Extent of Endoscopic Sinus Surgery for Odontogenic Sinusitis of Endodontic Origin with Ethmoid and Frontal Sinus Involvement
Abstract
1. Introduction
Aim
2. Materials and Methods
2.1. Data and Outcome Measures
2.2. Statistical Analysis
2.3. Statement of Ethics
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turfe, Z.; Ahmad, A.; Peterson, E.I.; Craig, J.R. Odontogenic sinusitis is a common cause of unilateral sinus disease with maxillary sinus opacification. Int. Forum Allergy Rhinol. 2019, 9, 1515–1520. [Google Scholar] [CrossRef]
- Goyal, V.K.; Ahmad, A.; Turfe, Z.; Peterson, E.I.; Craig, J.R. Predicting Odontogenic Sinusitis in Unilateral Sinus Disease: A Prospective, Multivariate Analysis. Am. J. Rhinol. Allergy 2021, 35, 164–171. [Google Scholar] [CrossRef]
- Craig, J.R. Odontogenic Sinusitis: A State-Of-The-Art Review. World J. Otorhinolaryngol. Head Neck Surg. 2022, 8, 8–15. [Google Scholar] [CrossRef]
- Craig, J.R.; Tataryn, R.W.; Aghaloo, T.L.; Pokorny, A.T.; Gray, S.T.; Mattos, J.L.; Poetker, D.M. Management of Odontogenic Sinusitis: Multidisciplinary Consensus Statement. In International Forum of Allergy and Rhinology; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2020; Volume 10, pp. 901–912. [Google Scholar]
- Tomomatsu, N.; Uzawa, N.; Aragaki, T.; Harada, K. Aperture width of the osteomeatal complex as a predictor of successful treatment of odontogenic maxillary sinusitis. Int. J. Oral. Maxillofac. Surg. 2014, 43, 1386–1390. [Google Scholar] [CrossRef]
- Psillas, G.; Papaioannou, D.; Petsali, S.; Dimas, G.G.; Constantinidis, J. Odontogenic maxillary sinusitis: A comprehensive review. J. Dent. Sci. 2021, 16, 474–481. [Google Scholar] [CrossRef]
- Craig, J.R.; McHugh, C.I.; Griggs, Z.H.; Peterson, E.I. Optimal timing of endoscopic sinus surgery for odontogenic sinusitis. Laryngoscope 2019, 129, 1976–1983. [Google Scholar] [CrossRef]
