Revealing the Unseen: Detecting Negative Symptoms in Students
Abstract
1. Introduction
2. Method
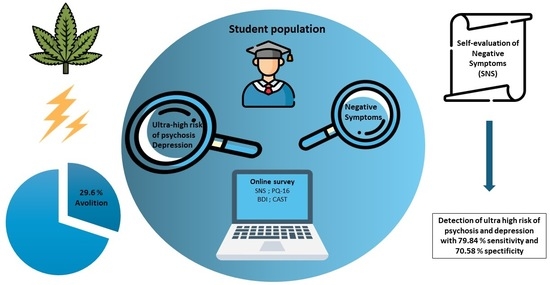
2.1. Population
2.2. Ethics
2.3. Assessments
2.4. Planned Statistical Analysis
3. Results
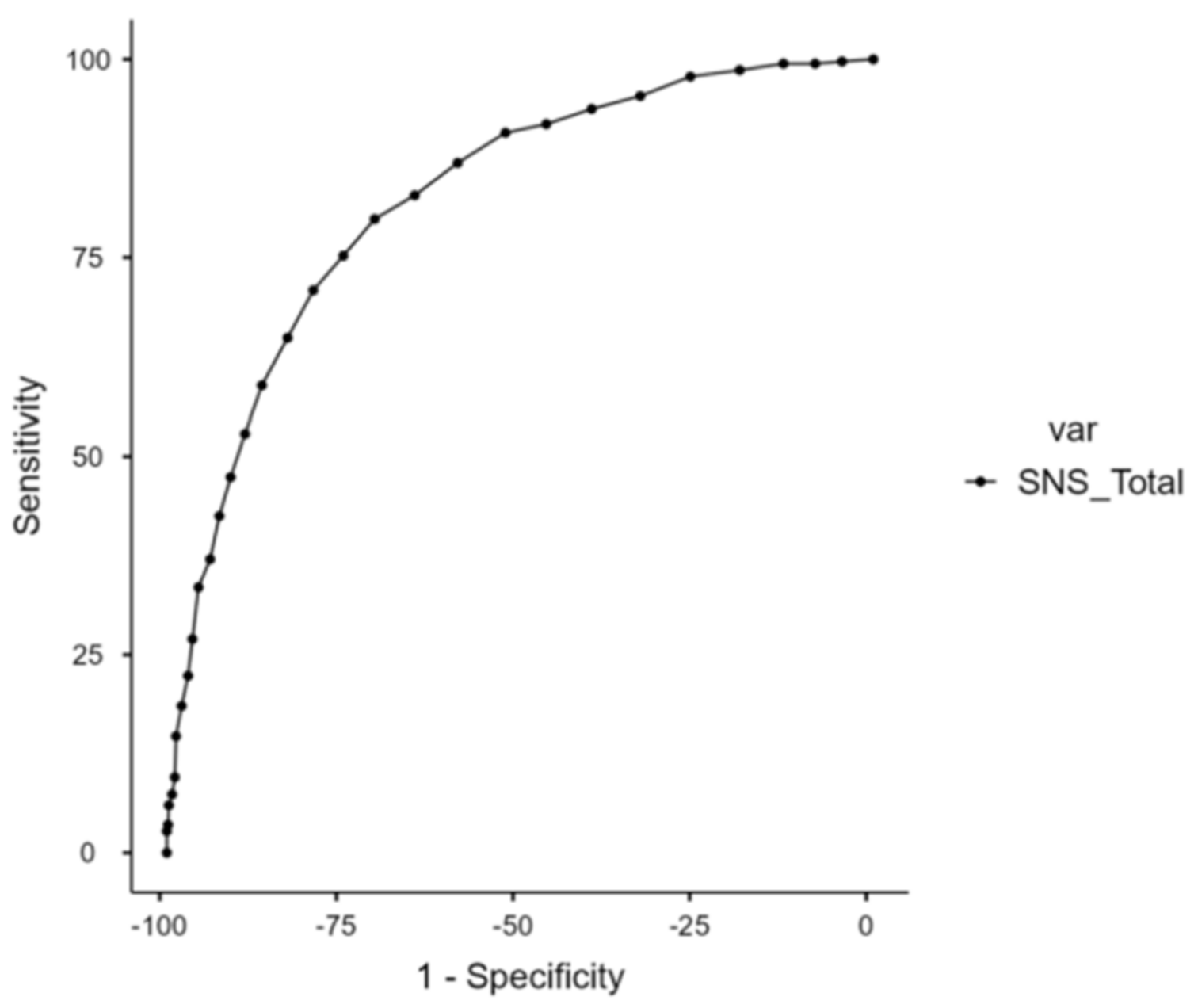
3.1. ROC Analysis of Total SNS Score
3.2. Frequency of Negative Symptoms in the Student Population
3.3. Stepwise Linear Regressions
3.3.1. Association of the Total SNS Score with Cannabis Use, Tobacco Use, and Clinical Variables
3.3.2. Association of the SNS Motivational Dimension with Cannabis Use, Tobacco Use, and Clinical Variables
3.3.3. Association of the SNS Emotional Expression Dimension with Cannabis Use, Tobacco Use, and Clinical Variables
| Dependent Variables | Independent Variables | β | Std.β | SE | 95% CI | R2 | p |
|---|---|---|---|---|---|---|---|
| SNS total score | BDI total score | 0.31 | 0.42 | 0.07 | [0.25–0.60] | 0.277 | <0.001 |
| PQ-16 distress score | 0.47 | 0.22 | 0.19 | [0.04–0.40] | 0.047 | 0.017 | |
| CAST score | 0.46 | 0.21 | 0.18 | [0.05–0.37] | 0.042 | 0.012 | |
| SNS motivational dimension | BDI total score | 0.30 | 0.45 | 0.05 | [0.29–0.60] | 0.363 | <0.001 |
| PQ-16 distress score | 0.68 | 0.35 | 0.15 | [0.19–0.50] | 0.110 | <0.001 | |
| CAST score | 0.41 | 0.20 | 0.14 | [0.06–0.34] | 0.039 | 0.006 | |
| SNS emotional expression dimension | BDI total score | 0.16 | 0.242 | 0.07 | [0.05–0.44] | 0.058 | 0.014 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ernst, M.; Korelitz, K.E. Cerebral Maturation in Adolescence: Behavioral Vulnerability. Encephale 2009, 35 (Suppl. S6), S182–S189. [Google Scholar] [CrossRef] [PubMed]
- World Drug Report. 2022. Available online: www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2022.html (accessed on 28 February 2024).
- Carton, L.; Bastien, A.; Chérot, N.; Caron, C.; Deheul, S.; Cottencin, O.; Gautier, S.; Moreau-Crépeaux, S.; Dondaine, T.; Bordet, R. An Overview of the Use of Psychoactive Substances among Students at the University of Lille during the COVID-19 Health Crisis: Results of the PETRA Study. Dialogues Clin. Neurosci. 2023, 25, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kreitzberg, D.S.; Pasch, K.E.; Loukas, A. Longitudinal Patterns of Cannabis and Tobacco Co-Administration and Concurrent Use among Young Adult College Students. Addict. Behav. 2024, 148, 107871. