disease-Mineral and Bone Disorder in Non-Dialysis Adult Patients. J. Clin. Med. 2023, 12, 6306. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Akihisa, T. The Importance of Phosphate Control in Chronic Kidney Disease. Nutrients 2021, 13, 1670. [Google Scholar] [CrossRef]
- Nistor, C.E.; Stanciu-Găvan, C.; Vasilescu, F.; Dumitru, A.V.; Ciuche, A. Attitude of the surgical approach in hyperparathyroidism: A retrospective study. Exp. Ther. Med. 2021, 22, 959. [Google Scholar] [CrossRef]
- Miyakoshi, M.; Kamoi, K.; Takano, T.; Nishihara, M.; Kawashima, T.; Sudo, N.; Togashi, K.; Emura, I.; Williams, D. Multiple brown tumors in primary hyperparathyroidism caused by an adenoma mimicking metastatic bone disease with false positive results on computed tomography and Tc-99m sestamibi imaging: MR findings. Endocr. J. 2007, 54, 205–210. [Google Scholar] [CrossRef]
- Baracaldo, R.M.; Bao, D.; Iampornpipopchai, P.; Fogel, J.; Rubinstein, S. Facial disfigurement due to osteitis fibrosa cystica or brown tumor from secondary hyperparathyroidism in patients on dialysis: A systematic review and an illustrative case report. Hemodial. Int. 2015, 19, 583–592. [Google Scholar] [CrossRef]
- Aspray, T.J.; Bowring, C.; Fraser, W.; Gittoes, N.; Javaid, M.K.; Macdonald, H.; Patel, S.; Selby, P.; Tanna, N.; Francis, R.M.; et al. National Osteoporosis Society vitamin D guideline summary. Age Ageing 2014, 43, 592–595. [Google Scholar] [CrossRef]
- Sevva, C.; Divanis, D.; Tsinari, A.; Grammenos, P.; Laskou, S.; Mantalobas, S.; Paschou, E.; Magra, V.; Kopsidas, P.; Kesisoglou, I.; et al. Pharmaceutical Management of Secondary Hyperparathyroidism and the Role of Surgery: A 5-Year Retrospective Study. Medicina 2024, 60, 812. [Google Scholar] [CrossRef]
- Tiong, M.K.D.; Toussaint, N.D. Severe secondary hyperparathyroidism: An increasing problem in CKD but the best management option is still unknown. J. Bras. Nefrol. 2024, 46, e2024E004. [Google Scholar] [CrossRef]
- Gao, D.; Liu, Y.; Cui, W.; Lu, X.; Lou, Y. A nomogram prediction model for hungry bone syndrome in dialysis patients with secondary hyperparathyroidism after total parathyroidectomy. Eur. J. Med. Res. 2024, 29, 208. [Google Scholar] [CrossRef]
- Jain, N.; Reilly, R.F. Hungry bone syndrome. Curr. Opin. Nephrol. Hypertens 2017, 26, 250–255. [Google Scholar] [CrossRef]