- Craig, J.R.; Tataryn, R.W.; Cha, B.Y.; Bhargava, P.; Pokorny, A.; Gray, S.T.; Mattos, J.L.; Poetker, D.M. Diagnosing odontogenic sinusitis of endodontic origin: A multidisciplinary literature review. Am. J. Otolaryngol. 2021, 42, 102925. [Google Scholar] [CrossRef]
- Sakkas, A.; Weiß, C.; Ebeling, M.; Pietzka, S.; Wilde, F.; Evers, T.; Thiele, O.C.; Mischkowski, R.A.; Scheurer, M. Factors Influencing Recurrence after Surgical Treatment of Odontogenic Maxillary Sinusitis: An Analysis from the Oral and Maxillofacial Surgery Point of View. J. Clin. Med. 2023, 12, 3670. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Corbella, S.; Sequeira-Byron, P.; Tsesis, I.; Rosen, E.; Lolato, A.; Taschieri, S. Endodontic procedures for retreatment of periapical lesions. In Cochrane Database of Systematic Reviews; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2016; Volume 2016. [Google Scholar]
- Cintra, L.T.A.; Estrela, C.; Azuma, M.M.; Queiroz Índia, O.d.A.; Kawai, T.; Gomes-Filho, J.E. Endodontic medicine: Interrelationships among apical periodontitis, systemic disorders, and tissue responses of dental materials. Braz. Oral. Res. 2018, 32, 66–81. [Google Scholar] [CrossRef]
- Bordagaray, M.J.; Fernández, A.; Garrido, M.; Astorga, J.; Hoare, A.; Hernández, M. Systemic and Extraradicular Bacterial Translocation in Apical Periodontitis. Front. Cell Infect. Microbiol. 2021, 19, 11. [Google Scholar] [CrossRef]
- Panzarella, F.K.; Coelho, M.S.; Estrela, C. Association between odontogenic conditions and maxillary sinus abnormalities. Ann. Palliat. Med. 2023, 12, 887–890. [Google Scholar] [CrossRef]
- Dumitrescu, A.; Martu, M.A.; Nemtoi, A.; Sirghe, A.; Chelaru, L.; Tatarciuc, D.; Dumitrescu, A.M.; Haba, D. Association between cone-beam computed tomography and histological and immunohistochemical features in periapical lesions correlated with thickened maxillary sinus mucosa. Medicina 2021, 57, 840. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I. European Position Paper on Rhinosinusitis and Nasal Polyps. Dieudonné Nyenbue Tshipukane 2020, 103. [Google Scholar]
