Effects of Probiotic Supplementation during Chronic Rhinosinusitis on the Microbiome
Abstract
1. Introduction
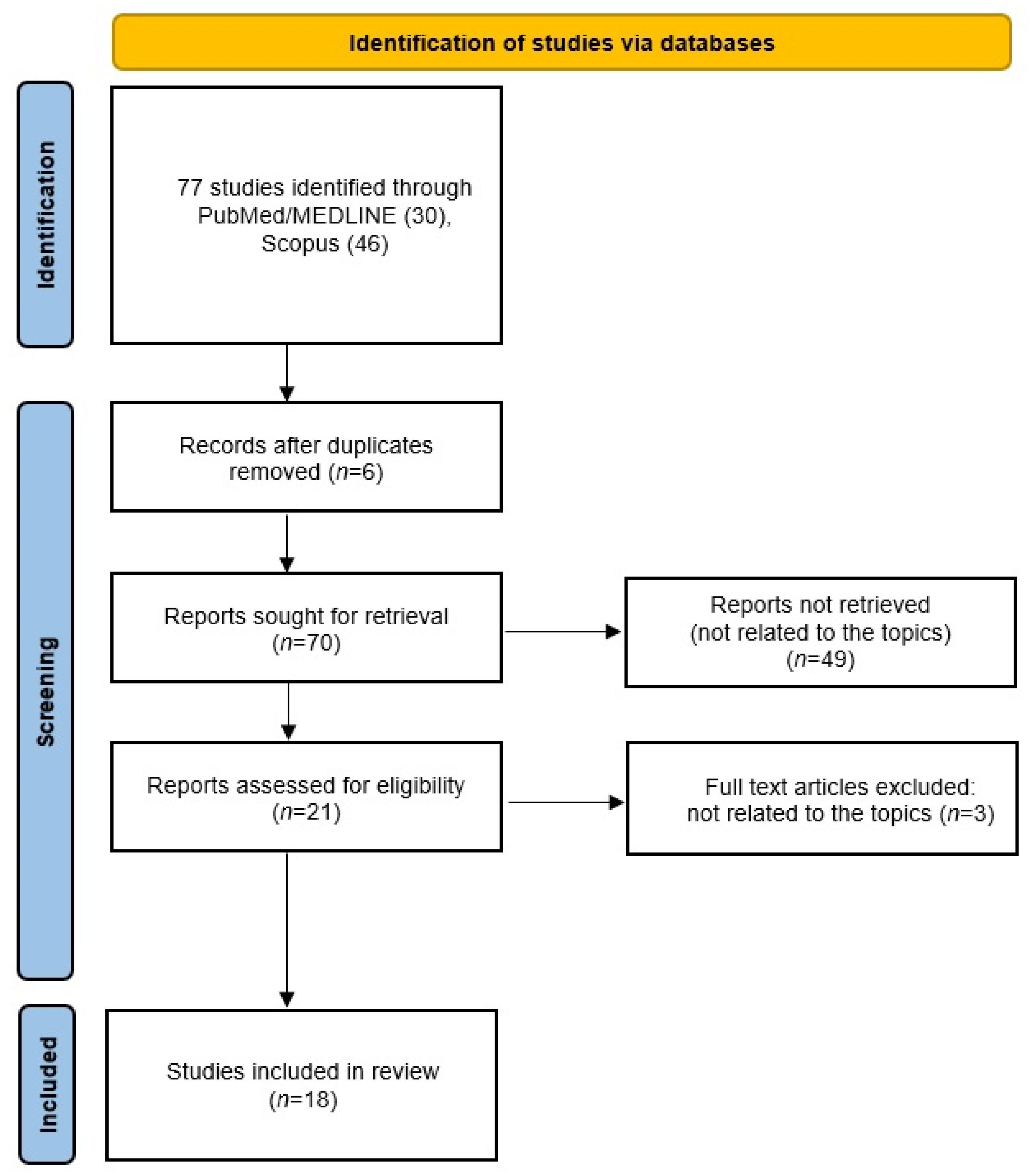
2. Methods
3. Probiotics in Chronic Rhinosinusitis
4. Microbiome in Chronic Rhinosinusitis
5. Therapeutic Applications of Lactic Acid Bacteria Probiotics
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Boeck, I.; Spacova, I.; Vanderveken, O.M.; Lebeer, S. Lactic Acid Bacteria as Probiotics for the Nose? Microb. Biotechnol. 2021, 14, 859–869. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, A.A.; Agu, R.U.; Massoud, E. Models for the Study of Nasal and Sinus Physiology in Health and Disease: A Review of the Literature. Laryngoscope Investig. Otolaryngol. 2017, 2, 398. [Google Scholar] [CrossRef]
- Berger, G.; Kogan, T.; Paker, M.; Berger-Achituv, S.; Ebner, Y. Pediatric Chronic Rhinosinusitis Histopathology. Otolaryngol. Neck Surg. 2011, 144, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Akdis, C.A.; Bachert, C.; Bousquet, J.; Pugin, B.; Adriaensen, G.; Advani, R.; Agache, I.; Anjo, C.; Anmolsingh, R.; et al. EUFOREA Rhinology Research Forum 2016: Report of the Brainstorming Sessions on Needs and Priorities in Rhinitis and Rhinosinusitis. Rhinology 2017, 55, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Arcimowicz, M.; Niemczyk, K. EPOS 2020: What’s New for Physician Practitioners? Pol. Przegląd Otorynolaryngologiczny 2020, 9, 7–17. [Google Scholar] [CrossRef]
- De Boeck, I.; Wittouck, S.; Martens, K.; Claes, J.; Jorissen, M.; Steelant, B.; van den Broek, M.F.L.; Seys, S.F.; Hellings, P.W.; Vanderveken, O.M.; et al. Anterior Nares Diversity and Pathobionts Represent Sinus Microbiome in Chronic Rhinosinusitis. mSphere 2019, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Andaloro, C.; Santagati, M.; Stefani, S.; La Mantia, I. Bacteriotherapy with Streptococcus Salivarius 24SMB and Streptococcus Oralis 89a Oral Spray for Children with Recurrent Streptococcal Pharyngotonsillitis: A Randomized Placebo-Controlled Clinical Study. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 879–887. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive Summary of EPOS 2020 Including Integrated Care Pathways. Rhinol. J. 2020, 58, 82–111. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Mackenzie, B.W.; Cope, E.K.; Ramakrishnan, V.R. Unraveling the Role of the Microbiome in Chronic Rhinosinusitis. J. Allergy Clin. Immunol. 2022, 149, 1513–1521. [Google Scholar] [CrossRef]
- Lambert, P.A.; Gill, A.L.; Gill, S.R.; Allen, P.D.; Man, L. Microbiomics of Irrigation with Xylitol or Lactococcus Lactis in Chronic Rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2021, 6, 64–70. [Google Scholar] [CrossRef]