- Witteveen, J.E.; van Thiel, S.; Romijn, J.A.; Hamdy, N.A. Hungry bone syndrome: Still a challenge in the post-operative management of primary hyperparathyroidism: A systematic review of the literature. Eur. J. Endocrinol. 2013, 168, R45–R53. [Google Scholar] [CrossRef]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients-Our Experience and Brief Review. Chirurgia 2022, 117, 317–327. [Google Scholar] [CrossRef]
- Aojula, N.; Ready, A.; Gittoes, N.; Hassan-Smith, Z. Management of Parathyroid Disease during the COVID-19 Pandemic. J. Clin. Med. 2021, 10, 920. [Google Scholar] [CrossRef]
- Alfadhli, E.M. Management of Primary Hyperparathyroidism with Severe Hypercalcemia During the COVID-19 Pandemic. Clin. Ther. 2021, 43, 711–719. [Google Scholar] [CrossRef]
- Nistor, C.E.; Pantile, D.; Gavan, C.S.; Ciuche, A. Pneumothorax on COVID-19 patients-retrospective clinical observations. Rom. J. Leg Med. 2022, 30, 112–116. [Google Scholar] [CrossRef]
- Alvarez-Payares, J.C.; Ribero, M.E.; Ramírez-Urrea, S.; Fragozo-Ramos, M.C.; Agámez-Gómez, J.E.; Román-González, A.; Arias, L.F.; Arenas, R.B.; López-Urbano, F. Giant Parathyroid Adenoma-Associated Fracture, Not All Lytic Bone Lesions are Cancer: A Case-Based Review. Case Rep. Med. 2022, 2022, 3969542. [Google Scholar] [CrossRef]
- Hamidi, S.; Mottard, S.; Berthiaume, M.J.; Doyon, J.; Bégin, M.J.; Bondaz, L. Brown tumor of the iliac crest initially misdiagnosed as a giant cell tumor of the bone. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 20-0029. [Google Scholar] [CrossRef]
- Gosavi, S.; Kaur, H.; Gandhi, P. Multifocal osteolytic lesions of jaw as a road map to diagnosis of brown tumor of hyperparathyroidism: A rare case report with review of literature. J. Oral Maxillofac. Pathol. 2020, 24 (Suppl. S1), S59–S66. [Google Scholar] [CrossRef]
- Bandeira, F.; Cassibba, S. Hyperparathyroidism and Bone Health. Curr. Rheumatol. Rep. 2015, 17, 48. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Skeletal abnormalities in Hypoparathyroidism and in Primary Hyperparathyroidism. Rev. Endocr. Metab. Disord. 2021, 22, 789–802. [Google Scholar] [CrossRef]
- Bandeira, F.; Cusano, N.E.; Silva, B.C.; Cassibba, S.; Almeida, C.B.; Machado, V.C.; Bilezikian, J.P. Bone disease in primary hyperparathyroidism. Arq. Bras. Endocrinol. Metabol. 2014, 58, 553–561. [Google Scholar] [CrossRef]