- Sato, K.; Chitose S ichi Sato, K.; Sato, F.; Ono, T.; Umeno, H. Pathophysiology of current odontogenic maxillary sinusitis and endoscopic sinus surgery preceding dental treatment. Auris Nasus Larynx. 2021, 48, 104–109. [Google Scholar] [CrossRef]
- Yassin-Kassab, A.; Peterson, E.L.; Craig, J.R. Total times to treatment completion and clinical outcomes in odontogenic sinusitis. Am. J. Otolaryngol. 2023, 44, 103921. [Google Scholar] [CrossRef]
- Ye, J.; Hu, S.; Bian, M.; Yuan, J.; Tang, J. Endoscopic sinus surgery plays an essential role in systematic treatment of odontogenic maxillary sinusitis. Laparosc. Endosc. Robot. Surg. 2018, 1, 19–23. [Google Scholar] [CrossRef]
- Dobroś, K.; Zarzecka, J. Dental assessment of odontogenic maxillary sinusitis, aided by Cone Beam Computed Tomography. Folia Med. Cracov. 2020, 60, 85–96. [Google Scholar]
- Saibene, A.M.; Collurà, F.; Pipolo, C.; Bulfamante, A.M.; Lozza, P.; Maccari, A.; Arnone, F.; Ghelma, F.; Allevi, F.; Biglioli, F.; et al. Odontogenic rhinosinusitis and sinonasal complications of dental disease or treatment: Prospective validation of a classification and treatment protocol. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 401–406. [Google Scholar] [CrossRef]
- Saibene, A.M.; Pipolo, C.; Borloni, R.; Felisati, G. Ent and dentist cooperation in the management of odontogenic sinusitis. A review. Acta Otorhinolaryngol. Ital. 2021, 41, 116–123. [Google Scholar] [CrossRef]
- Safadi, A.; Kleinman, S.; Oz, I.; Wengier, A.; Mahameed, F.; Vainer, I.; Ungar, O.J. Questioning the Justification of Frontal Sinusotomy for Odontogenic Sinusitis. J. Oral Maxillofac. Surg. 2020, 78, 762–770. [Google Scholar] [CrossRef]