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.B.; Myers, M.G.; Tully, L.K.; Doran, N. Co-Occurring Use of Cannabis and Tobacco and the Presence of Acute Respiratory Symptoms among Young Adult Light and Intermittent Smokers. Subst. Use Misuse 2020, 55, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, R.P.; Alonso, J.; Axinn, W.G.; Cuijpers, P.; Ebert, D.D.; Green, J.G.; Hwang, I.; Kessler, R.C.; Liu, H.; Mortier, P.; et al. Mental Disorders among College Students in the WHO World Mental Health Surveys. Psychol. Med. 2016, 46, 2955–2970. [Google Scholar] [CrossRef]
- Sheldon, E.; Simmonds-Buckley, M.; Bone, C.; Mascarenhas, T.; Chan, N.; Wincott, M.; Gleeson, H.; Sow, K.; Hind, D.; Barkham, M. Prevalence and Risk Factors for Mental Health Problems in University Undergraduate Students: A Systematic Review with Meta-Analysis. J. Affect. Disord. 2021, 287, 282–292. [Google Scholar] [CrossRef]
- Vardell, E. Global Health Observatory Data Repository. Med. Ref. Serv. Q. 2020, 39, 67–74. [Google Scholar] [CrossRef]
- Schultze-Lutter, F.; Michel, C.; Ruhrmann, S.; Schimmelmann, B.G. Prevalence and Clinical Relevance of Interview-Assessed Psychosis-Risk Symptoms in the Young Adult Community. Psychol. Med. 2018, 48, 1167–1178. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Salazar de Pablo, G.; Correll, C.U.; Meyer-Lindenberg, A.; Millan, M.J.; Borgwardt, S.; Galderisi, S.; Bechdolf, A.; Pfennig, A.; Kessing, L.V.; et al. Prevention of Psychosis: Advances in Detection, Prognosis, and Intervention. JAMA Psychiatry 2020, 77, 755–765. [Google Scholar] [CrossRef]
- Myran, D.T.; Harrison, L.D.; Pugliese, M.; Solmi, M.; Anderson, K.K.; Fiedorowicz, J.G.; Perlman, C.M.; Webber, C.; Finkelstein, Y.; Tanuseputro, P. Transition to Schizophrenia Spectrum Disorder Following Emergency Department Visits Due to Substance Use With and Without Psychosis. JAMA Psychiatry 2023, 80, 1169–1174. [Google Scholar] [CrossRef]
- Penttilä, M.; Jääskeläinen, E.; Hirvonen, N.; Isohanni, M.; Miettunen, J. Duration of Untreated Psychosis as Predictor of Long-Term Outcome in Schizophrenia: Systematic Review and Meta-Analysis. Br. J. Psychiatry 2014, 205, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Souaiby, L.; Gauthier, C.; Kazes, M.; Mam-Lam-Fook, C.; Daban, C.; Plaze, M.; Gaillard, R.; ICAAR Study Group; Krebs, M.-O. Individual Factors Influencing the Duration of Untreated Psychosis. Early Interv. Psychiatry 2019, 13, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Joa, I.; Bjornestad, J.; Johannessen, J.O.; Langeveld, J.; Stain, H.J.; Weibell, M.; Hegelstad, W.T.V. Early Detection of Ultra High Risk for Psychosis in a Norwegian Catchment Area: The Two Year Follow-Up of the Prevention of Psychosis Study. Front. Psychiatry 2021, 12, 573905. [Google Scholar] [CrossRef] [PubMed]