- Endam, L.M.; Alromaih, S.; Gonzalez, E.; Madrenas, J.; Cousineau, B.; Renteria, A.E.; Desrosiers, M. Intranasal Application of Lactococcus Lactis W136 Is Safe in Chronic Rhinosinusitis Patients With Previous Sinus Surgery. Front. Cell. Infect. Microbiol. 2020, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sun, Y.; Zhao, N.; Song, J.; Zhang, N.; Liu, L.; Liu, Q. Characteristics of the Bacterial Microbiota in the Upper Respiratory Tract of Children. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Elwany, S.; Gamea, M.A.; Talaat, I. Passive Smoking Induces Nasal Biofilms in Children. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110755. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.N.; Franks, Z.G.; McCrary, H.C.; Saleh, A.A.; Chang, E.H. A Systematic Review of the Association between Cigarette Smoke Exposure and Chronic Rhinosinusitis. Otolaryngology 2018, 158, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, A.A.; Abolhalaj, M.; Lindstedt, M.; Mårtensson, A.A.; Olofsson, T.C.; Vásquez, A.; Greiff, L.; Cervin, A. Clinical Efficacy of a Topical Lactic Acid Bacterial Microbiome in Chronic Rhinosinusitis: A Randomized Controlled Trial. Laryngoscope Investig. Otolaryngol. 2017, 2, 410. [Google Scholar] [CrossRef] [PubMed]
- Szaleniec, J.; Gibała, A.; Pobiega, M.; Parasion, S.; Składzień, J.; Stręk, P.; Gosiewski, T.; Szaleniec, M. Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy. Antibiotics 2019, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus Microbiome Diversity Depletion and Corynebacterium Tuberculostearicum Enrichment Mediates Rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124. [Google Scholar] [CrossRef]
- Calò, L.; Passàli, G.C.; Galli, J.; Fadda, G.; Paludetti, G. Role of Biofilms in Chronic Inflammatory Diseases of the Upper Airways. Adv. Otorhinolaryngol. 2011, 72, 93–96. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Spacova, I.; Dodiya, H.B.; Happel, A.U.; Strain, C.; Vandenheuvel, D.; Wang, X.; Reid, G. Future of Probiotics and Prebiotics and the Implications for Early Career Researchers. Front. Microbiol. 2020, 11, 1400. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Aragona, S.E.; Drago, L.; Mantia, I. La The Nutraceuticals: A New Therapeutic Strategy in the Management of Digestive and Respiratory Disorders. Acta Bio Medica Atenei Parm. 2019, 90, 5. [Google Scholar] [CrossRef]
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Copeland, E.; Leonard, K.; Carney, R.; Kong, J.; Forer, M.; Naidoo, Y.; Oliver, B.G.G.; Seymour, J.R.; Woodcock, S.; Burke, C.M.; et al. Chronic Rhinosinusitis: Potential Role of Microbial Dysbiosis and Recommendations for Sampling Sites. Front. Cell. Infect. Microbiol. 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, I.; van den Broek, M.F.L.; Allonsius, C.N.; Spacova, I.; Wittouck, S.; Martens, K.; Wuyts, S.; Cauwenberghs, E.; Jokicevic, K.; Vandenheuvel, D.; et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 2020, 31, 107674. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 550348. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 560612. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Dicks, L.M.T.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at War against Viruses: What Is Missing from the Picture? Front. Microbiol. 2020, 11, 560166. [Google Scholar] [CrossRef]