- Crovetto-Martínez, R.; Martin-Arregui, F.J.; Zabala-López-de-Maturana, A.; Tudela-Cabello, K.; Crovetto-de la Torre, M.A. Frequency of the odontogenic maxillary sinusitis extended to the anterior ethmoid sinus and response to surgical treatment. Med. Oral. Patol. Oral. Cir. Bucal. 2014, 19, e409. [Google Scholar] [CrossRef]
- Mattos, J.L.; Ferguson, B.J.; Lee, S. Predictive factors in patients undergoing endoscopic sinus surgery for odontogenic sinusitis. Int. Forum Allergy Rhinol. 2016, 6, 697–700. [Google Scholar] [CrossRef]
- Sato, K.; Chitose S ichi Sato, K.; Sato, F.; Ono, T.; Umeno, H. Histopathology of maxillary sinus mucosa with odontogenic maxillary sinusitis. Laryngoscope Investig. Otolaryngol. 2020, 5, 205–209. [Google Scholar] [CrossRef]
- Soler, Z.M.; Jones, R.; Le, P.; Rudmik, L.; Mattos, J.L.; Nguyen, S.A.; Schlosser, R.J. Sino-Nasal outcome test-22 outcomes after sinus surgery: A systematic review and meta-analysis. Laryngoscope 2018, 128, 581–592. [Google Scholar] [CrossRef]
- Simuntis, R.; Vaitkus, J.; Kubilius, R.; Padervinskis, E.; Tušas, P.; Leketas, M.; Šiupšinskienė, N.; Vaitkus, S. Comparison of Sino-Nasal Outcome Test 22 Symptom Scores in Rhinogenic and Odontogenic Sinusitis. Am. J. Rhinol. Allergy 2019, 33, 44–50. [Google Scholar] [CrossRef]
- Skośkiewicz-Malinowska, K.; Kaczmarek, U.; Ziętek, M.; Malicka, B. Validation of the Polish version of the oral health impact profile-14. Adv. Clin. Exp. Med. 2015, 24, 129–137. [Google Scholar] [CrossRef]
- Georgalas, C.; Detsis, M.; Geramas, I.; Terzakis, D.; Liodakis, A. Quality of life outcomes in frontal sinus surgery. J. Clin. Med. 2020, 9, 2145. [Google Scholar] [CrossRef]
- Manfredini, M.; Pellegrini, M.; Rigoni, M.; Veronesi, V.; Beretta, M.; Maiorana, C.; Poli, P.P. Oral health-related quality of life in implant-supported rehabilitations: A prospective single-center observational cohort study. BMC Oral Health 2024, 24, 1. [Google Scholar] [CrossRef]