- Rejnmark, L.; Ejlsmark-Svensson, H. Effects of PTH and PTH Hypersecretion on Bone: A Clinical Perspective. Curr. Osteoporos. Rep. 2020, 18, 103–114. [Google Scholar] [CrossRef]
- Cusano, N.E.; Cetani, F. Normocalcemic primary hyperparathyroidism. Arch. Endocrinol. Metab. 2022, 66, 666–677. [Google Scholar] [CrossRef]
- Shaker, J.L.; Wermers, R.A. The Eucalcemic Patient with Elevated Parathyroid Hormone Levels. J. Endocr. Soc. 2023, 7, bvad013. [Google Scholar] [CrossRef]
- Kulkarni, P.; Goldenberg, D. Surgery for Normocalcemic Hyperparathyroidism. Otolaryngol. Clin. N. Am. 2024, 57, 111–116. [Google Scholar] [CrossRef]
- Yigit, B.; Tanal, M.; Citgez, B. Giant parathyroid adenoma diagnosed by brown tumour, a clinical manifestation of primary hyperparathyroidism: A case report. J. Pak. Med. Assoc. 2021, 71, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Habas, E.S.; Eledrisi, M.; Khan, F.; Elzouki, A.Y. Secondary Hyperparathyroidism in Chronic Kidney Disease: Pathophysiology and Management. Cureus 2021, 13, e16388. [Google Scholar] [CrossRef]
- Keiler, A.; Dammerer, D.; Liebensteiner, M.; Schmitz, K.; Kaiser, P.; Wurm, A. Pathological Fracture of the Tibia as a First Sign of Hyperparathyroidism-A Case Report and Systematic Review of the Current Literature. Anticancer Res. 2021, 41, 3083–3089. [Google Scholar] [CrossRef]
- Gallo, D.; Rosetti, S.; Marcon, I.; Armiraglio, E.; Parafioriti, A.; Pinotti, G.; Perrucchini, G.; Patera, B.; Gentile, L.; Tanda, M.L.; et al. When primary hyperparathyroidism comes as good news. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 20-0046. [Google Scholar] [CrossRef]
- Marcocci, C.; Cianferotti, L.; Cetani, F. Bone disease in primary hyperparathyrodism. Ther. Adv. Musculoskelet. Dis. 2012, 4, 357–368. [Google Scholar] [CrossRef]
- Haarhaus, M.; Cianciolo, G.; Barbuto, S.; La Manna, G.; Gasperoni, L.; Tripepi, G.; Plebani, M.; Fusaro, M.; Magnusson, P. Alkaline Phosphatase: An Old Friend as Treatment Target for Cardiovascular and Mineral Bone Disorders in Chronic Kidney Disease. Nutrients 2022, 14, 2124. [Google Scholar] [CrossRef]
- Sardiwal, S.; Magnusson, P.; Goldsmith, D.J.; Lamb, E.J. Bone alkaline phosphatase in CKD-mineral bone disorder. Am. J. Kidney Dis. 2013, 62, 810–822. [Google Scholar] [CrossRef]