- Van Den Broek, M.F.L.; De Boeck, I.; Kiekens, F.; Boudewyns, A.; Vanderveken, O.M.; Lebeer, S. Translating Recent Microbiome Insights in Otitis Media into Probiotic Strategies. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef]
- Man, W.H.; De Steenhuijsen Piters, W.A.A.; Bogaert, D. The Microbiota of the Respiratory Tract: Gatekeeper to Respiratory Health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Martens, K.; Pugin, B.; De Boeck, I.; Spacova, I.; Steelant, B.; Seys, S.F.; Lebeer, S.; Hellings, P.W. Probiotics for the Airways: Potential to Improve Epithelial and Immune Homeostasis. Allergy 2018, 73, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Martens, K.; De Boeck, I.; Jokicevic, K.; Kiekens, F.; Farré, R.; Vanderveken, O.M.; Seys, S.F.; Lebeer, S.; Hellings, P.W.; Steelant, B. Lacticaseibacillus Casei AMBR2 Restores Airway Epithelial Integrity in Chronic Rhinosinusitis with Nasal Polyps. Allergy. Asthma Immunol. Res. 2021, 13, 560–575. [Google Scholar] [CrossRef]
- Suzuki, M.; Cooksley, C.; Suzuki, T.; Ramezanpour, M.; Nakazono, A.; Nakamaru, Y.; Homma, A.; Vreugde, S. TLR Signals in Epithelial Cells in the Nasal Cavity and Paranasal Sinuses. Front. Allergy 2021, 2, 780425. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Chang, A.; Hoggard, M.; Radcliff, F.J.; Jiang, Y.; Taylor, M.W.; Darveau, R.; Douglas, R.G. Toll-like Receptor Activation by Sino-Nasal Mucus in Chronic Rhinosinusitis. Rhinology 2017, 55, 59–69. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.P.; O’Connell Motherway, M.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of Probiotic Effector Molecules: Present State and Future Perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Li, P.; Pang, K.; Liu, H.; Tian, L. Epithelial Barrier in the Nasal Mucosa, Related Risk Factors and Diseases. Int. Arch. Allergy Immunol. 2023, 184, 481–501. [Google Scholar] [CrossRef]
- De Rudder, C.; Garcia-Tímermans, C.; De Boeck, I.; Lebeer, S.; Van de Wiele, T.; Calatayud Arroyo, M. Lacticaseibacillus Casei AMBR2 Modulates the Epithelial Barrier Function and Immune Response in a Donor-Derived Nasal Microbiota Manner. Sci. Rep. 2020, 10, 16939. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell. Microbiol. 2014, 16, 1024. [Google Scholar] [CrossRef]
- Noecker, C.; McNally, C.P.; Eng, A.; Borenstein, E. High-Resolution Characterization of the Human Microbiome. Transl. Res. 2017, 179, 7. [Google Scholar] [CrossRef]