- Piskulic, D.; Addington, J.; Cadenhead, K.S.; Cannon, T.D.; Cornblatt, B.A.; Heinssen, R.; Perkins, D.O.; Seidman, L.J.; Tsuang, M.T.; Walker, E.F.; et al. Negative Symptoms in Individuals at Clinical High Risk of Psychosis. Psychiatry Res. 2012, 196, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Carrión, R.E.; Correll, C.U.; Auther, A.M.; Cornblatt, B.A. A Severity-Based Clinical Staging Model for the Psychosis Prodrome: Longitudinal Findings From the New York Recognition and Prevention Program. Schizophr. Bull. 2017, 43, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Salazar de Pablo, G.; Catalan, A.; Vaquerizo Serrano, J.; Pedruzo, B.; Alameda, L.; Sandroni, V.; Armendariz, A.; Rodriguez, V.; Arango, C.; Moreno, C.; et al. Negative Symptoms in Children and Adolescents with Early-Onset Psychosis and at Clinical High-Risk for Psychosis: Systematic Review and Meta-Analysis. Br. J. Psychiatry 2023, 223, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Cowan, H.R.; Mittal, V.A. Transdiagnostic Dimensions of Psychiatric Comorbidity in Individuals at Clinical High Risk for Psychosis: A Preliminary Study Informed by HiTOP. Front. Psychiatry 2021, 11, 614710. [Google Scholar] [CrossRef] [PubMed]
- Velthorst, E.; Nieman, D.H.; Becker, H.E.; van de Fliert, R.; Dingemans, P.M.; Klaassen, R.; de Haan, L.; van Amelsvoort, T.; Linszen, D.H. Baseline Differences in Clinical Symptomatology between Ultra High Risk Subjects with and without a Transition to Psychosis. Schizophr. Res. 2009, 109, 60–65. [Google Scholar] [CrossRef]
- Werbeloff, N.; Dohrenwend, B.P.; Yoffe, R.; van Os, J.; Davidson, M.; Weiser, M. The Association between Negative Symptoms, Psychotic Experiences and Later Schizophrenia: A Population-Based Longitudinal Study. PLoS ONE 2015, 10, e0119852. [Google Scholar] [CrossRef]
- Rodríguez-Testal, J.F.; Perona-Garcelán, S.; Dollfus, S.; Valdés-Díaz, M.; García-Martínez, J.; Ruíz-Veguilla, M.; Senín-Calderón, C. Spanish Validation of the Self-Evaluation of Negative Symptoms Scale SNS in an Adolescent Population. BMC Psychiatry 2019, 19, 327. [Google Scholar] [CrossRef]
- Kaiser, S.; Heekeren, K.; Simon, J.J. The Negative Symptoms of Schizophrenia: Category or Continuum? Psychopathology 2011, 44, 345–353. [Google Scholar] [CrossRef]
- Pacheco-Colón, I.; Limia, J.M.; Gonzalez, R. Nonacute Effects of Cannabis Use on Motivation and Reward Sensitivity in Humans: A Systematic Review. Psychol. Addict. Behav. 2018, 32, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Buchanan, R.W.; Breier, A.; Carpenter, W.T. Case Identification and Stability of the Deficit Syndrome of Schizophrenia. Psychiatry Res. 1993, 47, 47–56. [Google Scholar] [CrossRef]