- Nizet, A.; Cavalier, E.; Stenvinkel, P.; Haarhaus, M.; Magnusson, P. Bone alkaline phosphatase: An important biomarker in chronic kidney disease-mineral and bone disorder. Clin. Chim. Acta 2020, 501, 198–206. [Google Scholar] [CrossRef]
- Gao, D.; Lou, Y.; Cui, Y.; Liu, S.; Cui, W.; Sun, G. Risk factors for hypocalcemia in dialysis patients with refractory secondary hyperparathyroidism after parathyroidectomy: A meta-analysis. Ren. Fail 2022, 44, 503–512. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, N.; Tang, Y.; Zhan, Z.; Yang, M.; Lu, Y.; Li, G.S.; Zhang, L. Predictive markers for severe hypocalcemia in dialysis patients with secondary hyperparathyroidism after near-total parathyroidectomy. Ann. Palliat. Med. 2021, 10, 10712–10719. [Google Scholar] [CrossRef]
- Bi, T.; Bai, S.J.; Cheng, G.M.; Feng, X.D.; Zhang, W. Predictive analysis of severe hypocalcemia following total parathyroidectomy for renal secondary hyperparathyroidism. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 2217–2223. [Google Scholar] [CrossRef]
- Ciobîcă, M.L.; Ionescu, O.P.; Săndulescu, B.A. Osteoporosis and the fracture risk in systemic lupus erythematosus. Rom. J. Mil. Med. 2020, CXXIII, 341–347. [Google Scholar]
- Panchagnula, R.; Amarnath, S.S. Osteoporosis: Investigations and Monitoring. Indian J. Orthop. 2023, 57 (Suppl. S1), 70–81. [Google Scholar] [CrossRef]
- Tamimi, R.; Bdair, A.; Shratih, A.; Abdalla, M.; Sarsour, A.; Hamdan, Z.; Nazzal, Z. Bone mineral density and related clinical and laboratory factors in peritoneal dialysis patients: Implications for bone health management. PLoS ONE 2024, 19, e0301814. [Google Scholar] [CrossRef]
- Rajab, H.A. The Effect of Vitamin D Level on Parathyroid Hormone and Alkaline Phosphatase. Diagnostics 2022, 12, 2828. [Google Scholar] [CrossRef]
- Ionescu, O.P.; Stanciu, S.M.; Ciobîcă, M.L. Atherosclerosis in rheumatoid arthritis–The importance of imaging testing. Rom. J. Mil. Med. 2020, CXXIII, 26–31. [Google Scholar]
- Tram, L.; Kubik, M.; Kvist Jensen, K.; Almasi, C.E. Brown tumor mimicking metastases-the late manifestation of hyperparathyroidism. Acta Radiol. Open 2022, 11, 20584601221128415. [Google Scholar] [CrossRef]