- Desrosiers, M.; Pereira Valera, F.C. Brave New (Microbial) World: Implications for Nasal and Sinus Disorders. Braz. J. Otorhinolaryngol. 2019, 85, 675. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Garaiova, I.; Paduchová, Z.; Nagyová, Z.; Wang, D.; Michael, D.R.; Plummer, S.F.; Marchesi, J.R.; Ďuračková, Z.; Muchová, J. Probiotics with Vitamin C for the Prevention of Upper Respiratory Tract Symptoms in Children Aged 3-10 Years: Randomised Controlled Trial. Benef. Microbes 2021, 12, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, M.; Keshavarzian, A.; Tobin, M.C.; Landay, A.L.; Schleimer, R.P. A Comprehensive Review of the Nasal Microbiome in Chronic Rhinosinusitis (CRS). Clin. Exp. Allergy 2016, 46, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Losol, P.; Park, H.S.; Song, W.J.; Hwang, Y.K.; Kim, S.H.; Holloway, J.W.; Chang, Y.S. Association of Upper Airway Bacterial Microbiota and Asthma: Systematic Review. Asia Pac. Allergy 2022, 12, e32. [Google Scholar] [CrossRef]
- Azevedo, A.C.; Hilário, S.; Gonçalves, M.F.M. Microbiome in Nasal Mucosa of Children and Adolescents with Allergic Rhinitis: A Systematic Review. Children 2023, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.; Bork, P.; Fraser, C.; Knight, R.; Wang, J. The Microbiome Explored: Recent Insights and Future Challenges. Nat. Rev. Microbiol. 2013, 11, 213–217. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Kumpitsch, C.; Koskinen, K.; Schöpf, V.; Moissl-Eichinger, C. The Microbiome of the Upper Respiratory Tract in Health and Disease. BMC Biol. 2019, 17, 87. [Google Scholar] [CrossRef]
- Neuman, H.; Forsythe, P.; Uzan, A.; Avni, O.; Koren, O. Antibiotics in Early Life: Dysbiosis and the Damage Done. FEMS Microbiol. Rev. 2018, 42, 489–499. [Google Scholar] [CrossRef]
- Hoggard, M.; Biswas, K.; Zoing, M.; Wagner Mackenzie, B.; Taylor, M.W.; Douglas, R.G. Evidence of Microbiota Dysbiosis in Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 230–239. [Google Scholar] [CrossRef]
- Koeller, K.; Herlemann, D.P.R.; Schuldt, T.; Ovari, A.; Guder, E.; Podbielski, A.; Kreikemeyer, B.; Olzowy, B. Microbiome and Culture Based Analysis of Chronic Rhinosinusitis Compared to Healthy Sinus Mucosa. Front. Microbiol. 2018, 9, 335624. [Google Scholar] [CrossRef]
- Dimitri-Pinheiro, S.; Soares, R.; Barata, P. The Microbiome of the Nose—Friend or Foe? Allergy Rhinol. 2020, 11, 215265672091160. [Google Scholar] [CrossRef]
- Lal, D.; Keim, P.; Delisle, J.; Barker, B.; Rank, M.A.; Chia, N.; Schupp, J.M.; Gillece, J.D.; Cope, E.K. Mapping and Comparing Bacterial Microbiota in the Sinonasal Cavity of Healthy, Allergic Rhinitis, and Chronic Rhinosinusitis Subjects. Int. Forum Allergy Rhinol. 2017, 7, 561–569. [Google Scholar] [CrossRef]