- Kim, S.; Kratchman, S. Modern Endodontic Surgery Concepts and Practice: A Review. J. Endod. 2006, 32, 601–623. [Google Scholar] [CrossRef]
- Kwiatkowska, M.A.; Szczygielski, K.; Brociek-Piłczyńska, A.; Chloupek, A.; Jurkiewicz, D. The Influence of Endodontic Lesions on The Clinical Evolution of Odontogenic Sinusitis—A Cohort Study. J. Clin. Med. 2023, 12, 1103. [Google Scholar] [CrossRef]
- Rangics, A.; Répássy, G.D.; Gyulai-Gaál, S.; Dobó-Nagy, C.; Tamás, L.; Simonffy, L. Management of Odontogenic Sinusitis: Results with Single-Step FESS and Dentoalveolar Surgery. J. Pers. Med. 2023, 13, 1291. [Google Scholar] [CrossRef]
- Little, R.E.; Long, C.M.; Loehrl, T.A.; Poetker, D.M. Odontogenic sinusitis: A review of the current literature. Laryngoscope Investig. Otolaryngol. 2018, 3, 110–114. [Google Scholar] [CrossRef]
- Craig, J.R.; Saibene, A.M.; Adappa, N.D.; Douglas, J.E.; Eide, J.G.; Felisati, G.; Kohanski, M.A.; Kshirsagar, R.S.; Kwiecien, C.; Lee, D.; et al. Maxillary Antrostomy Versus Complete Sinus Surgery for Odontogenic Sinusitis with Frontal Sinus Extension. Laryngoscope 2024, 27. [Google Scholar] [CrossRef]
- Gomes, P.; Gomes, A.; Salvador, P.; Lombo, C.; Caselhos, S.; Fonseca, R. Clinical assessment, diagnosis and management of patients with unilateral sinonasal disease. Acta Otorrinolaringol. Esp. 2020, 71, 16–25. [Google Scholar] [CrossRef]
- Allevi, F.; Fadda, G.L.; Rosso, C.; Martino, F.; Pipolo, C.; Cavallo, G.; Felisati, G.; Saibene, A.M. Treatment of Sinusitis Following Dental Implantation: A Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2022, 36, 539–549. [Google Scholar] [CrossRef]
- Ungar, O.J.; Yafit, D.; Kleinman, S.; Raiser, V.; Safadi, A. Odontogenic sinusitis involving the frontal sinus: Is middle meatal antrostomy enough? Eur. Arch. Otorhinolaryngol. 2018, 275, 2291–2295. [Google Scholar] [CrossRef]