- Messinger, J.W.; Trémeau, F.; Antonius, D.; Mendelsohn, E.; Prudent, V.; Stanford, A.D.; Malaspina, D. Avolition and Expressive Deficits Capture Negative Symptom Phenomenology: Implications for DSM-5 and Schizophrenia Research. Clin. Psychol. Rev. 2011, 31, 161–168. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and Theoretical Foundations. Br. J. Psychiatry Suppl. 1989, 155 (Suppl. S7), 49–58. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Buchanan, R.W.; McKenney, P.D.; Alphs, L.D.; Carpenter, W.T. The Schedule for the Deficit Syndrome: An Instrument for Research in Schizophrenia. Psychiatry Res. 1989, 30, 119–123. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dollfus, S.; Lyne, J. Current Developments and Challenges in the Assessment of Negative Symptoms. Schizophr. Res. 2017, 186, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Strauss, G.P.; Nguyen, L.; Fischer, B.A.; Daniel, D.G.; Cienfuegos, A.; Marder, S.R. The Brief Negative Symptom Scale: Psychometric Properties. Schizophr. Bull. 2011, 37, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Dollfus, S.; Nordentoft, M.; Falkai, P.; Kaiser, S.; Giordano, G.M.; Vandevelde, A.; Nielsen, M.Ø.; Glenthøj, L.B.; et al. EPA Guidance on Assessment of Negative Symptoms in Schizophrenia. Eur. Psychiatry 2021, 64, e23. [Google Scholar] [CrossRef]
- Miller, T.J.; McGlashan, T.H.; Woods, S.W.; Stein, K.; Driesen, N.; Corcoran, C.M.; Hoffman, R.; Davidson, L. Symptom Assessment in Schizophrenic Prodromal States. Psychiatry Q. 1999, 70, 273–287. [Google Scholar] [CrossRef]
- Yung, A.R.; Yuen, H.P.; McGorry, P.D.; Phillips, L.J.; Kelly, D.; Dell’Olio, M.; Francey, S.M.; Cosgrave, E.M.; Killackey, E.; Stanford, C.; et al. Mapping the Onset of Psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Z. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Niv, N.; Cohen, A.N.; Mintz, J.; Ventura, J.; Young, A.S. The Validity of Using Patient Self-Report to Assess Psychotic Symptoms in Schizophrenia. Schizophr. Res. 2007, 90, 245–250. [Google Scholar] [CrossRef]
- Kelleher, I.; Harley, M.; Murtagh, A.; Cannon, M. Are Screening Instruments Valid for Psychotic-like Experiences? A Validation Study of Screening Questions for Psychotic-like Experiences Using in-Depth Clinical Interview. Schizophr. Bull. 2011, 37, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Nemoto, T.; Koshikawa, H.; Osono, Y.; Yamazawa, R.; Murakami, M.; Kashima, H.; Mizuno, M. A Self-Reported Instrument for Prodromal Symptoms of Psychosis: Testing the Clinical Validity of the PRIME Screen-Revised (PS-R) in a Japanese Population. Schizophr. Res. 2008, 106, 356–362. [Google Scholar] [CrossRef]