- Anghel, D.; Ciobîcă, L.M.; Stanciu, S.M.; Jurcuț, C.V.; Stoicescu, G.D.; Răduță, I.A.; Coca, A. Ankylosing spondylitis and cardiovascular risk–case report. Rom. J. Mil. Med. 2016, CXIX, 39–42. [Google Scholar]
- Jacquet-Francillon, N.; Prevot, N. Brown tumors in nuclear medicine: A systematic review. Ann. Nucl. Med. 2023, 37, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Ciobîcă, L.M.; Sârbu, I.; Stanciu, S.M.; Coca, A. Behçet disease–Case presentation. Rom. J. Mil. Med. 2016, CXIX, 43–46. [Google Scholar] [CrossRef]
- Nistor, C.; Ranetti, A.E.; Ciuche, A.; Pantile, D.; Constatin, L.M.; Brincoveanu, R. Betadine in chemical pleurodesis. Farmacia 2014, 62, 897–906. [Google Scholar]
- Jinga, M.; Jurcuţ, C.; Vasilescu, F.; Becheanu, G.; Stancu, S.H.; Ciobaca, L.; Mircescu, G.; Jinga, V. A rare case of digestive hemorrhage in an elderly patient: Diagnosis and treatment difficulties. Rom J. Morphol. Embryol. 2012, 53 (Suppl. S3), 831–834. [Google Scholar] [PubMed]
- Prodana, M.; Nistor, C.E.; Stoian, A.B.; Ionita, D.; Burnei, C. Dual nanofibrous bioactive coarings on TiZr implants. Coatings 2020, 10, 526. [Google Scholar] [CrossRef]
- Nistor, C.E.; Ciuche, A.; Cucu, A.P.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Clavicular Malignancies: A Borderline Surgical Management. Medicina 2022, 58, 910. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.L.; Medawar, S.; Younan, T.; Abboud, H.; Trak-Smayra, V. Osteitis fibrosa cystica mimicking bone tumor, a case report. BMC Musculoskelet. Disord. 2021, 22, 479. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, M.A.; Stolzmann, P.; Huber, G.F.; Schmid, C. A patient with a history of breast cancer and multiple bone lesions: A case report. J. Med. Case Rep. 2017, 11, 127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sandru, F.; Carsote, M.; Dumitrascu, M.C.; Albu, S.E.; Valea, A. Glucocorticoids and Trabecular Bone Score. J Med Life. 2020, 13, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Mirzashahi, B.; Vosoughi, F.; Besharaty, S.; Satehi, S.H. Missed C5 vertebral brown tumor causing spinal cord compression and myelopathy: A case report and literature review. Clin. Case Rep. 2022, 10, e05331. [Google Scholar] [CrossRef]
- Queiroz, I.V.; Queiroz, S.P.; Medeiros RJr Ribeiro, R.B.; Crusoé-Rebello, I.M.; Leão, J.C. Brown tumor of secondary hyperparathyroidism: Surgical approach and clinical outcome. Oral Maxillofac. Surg. 2016, 20, 435–439. [Google Scholar] [CrossRef]