- Craig, J.R.; Tataryn, R.W.; Sibley, H.C.; Mason, W.D.; Deuel, J.A.; Loyd, G.E.; Nerenz, D.R.; Goyal, P. Expected Costs of Primary Dental Treatments and Endoscopic Sinus Surgery for Odontogenic Sinusitis. Laryngoscope 2022, 132, 1346–1355. [Google Scholar] [CrossRef]
- Yoshida, H.; Sakashita, M.; Adachi, N.; Matsuda, S.; Fujieda, S.; Yoshimura, H. Relationship between infected tooth extraction and improvement of odontogenic maxillary sinusitis. Laryngoscope Investig. Otolaryngol. 2022, 7, 335–341. [Google Scholar] [CrossRef]
- Kato, T.; Sakagami, H. Augmentation of Therapeutic Efficacy of Extraction of Causative Teeth by Irrigation for Odontogenic Maxillary Sinusitis. In Vivo 2024, 38, 1236–1242. [Google Scholar] [CrossRef]
- Shukairy, M.K.; Burmeister, C.; Ko, A.B.; Craig, J.R. Recognizing odontogenic sinusitis: A national survey of otolaryngology chief residents. Am. J. Otolaryngol. 2020, 41, 102635. [Google Scholar] [CrossRef]

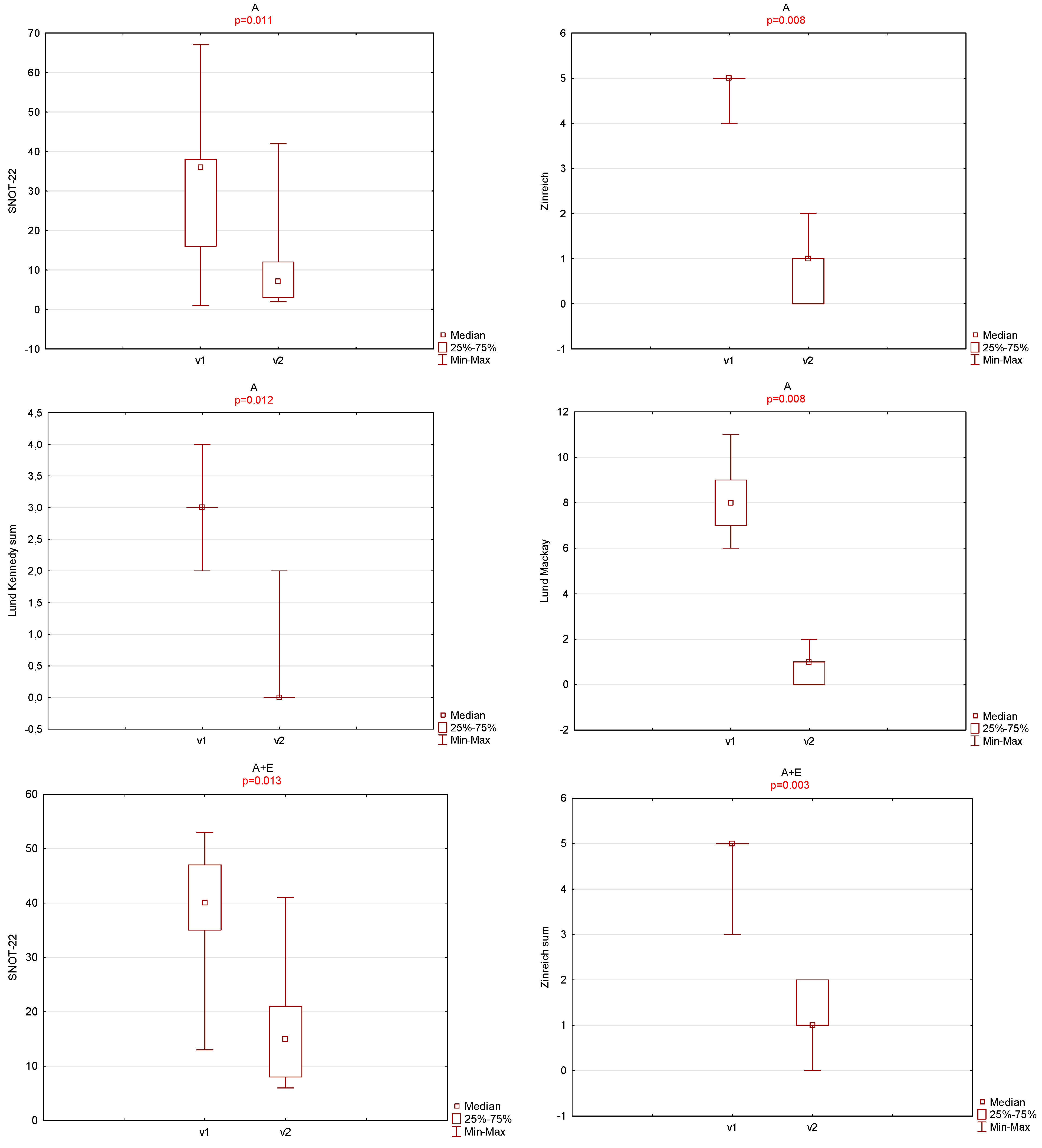
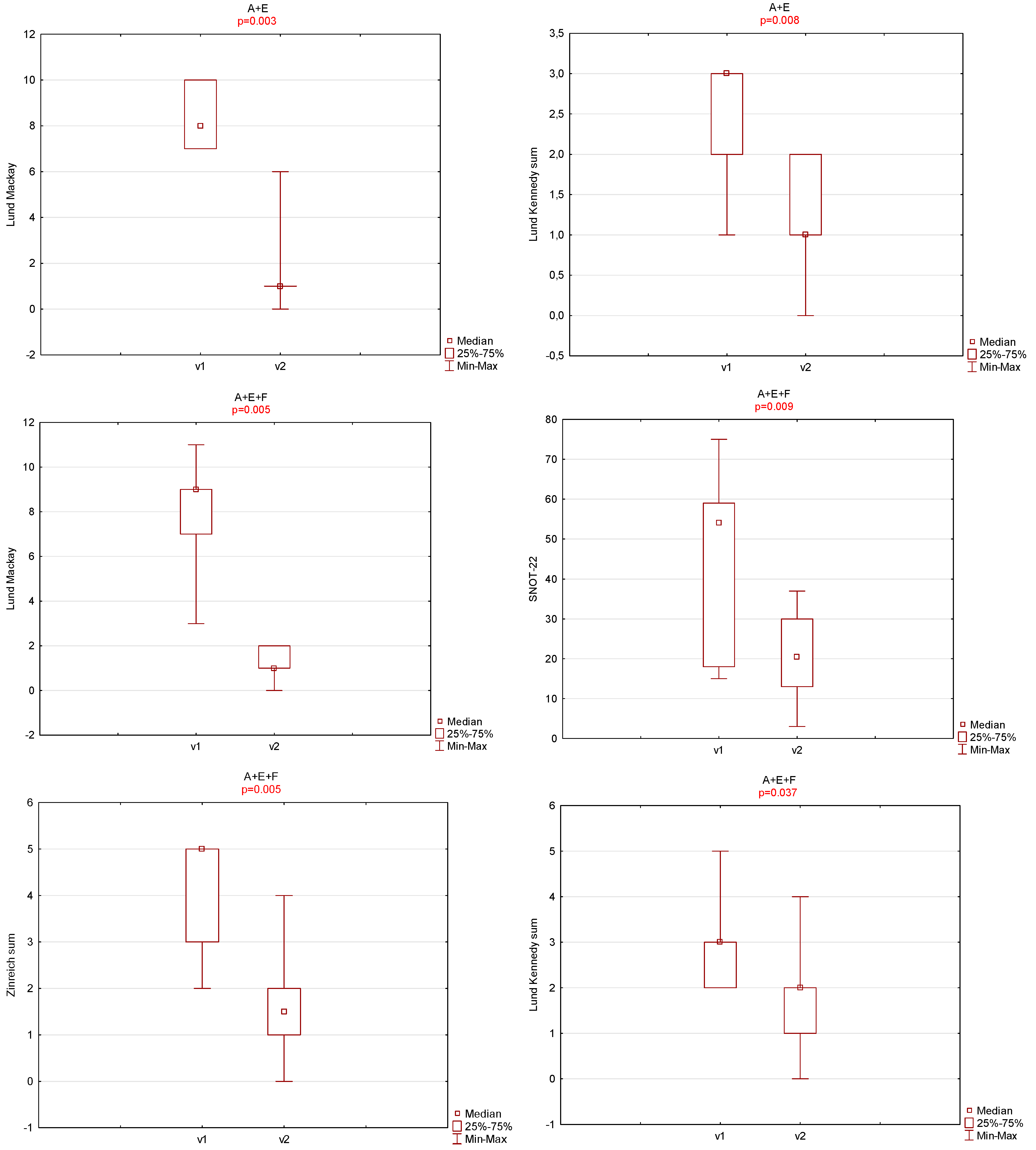
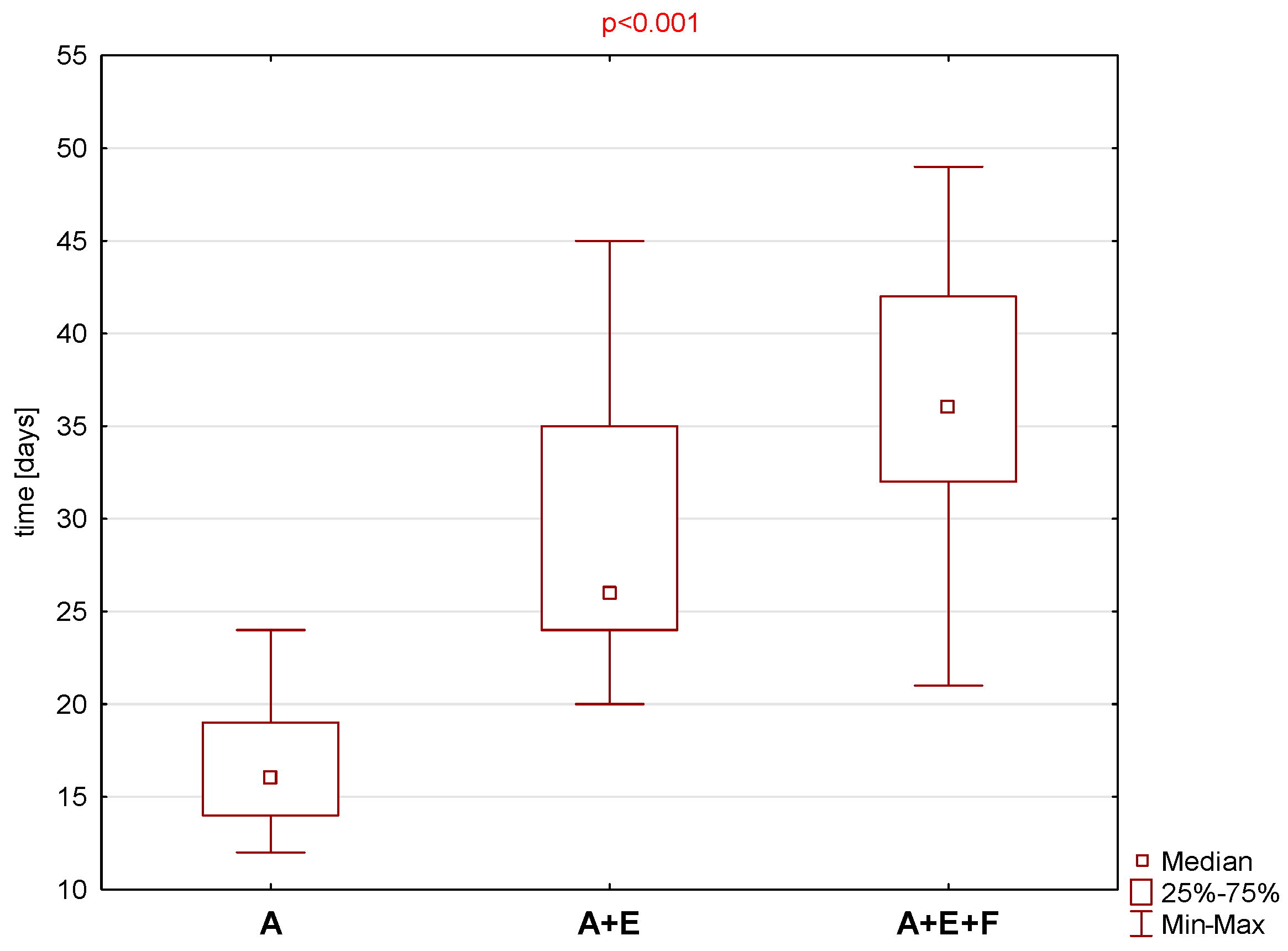
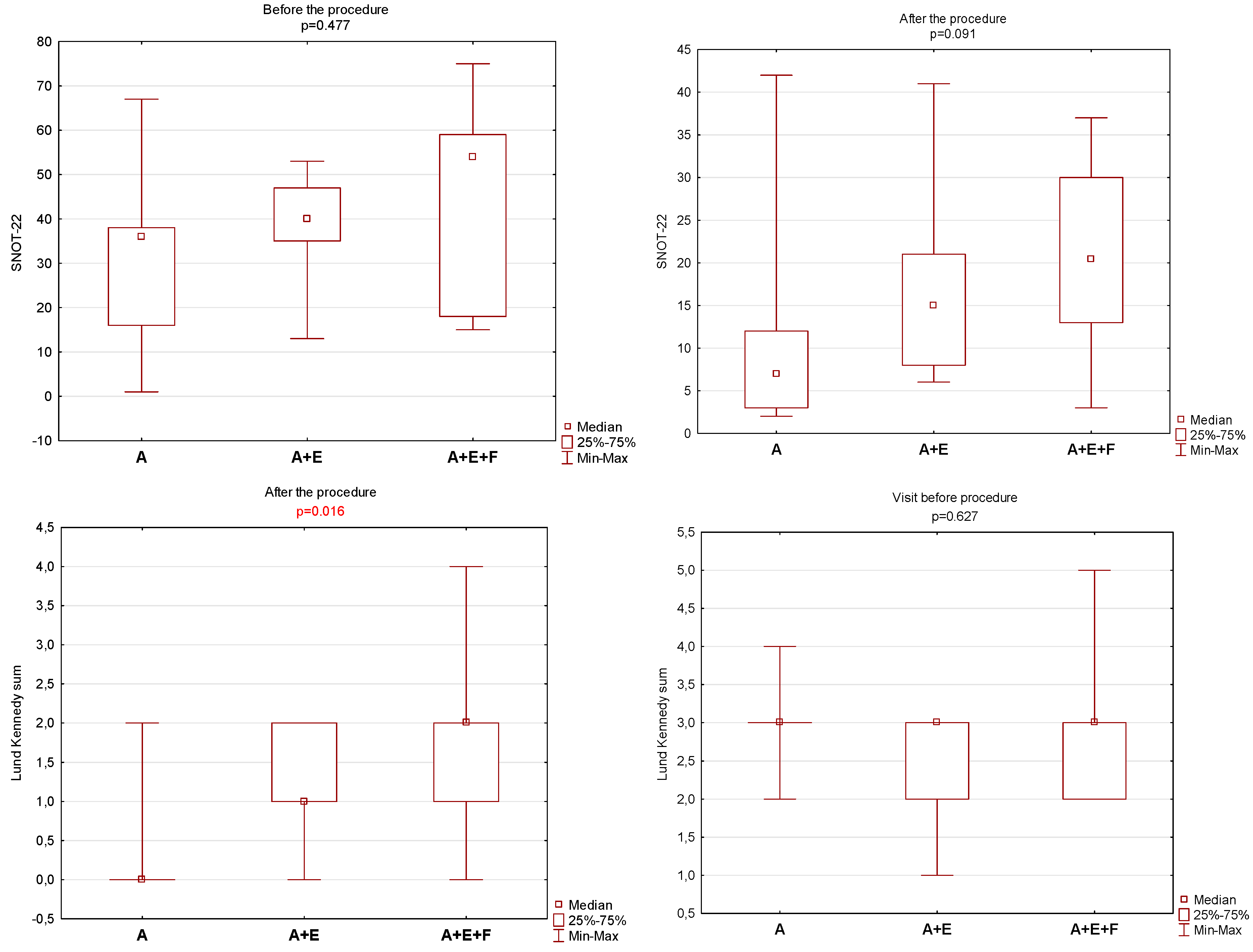
| OHIP-14 | SNOT-22 | Zinreich Scale Overall | Lund Kennedy Overall | BMI | Time to Total Recovery (Days) | |
|---|---|---|---|---|---|---|
| First visit | Groups A, A + E, A + E + F n = 30 | |||||
| Mean ± SD | 11.1 ± 11.0 | 38.8 ± 19.1 | 4.5 ± 0.9 | 2.8 ± 0.8 | 26.6 ± 5.0 | 28.0 ± 10.4 |
| Median (Min–Max) | 7 (0–46) | 37.5 (1–75) | 5 (2–5) | 3 (1–5) | 25.0 (18.4–40.5) | 26.0 (12.0–49.0) |
| Second visit | ||||||
| Mean ± SD | 5.0 ± 5.4 | 16.9 ± 11.8 | 1.3 ± 0.9 | 1.1 ± 1.1 | n/a | n/a |
| Median (Min–Max) | 3.5 (0–18) | 13.5 (2–42) | 1 (0–4) | 1 (0–4) | n/a | n/a |
| First visit | Group A n = 9 | |||||
| Mean ± SD | 6.2 ± 3.7 | 31.7 ± 22.6 | 4.8 ± 0.4 | 2.9 ± 0.6 | 26.3 ± 6.5 | 16.9 ± 3.9 |
| Median (Min–Max) | 7 (0–12) | 36 (1–67) | 5 (4–5) | 3 (2–4) | 24.5 (18.4–40.5) | 16 (12–24) |
| Second visit | ||||||