- Stearns, J.C.; Davidson, C.J.; Mckeon, S.; Whelan, F.J.; Fontes, M.E.; Schryvers, A.B.; Bowdish, D.M.E.; Kellner, J.D.; Surette, M.G. Culture and Molecular-Based Profiles Show Shifts in Bacterial Communities of the Upper Respiratory Tract That Occur with Age. ISME J. 2015, 9, 1246. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Luo, Y.; Yuan, L.; Nelson, K.E.; Wang, Y.; Xiang, C.; Li, L. Pyrosequencing Analysis of the Human Microbiota of Healthy Chinese Undergraduates. BMC Genom. 2013, 14, 390. [Google Scholar] [CrossRef]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early Respiratory Microbiota Composition Determines Bacterial Succession Patterns and Respiratory Health in Children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef]
- Jensen, A.; Fagö-Olsen, H.; Sørensen, C.H.; Kilian, M. Molecular Mapping to Species Level of the Tonsillar Crypt Microbiota Associated with Health and Recurrent Tonsillitis. PLoS ONE 2013, 8, e56418. [Google Scholar] [CrossRef]
- Paramasivan, S.; Bassiouni, A.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.; et al. The International Sinonasal Microbiome Study: A Multicentre, Multinational Characterization of Sinonasal Bacterial Ecology. Allergy 2020, 75, 2033–2045. [Google Scholar] [CrossRef]
- Bassiouni, A.; Paramasivan, S.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.S.; et al. Microbiotyping the Sinonasal Microbiome. Front. Cell. Infect. Microbiol. 2020, 10, 137. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Shilts, M.H.; Tovchigrechko, A.; Schobel, S.; Chappell, J.D.; Larkin, E.K.; Gebretsadik, T.; Halpin, R.A.; Nelson, K.E.; Moore, M.L.; et al. Nasopharyngeal Lactobacillus Is Associated with a Reduced Risk of Childhood Wheezing Illnesses Following Acute Respiratory Syncytial Virus Infection in Infancy. J. Allergy Clin. Immunol. 2018, 142, 1447. [Google Scholar] [CrossRef]
- Cope, E.K.; Goldberg, A.N.; Pletcher, S.D.; Lynch, S.V. Compositionally and Functionally Distinct Sinus Microbiota in Chronic Rhinosinusitis Patients Have Immunological and Clinically Divergent Consequences. Microbiome 2017, 5, 53. [Google Scholar] [CrossRef]
- van den Broek, M.F.L.; de Boeck, I.; Claes, I.J.J.; Nizet, V.; Lebeer, S. Multifactorial Inhibition of Lactobacilli against the Respiratory Tract Pathogen Moraxella Catarrhalis. Benef. Microbes 2018, 9, 429–439. [Google Scholar] [CrossRef]
- Spacova, I.; De Boeck, I.; Cauwenberghs, E.; Delanghe, L.; Bron, P.A.; Henkens, T.; Simons, A.; Gamgami, I.; Persoons, L.; Claes, I.; et al. Development of a Live Biotherapeutic Throat Spray with Lactobacilli Targeting Respiratory Viral Infections. Microb. Biotechnol. 2023, 16, 99–115. [Google Scholar] [CrossRef]
- De Boeck, I.; Wittouck, S.; Wuyts, S.; Oerlemans, E.F.M.; van den Broek, M.F.L.; Vandenheuvel, D.; Vanderveken, O.; Lebeer, S. Comparing the Healthy Nose and Nasopharynx Microbiota Reveals Continuity as Well as Niche-Specificity. Front. Microbiol. 2017, 8, 290805. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Salvetti, E.; O’Toole, P.W. When Regulation Challenges Innovation: The Case of the Genus Lactobacillus. Trends Food Sci. Technol. 2017, 66, 187–194. [Google Scholar] [CrossRef]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; Linder, J.A.; Shane, A.L.; Merenstein, D. Does Probiotic Consumption Reduce Antibiotic Utilization for Common Acute Infections? A Systematic Review and Meta-Analysis. Eur. J. Public Health 2019, 29, 494–499. [Google Scholar] [CrossRef]