- Uysal, C.; Yilmaz, T.; Ozkan, H.; Canoz, O.; Tokgoz, B. The refractory secondary hyperparathyroidism presenting with retro-orbital brown tumor: A case report. BMC Nephrol. 2024, 25, 15. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Ren, H.; Wang, W.; Shi, H.; Li, X.; Chen, X.; Shen, P.; Wu, X.; Xie, J.; et al. Evaluation of anemia and serum iPTH, calcium, and phosphorus in patients with primary glomerulonephritis. Contrib. Nephrol. 2013, 181, 31–40. [Google Scholar] [CrossRef]
- Li, F.; Ye, X.; Yang, G.; Huang, H.; Bian, A.; Xing, C.; Tang, S.; Zhang, J.; Jiang, Y.; Chen, H.; et al. Relationships between blood bone metabolic biomarkers and anemia in patients with chronic kidney disease. Ren. Fail. 2023, 45, 2210227. [Google Scholar] [CrossRef] [PubMed]
- Zenoaga-Barbăroșie, C.; Berca, L.; Vassu-Dimov, T.; Toma, M.; Nica, M.I.; Alexiu-Toma, O.A.; Ciornei, C.; Albu, A.; Nica, S.; Nistor, C.; et al. The Predisposition for Type 2 Diabetes Mellitus and Metabolic Syndrome. Balkan J. Med. Genet. 2023, 26, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kır, S.; Polat, C. Haematological manifestations in primary hyperparathyroidism. Indian J. Med. Res. 2022, 155, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.A.; Bula-Ibula, D. Diagnosis and management of primary hyperparathyroidism during pregnancy: A systematic review and a longitudinal case study. Gynecol. Obstet. Fertil. Senol. 2023, 51, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Corral de la Calle, M.Á.; Encinas de la Iglesia, J.; Fernández Pérez, G.C.; Fraino, A.; Repollés Cobaleda, M. Multiple and hereditary renal tumors: A review for radiologists. Radiologia 2024, 66, 132–154. [Google Scholar] [CrossRef]
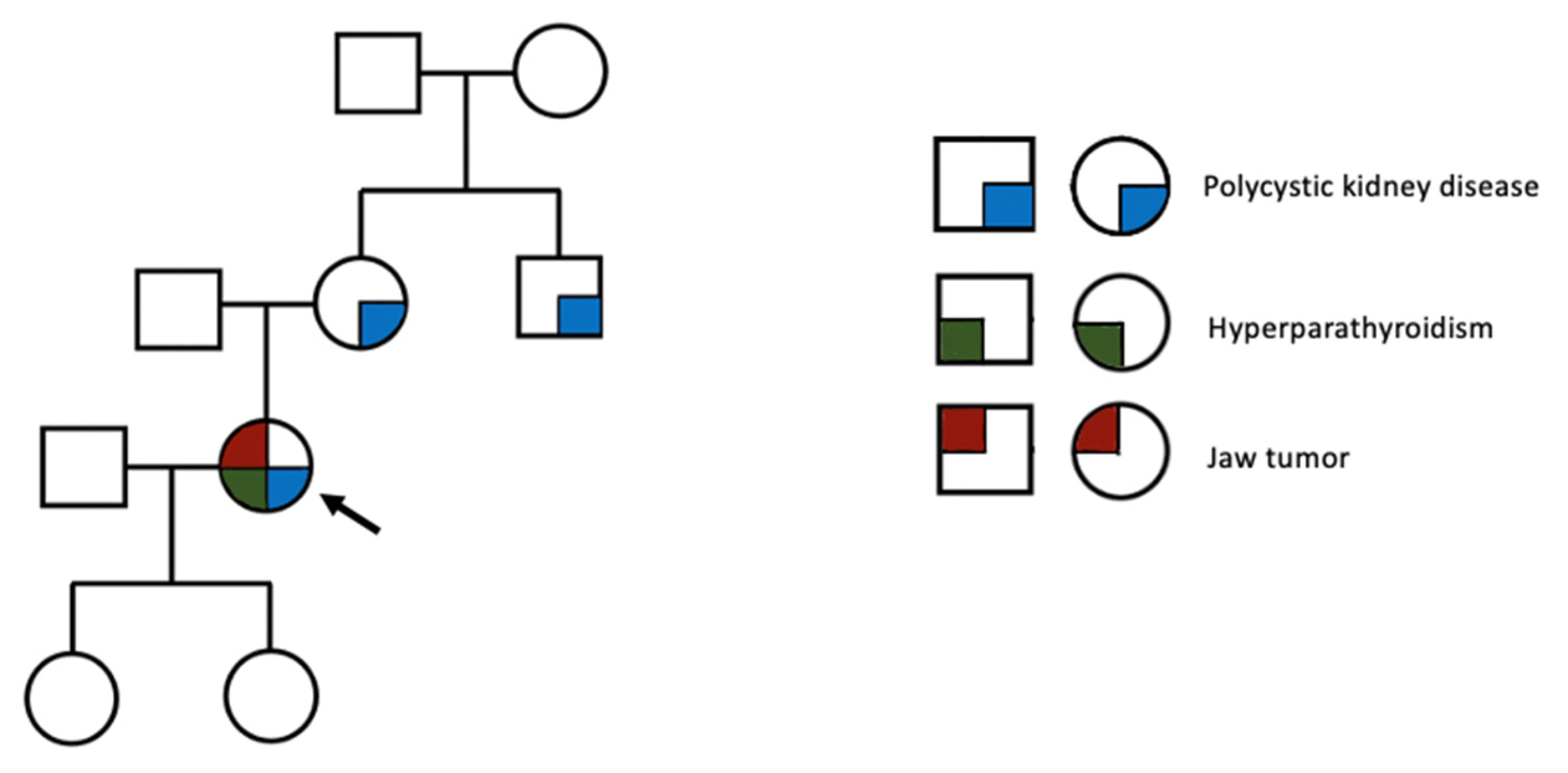
- Cetani, F.; Dinoi, E.; Pierotti, L.; Pardi, E. Familial states of primary hyperparathyroidism: An update. J. Endocrinol. Investig. 2024. [Google Scholar] [CrossRef]
- Zama, A.; Kruger, E.C.; Zemlin, A.E.; Conradie, M. Hyperparathyroidism Jaw Tumor Syndrome, an Unforeseen Diagnosis. JCEM Case Rep. 2024, 2, luae020. [Google Scholar] [CrossRef]