| Mean ± SD | 3.4 ± 5.8 | 11.8 ± 13.0 | 0.9 ± 0.8 | 0.3 ± 0.7 | n/a | n/a |
| Mediana (Min–Max) | 1 (0–18) | 7 (2–42) | 1 (0–2) | 0 (0–12) | n/a | n/a |
| First visit | Group A + E n = 11 | |||||
| Mean ± SD | 10.0 ± 9.4 | 39.0 ± 10.9 | 4.7 ± 0.6 | 2.5 ± 0.7 | 27.8 ± 4.8 | 29.9 ± 8.2 |
| Median (Min–Max) | 7 (0–29) | 40 (13–53) | 5 (3–5) | 3 (1–3) | 26.7 (22.1–37.2) | 26 (20–45) |
| Second visit | ||||||
| Mean ± SD | 6.4 ± 6.6 | 17.6 ± 10.9 | 1.3 ± 0.6 | 1.2 ± 0.8 | n/a | n/a |
| Median (Min–Max) | 5 (0–18) | 15 (6–41) | 1 (0–2) | 1 (0–2) | n/a | n/a |
| First visit | Group A + E + F n = 10 | |||||
| Mean ± SD | 45 ± 22.2 | 4.1 ± 1.3 | 2.9 ± 1.0 | 25.6 ± 3.9 | 35.8 ± 7.9 | |
| Median (Min–Max) | 54 (15–75) | 5 (2–5) | 3 (2–5) | 25.3 (18.7–31.0) | 36 (21–49) | |
| Second visit | ||||||
| Mean ± SD | 20.6 ± 11.0 | 1.6 ± 1.1 | 1.7 ± 1.3 | n/a | n/a | |
| Median (Min–Max) | 20.5 (3–37) | 1.5 (0–4) | 2 (0–4) | n/a | n/a | |
| Groups | Gender p = 0.753 | Affected Side p = 0.679 | |||
|---|---|---|---|---|---|
| Female | Male | Right | Left | ||
| A | 3 (33.3%) | 6 (66.7%) | 7 (77.8%) | 2 (22.2%) | |
| A + E | 5 (45.5%) | 6 (54.5%) | 8 (72.7%) | 3 (27.3%) | |
| A + E + F | 5 (50%) | 5 (50%) | 6 (60%) | 4 (40%) | |
| Comorbidities p = 0.396 | |||||
| None reported | Asthma | Allergy | Diabetes | Active Smoker | |
| A | 6 (66.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (33.3%) |
| A + E | 5 (45.4%) | 1 (9.1%) | 0 (0%) | 1 (9.1%) | 4 (36.4%) |
| A + E + F | 6 (60%) | 0 (0%) | 2 (20%) | 0 (0%) | 2 (20%) |
| Extent of Sinus Surgery | Crusting—0 | Crusting—1 | p-Value | Scarring—0 | Scarring—1 | p-Value |
|---|---|---|---|---|---|---|
| A | 9 (100%) | 0 | <0.001 | 8 (88.9%) | 1 (11.1%) | 0.363 |
| A + E | 11 (100%) | 0 | 8 (72.7%) | 3 (27.3%) | ||
| A + E + F | 4 (40%) | 6 (60%) | 6 (60%) | 4 (40%) |
| Extent of Surgical Treatment | Dental Pain (0—Absent/1—Present) | p-Value | |||