- La Mantia, I.; Gelardi, M.; Drago, L.; Aragona, S.E.; Cupido, G.; Vicini, C.; Berardi, C.; Ciprandi, G. Probiotics in the Add-on Treatment of Pharyngotonsillitis: A Clinical Experience. J. Biol. Regul. Homeost. Agents 2020, 34, 11–18. [Google Scholar]
- La Mantia, I.; Gelardi, M.; Drago, L.; Aragona, S.E.; Cupido, G.; Vicini, C.; Berardi, C.; Ciprandi, G. Probiotics in the Add-on Treatment of Laryngotracheitis: A Clinical Experience. J. Biol. Regul. Homeost. Agents 2020, 34, 35–40. [Google Scholar]
- Gelardi, M.; La Mantia, I.; Drago, L.; Meroni, G.; Aragona, S.E.; Cupido, G.; Vicini, C.; Berardi, C.; Ciprandi, G. Probiotics in the Add-on Treatment of Otitis Media in Clinical Practice. J. Biol. Regul. Homeost. Agents 2020, 34, 19–26. [Google Scholar]
- Gan, W.; Yang, F.; Tang, Y.; Zhou, D.; Qing, D.; Hu, J.; Liu, S.; Liu, F.; Meng, J. The Difference in Nasal Bacterial Microbiome Diversity between Chronic Rhinosinusitis Patients with Polyps and a Control Population. Int. Forum Allergy Rhinol. 2019, 9, 582–592. [Google Scholar] [CrossRef]
- Lappan, R.; Imbrogno, K.; Sikazwe, C.; Anderson, D.; Mok, D.; Coates, H.; Vijayasekaran, S.; Bumbak, P.; Blyth, C.C.; Jamieson, S.E.; et al. A Microbiome Case-Control Study of Recurrent Acute Otitis Media Identified Potentially Protective Bacterial Genera. BMC Microbiol. 2018, 18, 13. [Google Scholar] [CrossRef]
- Brugger, S.D.; Eslami, S.M.; Pettigrew, M.M.; Escapa, I.F.; Henke, M.T.; Kong, Y.; Lemon, K.P. Dolosigranulum Pigrum Cooperation and Competition in Human Nasal Microbiota. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standyło, A.; Obuchowska, A.; Horaczyńska-Wojtaś, A.; Mielnik-Niedzielska, G. Effects of Probiotic Supplementation during Chronic Rhinosinusitis on the Microbiome. J. Clin. Med. 2024, 13, 1726. https://doi.org/10.3390/jcm13061726
Standyło A, Obuchowska A, Horaczyńska-Wojtaś A, Mielnik-Niedzielska G. Effects of Probiotic Supplementation during Chronic Rhinosinusitis on the Microbiome. Journal of Clinical Medicine. 2024; 13(6):1726. https://doi.org/10.3390/jcm13061726
Chicago/Turabian StyleStandyło, Arkadiusz, Aleksandra Obuchowska, Anna Horaczyńska-Wojtaś, and Grażyna Mielnik-Niedzielska. 2024. "Effects of Probiotic Supplementation during Chronic Rhinosinusitis on the Microbiome" Journal of Clinical Medicine 13, no. 6: 1726. https://doi.org/10.3390/jcm13061726
APA StyleStandyło, A., Obuchowska, A., Horaczyńska-Wojtaś, A., & Mielnik-Niedzielska, G. (2024). Effects of Probiotic Supplementation during Chronic Rhinosinusitis on the Microbiome. Journal of Clinical Medicine, 13(6), 1726. https://doi.org/10.3390/jcm13061726