- Popow, M.; Kaszczewska, M.; Góralska, M.; Kaszczewski, P.; Skwarek-Szewczyk, A.; Chudziński, W.; Jażdżewski, K.; Kolanowska, M.; Bogdańska, M.; Starzyńska-Kubicka, A.; et al. Association between Parafibromin Expression and Presence of Brown Tumors and Jaw Tumors in Patients with Primary Hyperparathyroidism: Series of Cases with Review of the Literature. Am. J. Case Rep. 2022, 23, e936135. [Google Scholar] [CrossRef]
- English, K.A.; Lines, K.E.; Thakker, R.V. Genetics of hereditary forms of primary hyperparathyroidism. Hormones 2024, 23, 3–14. [Google Scholar] [CrossRef]



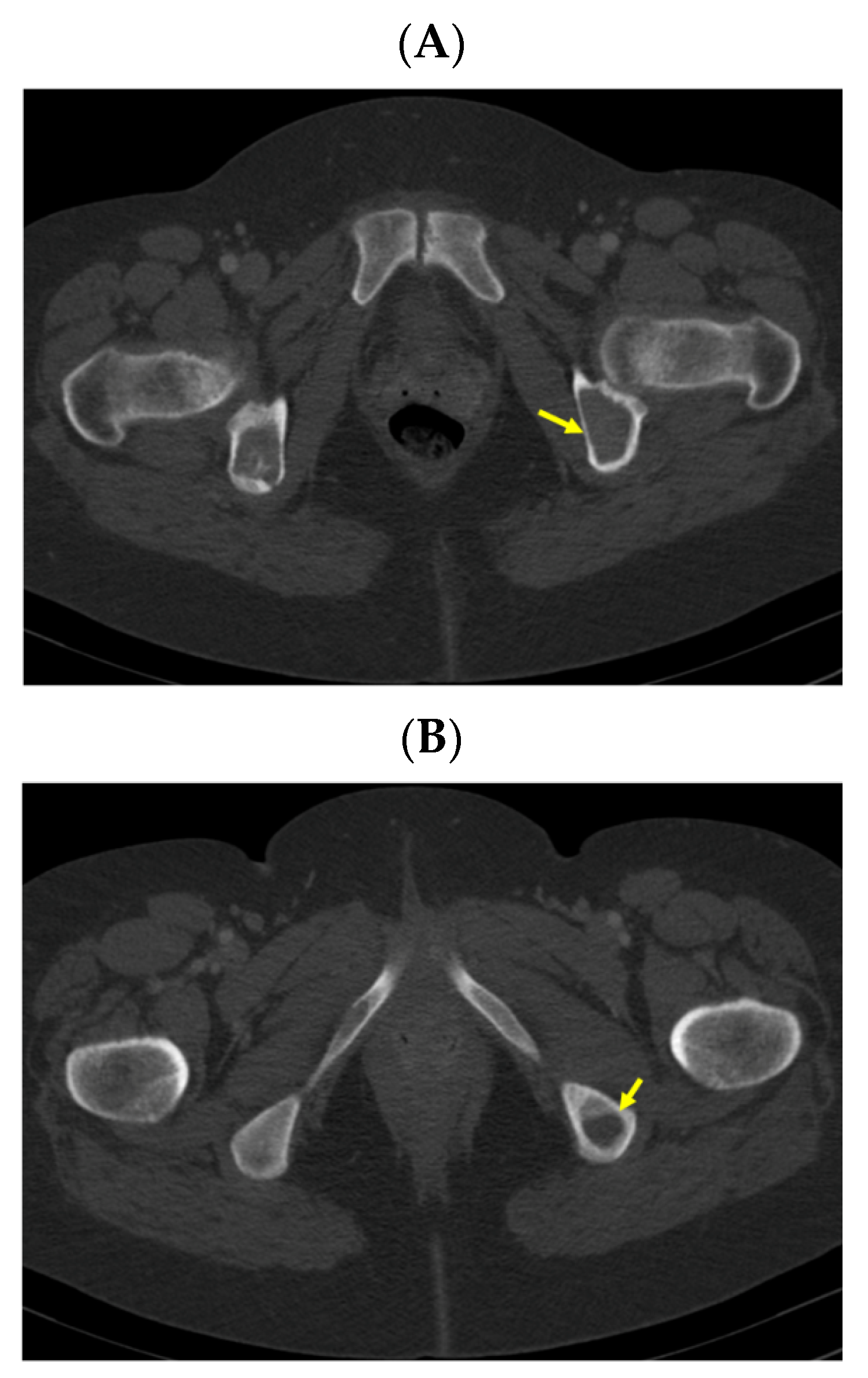
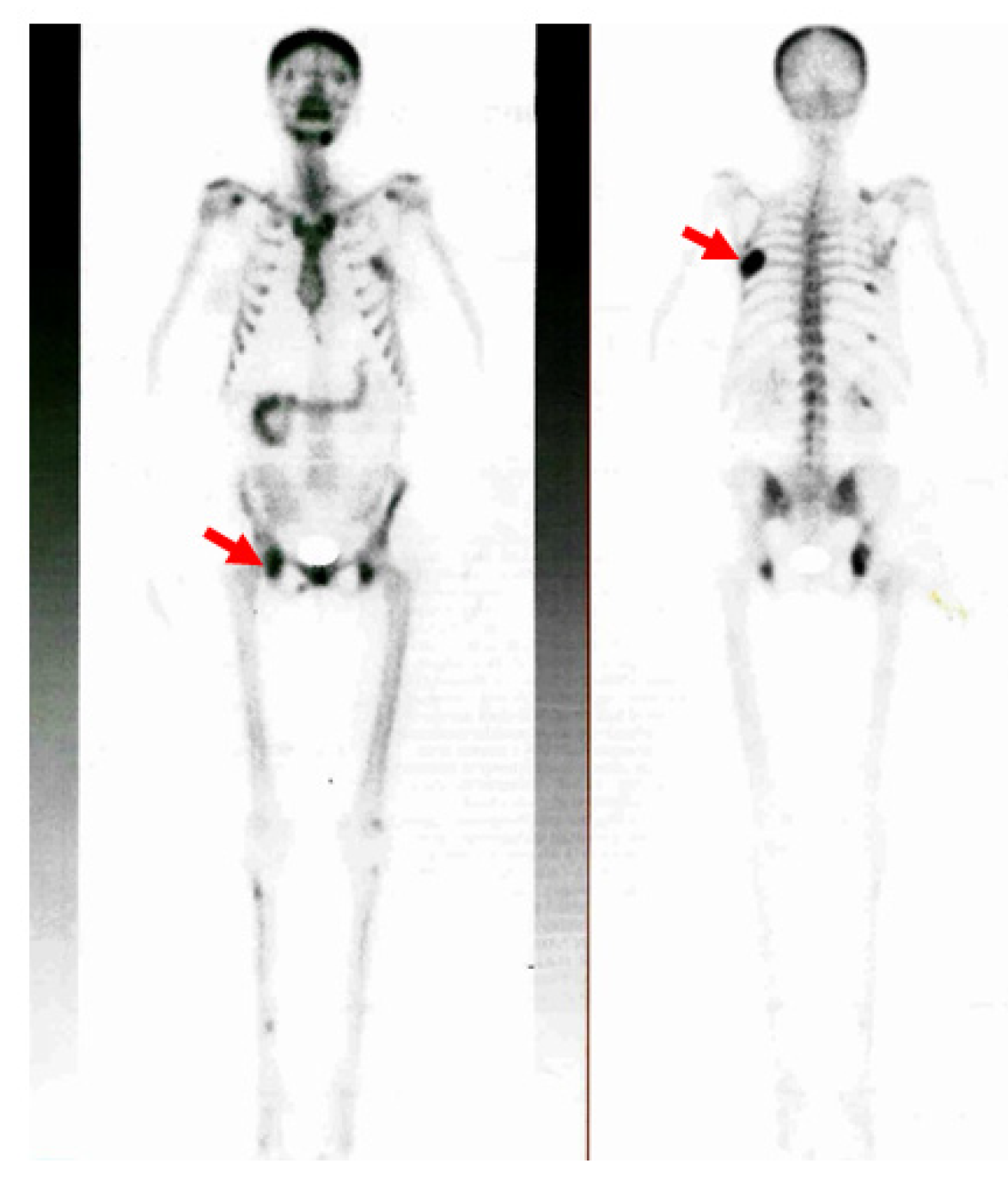
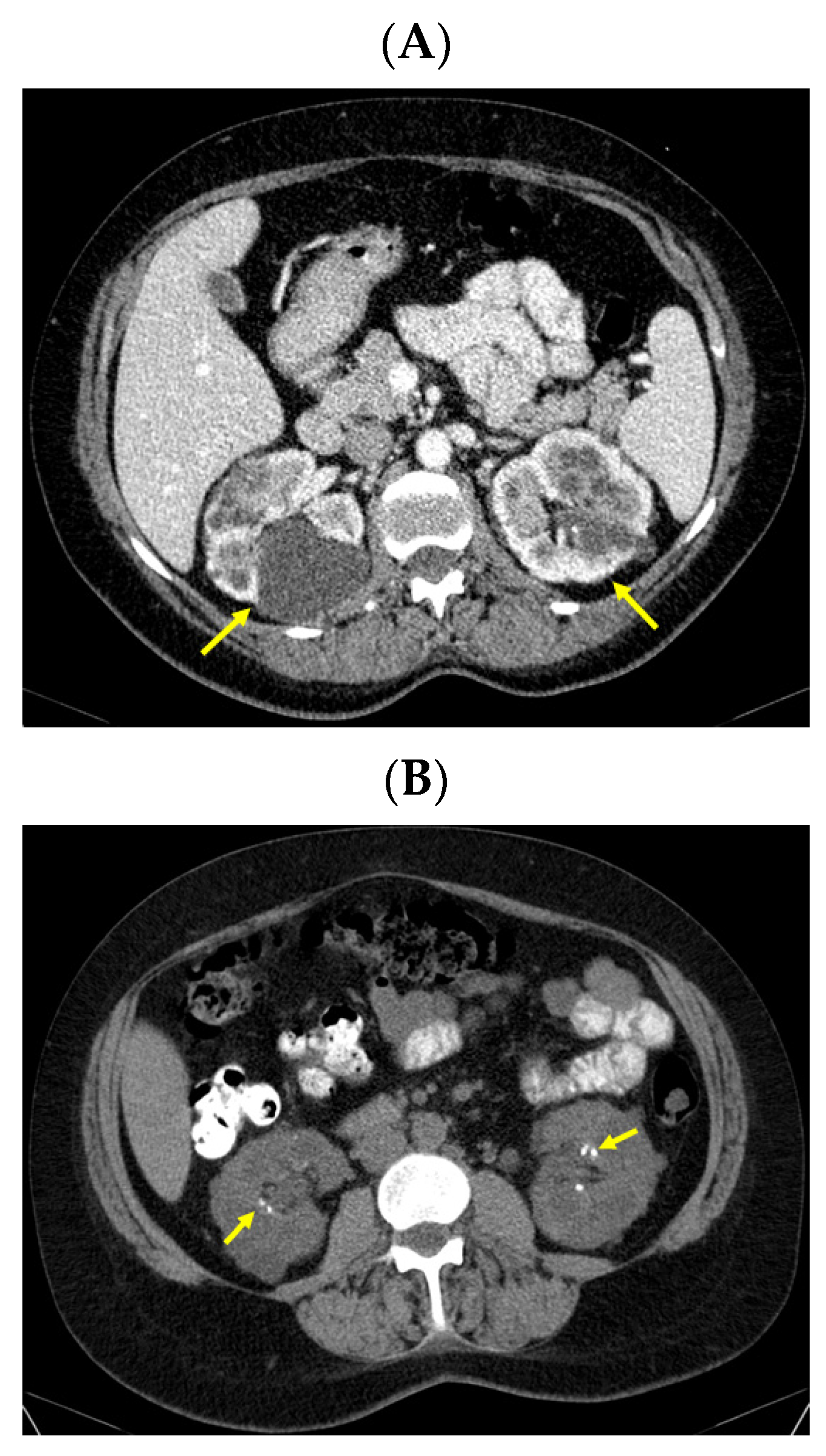
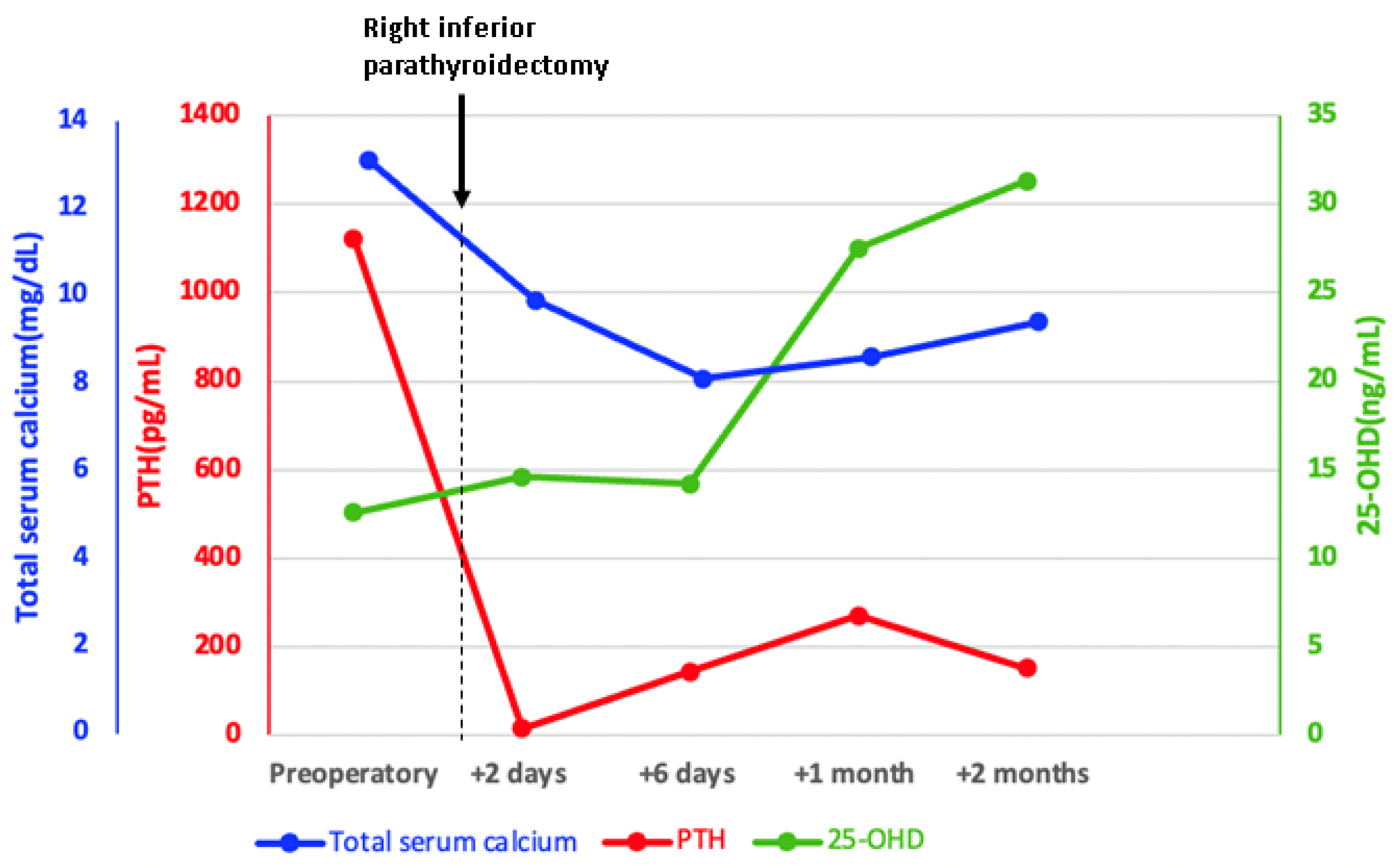

| Parameter | On Admission * | 2 Days after PT Surgery | 6 Days after PT Surgery | 1 Month after PT Surgery | 2 Months after PT Surgery | Normal Range |
|---|---|---|---|---|---|---|
| Total serum calcium (mg/dL) | 10 | 9.7 | 8.1 | 8.6 | 9.4 | 8.4–10.2 |
| Ionized calcium (mg/dL) | 4.5 | 4.5 | 3.9 | 3.7 | 4 | 3.9–4.9 |
| Total proteins (g/dL) | 6.8 | 6.7 | 6 | 7.2 | 7.4 | 6.4–8.3 |
| Phosphorus (mg/dL) | 1.9 | 3.6 | 4.1 | 3 | 3.9 | 2.3–4.7 |
| Serum creatinine (mg/dL) | 1 | 1.1 | 1.2 | 1.1 | 1.05 | 0.5–1.2 |
| Serum urea (mg/dL) | 41.3 | 60 | 65 | 50.1 | 43 | 15–50 |
| PTH (pg/mL) | 1123 * | 15.96 | 144.1 | 271.4 | 151.9 | 15–65 |
| 25OHD (ng/mL) | 12.6 | 14.6 | 14.2 | 27.5 | 31.3 | 30–100 |
| Parameter | On Admission | 1 Month after PT Surgery | 2 Months after PT Surgery | Normal Range |
|---|---|---|---|---|
| Uric acid (mg/dL) | 5.1 | 4.1 | 3.6 | 2.6–6 |
| Alanine aminotransferase (U/L) | 18.1 | 15 | 11 | 0–31 |
| Aspartate aminotransferase (U/L) | 12.3 | 14 | 11 | 0–32 |
| Total cholesterol (mg/dL) | 199.2 | 196 | 221 | 0–200 |
| HDL-cholesterol (mg/dL) | 39 | 40.6 | 47 | 40–60 |
| Triglycerides (mg/dL) | 112 | 141 | 122 | 0–149 |
| Fasting glycemia (mg/dL) | 110 | 84.3 | 87 | 70–105 |
| Glycated haemoglobin A1c (%) | 4.7 | 5.5 | NA | 4.8–5.9 |
| Sodium (mmol/L) | 143 | 141 | 139 | 136–145 |
| Potassium (mmol/L) | 4.62 | 4.53 | 4.1 | 3.5–5.1 |
| Magnesium (mg/dL) | 1.84 | 1.81 | 1.7 | 1.6–2.6 |
| Haemoglobin (g/dL) | 12.1 | 11.2 | 11.4 | 12–15.5 |
| Serum iron (μg/dL) | 47 | 56.8 | 57 | 50–170 |
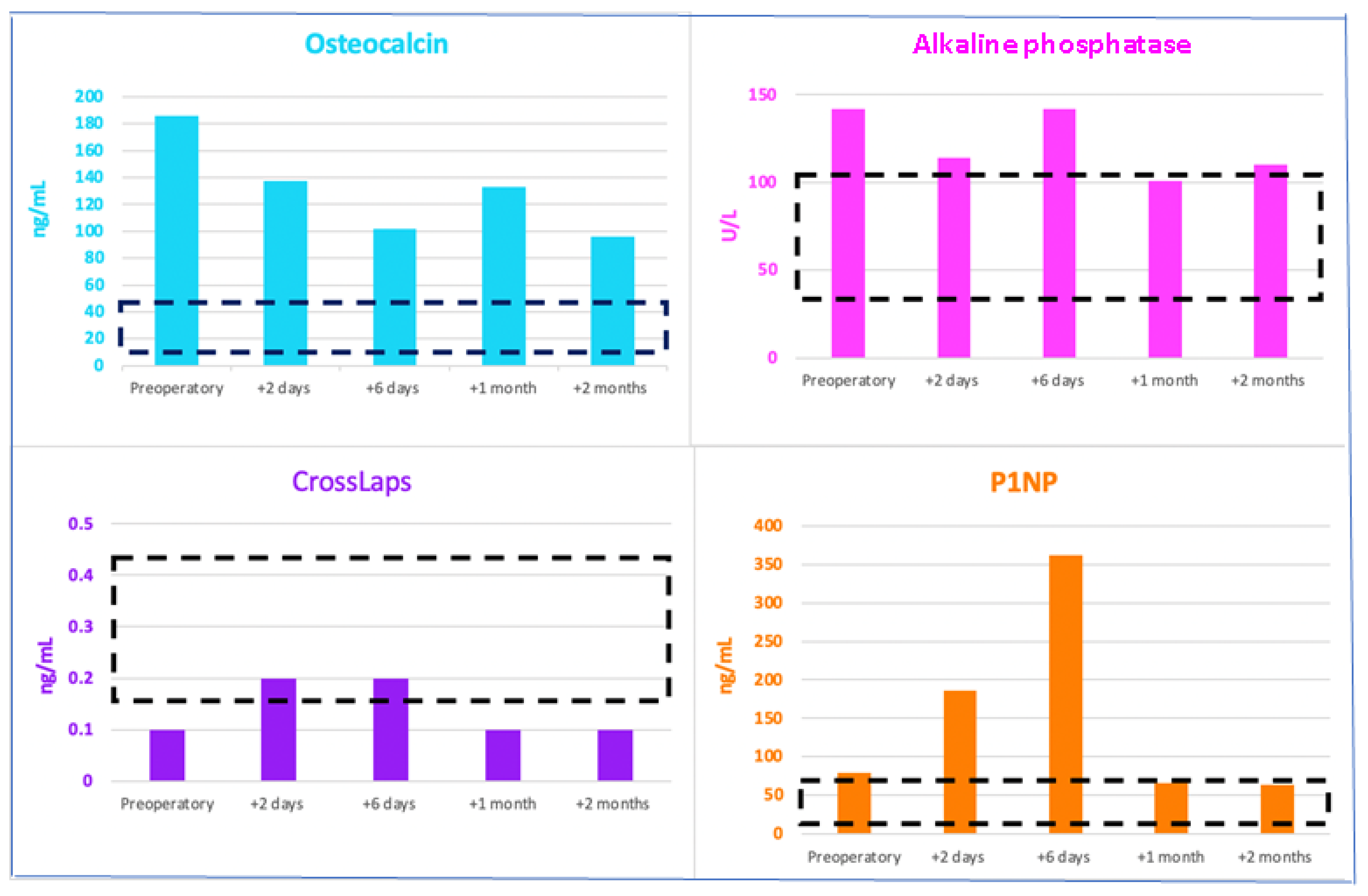
| Serum Bone Turnover Markers | On Admission | 2 Days after Surgery | 6 Days after Surgery | 1 Month after Surgery | 2 Months after Surgery | Normal Range |