|---|---|---|---|---|---|
| V1 | V2 | ||||
| 0 | 1 | 0 | 1 | ||
| A | 7 (77.8%) | 2 (22.2%) | 9 (100%) | 0 (0%) | p = 0.471 |
| A + E | 7 (63.6%) | 4 (36.4%) | 8 (72.7%) | 3 (27.3%) | p = 0.987 |
| A + E + F | 4 (40%) | 6 (60%) | 10 (100%) | 0 (0%) | p = 0.011 |
| Overall | 18 (60%) | 12 (40%) | 27 (90%) | 3 (10%) | p = 0.0153 |
| Extent of Surgical Treatment | Foul Smell (0—Absent/1—Present) | p-Value | |||
| V1 | V2 | ||||
| 0 | 1 | 0 | 1 | ||
| A | 1 (11.1%) | 8 (88.9%) | 9 (100%) | 0 (0%) | p < 0.001 |
| A + E | 3 (27.3%) | 8 (72.7%) | 10 (90.9%) | 1 (9.1%) | p = 0.001 |
| A + E + F | 4 (40%) | 6 (60%) | 10 (100%) | 0 (0%) | p = 0.011 |
| Overall | 8 (26.7%) | 22 (73.3%) | 29 (96.7%) | 1 (3.3%) | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, M.A.; Szczygielski, K.; Jurkiewicz, D.; Rot, P. Extent of Endoscopic Sinus Surgery for Odontogenic Sinusitis of Endodontic Origin with Ethmoid and Frontal Sinus Involvement. J. Clin. Med. 2024, 13, 6204. https://doi.org/10.3390/jcm13206204
Kwiatkowska MA, Szczygielski K, Jurkiewicz D, Rot P. Extent of Endoscopic Sinus Surgery for Odontogenic Sinusitis of Endodontic Origin with Ethmoid and Frontal Sinus Involvement. Journal of Clinical Medicine. 2024; 13(20):6204. https://doi.org/10.3390/jcm13206204
Chicago/Turabian StyleKwiatkowska, Marta Aleksandra, Kornel Szczygielski, Dariusz Jurkiewicz, and Piotr Rot. 2024. "Extent of Endoscopic Sinus Surgery for Odontogenic Sinusitis of Endodontic Origin with Ethmoid and Frontal Sinus Involvement" Journal of Clinical Medicine 13, no. 20: 6204. https://doi.org/10.3390/jcm13206204
APA StyleKwiatkowska, M. A., Szczygielski, K., Jurkiewicz, D., & Rot, P. (2024). Extent of Endoscopic Sinus Surgery for Odontogenic Sinusitis of Endodontic Origin with Ethmoid and Frontal Sinus Involvement. Journal of Clinical Medicine, 13(20), 6204. https://doi.org/10.3390/jcm13206204