|---|---|---|---|---|---|---|
| Bone Formation Markers | ||||||
| Osteocalcin (ng/mL) | 185.6 | 137.7 | 101.8 | 132.7 | 95.9 | 11–43 |
| Alkaline phosphatase (U/L) | 142 | 114 | 142 | 101 | 110 | 38–105 |
| P1NP (ng/mL) | 79.23 | 186.1 | 362.1 | 65.6 | 63.7 | 15.13–58.59 |
| Bone Resorption Marker | ||||||
| CrossLaps (ng/mL) | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.162–0.436 |
| Parameters | First Admission | 7 Months Later | 2 Years Later | Normal Values |
|---|---|---|---|---|
| Total serum calcium (mg/dL) | 7.3 | 8.4 | 9.75 | 8.8–10.6 |
| Phosphorus (mg/dL) | 3.7 | 3.4 | 3.3 | 2.5–4.5 |
| iPTH (pg/mL) | 2008 | 1605 | 44 | 15–65 |
| Alkaline phosphatase (U/L) | 1893 | 933 | 211 | 30–120 |
| pH | 7.1 | 7.37 | 7.36 | 7.35–7.45 |
| Bicarbonate level (mmol/L) | 12 | 17 | 18 | 22–29 |
| Hemoglobin (g/dL) | 6 | 11.5 | 12 | 12–15.5 |
| Albumin (g/dL) | 3.2 | 3.7 | 4.1 | 3.5–5.2 |
| Recommended Therapy with Vitamin D Analogs | ||||
| Paricalcitol (mg/day) | 1 | 2 | 2 | |
| Calcitriol (µg/day) | 0.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsote, M.; Ciobica, M.-L.; Sima, O.-C.; Valea, A.; Bondor, C.I.; Geleriu, A.; Ticolea, M.; Nistor, C.; Rusu, C.C. Brown Tumors: The Hidden Face of Primary and Renal Hyperparathyroidism Amid Real-Life Settings. J. Clin. Med. 2024, 13, 3847. https://doi.org/10.3390/jcm13133847
Carsote M, Ciobica M-L, Sima O-C, Valea A, Bondor CI, Geleriu A, Ticolea M, Nistor C, Rusu CC. Brown Tumors: The Hidden Face of Primary and Renal Hyperparathyroidism Amid Real-Life Settings. Journal of Clinical Medicine. 2024; 13(13):3847. https://doi.org/10.3390/jcm13133847
Chicago/Turabian StyleCarsote, Mara, Mihai-Lucian Ciobica, Oana-Claudia Sima, Ana Valea, Cosmina Ioana Bondor, Andreea Geleriu, Madalina Ticolea, Claudiu Nistor, and Crina Claudia Rusu. 2024. "Brown Tumors: The Hidden Face of Primary and Renal Hyperparathyroidism Amid Real-Life Settings" Journal of Clinical Medicine 13, no. 13: 3847. https://doi.org/10.3390/jcm13133847
APA StyleCarsote, M., Ciobica, M.-L., Sima, O.-C., Valea, A., Bondor, C. I., Geleriu, A., Ticolea, M., Nistor, C., & Rusu, C. C. (2024). Brown Tumors: The Hidden Face of Primary and Renal Hyperparathyroidism Amid Real-Life Settings. Journal of Clinical Medicine, 13(13), 3847. https://doi.org/10.3390/jcm13133847