- Dollfus, S.; Mach, C.; Morello, R. Self-Evaluation of Negative Symptoms: A Novel Tool to Assess Negative Symptoms. Schizophr. Bull. 2016, 42, 571–578. [Google Scholar] [CrossRef]
- Dollfus, S.; Mucci, A.; Giordano, G.M.; Bitter, I.; Austin, S.F.; Delouche, C.; Erfurth, A.; Fleischhacker, W.W.; Movina, L.; Glenthøj, B.; et al. European Validation of the Self-Evaluation of Negative Symptoms (SNS): A Large Multinational and Multicenter Study. Front. Psychiatry 2022, 13, 826465. [Google Scholar] [CrossRef] [PubMed]
- Dollfus, S.; Delouche, C.; Hervochon, C.; Mach, C.; Bourgeois, V.; Rotharmel, M.; Tréhout, M.; Vandevelde, A.; Guillin, O.; Morello, R. Specificity and Sensitivity of the Self-Assessment of Negative Symptoms (SNS) in Patients with Schizophrenia. Schizophr. Res. 2019, 211, 51–55. [Google Scholar] [CrossRef]
- Mallet, J.; Guessoum, S.B.; Tebeka, S.; Le Strat, Y.; Dubertret, C. Self-Evaluation of Negative Symptoms in Adolescent and Young Adult First Psychiatric Episodes. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 103, 109988. [Google Scholar] [CrossRef]
- Hamel-Sénécal, L.; Chrétien, B.; Cabé, N.; Ritz, L.; Mange, J.; Sénemeaud, C.; NicolasMargas; Leconte, P.; Bazire, A.; Marchand, J.B.; et al. Alcool et drogues à l’université de Caen Normandie (ADUC): Une contribution utile pour l’addictovigilance sur la population estudiantine. Therapies 2018, 73, 576. [Google Scholar] [CrossRef]
- LimeSurvey—Free Online Survey Tool. Available online: https://www.limesurvey.org/fr (accessed on 28 February 2024).
- Mauduy, M.; Mauny, N.; Mange, J. Tobacco Dependence Among French University Students: A Cluster Analytic Approach to Identifying Distinct Psychological Profiles of Smokers. J. Drug Issues 2022, 53, 226–246. [Google Scholar] [CrossRef]
- Legleye, S.; Piontek, D.; Kraus, L. Psychometric Properties of the Cannabis Abuse Screening Test (CAST) in a French Sample of Adolescents. Drug Alcohol. Depend. 2011, 113, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.-F.; Le Houezec, J.; Perneger, T.V. A Self-Administered Questionnaire to Measure Dependence on Cigarettes: The Cigarette Dependence Scale. Neuropsychopharmacology 2003, 28, 359–370. [Google Scholar] [CrossRef]
- Etter, J.-F. A Comparison of the Content-, Construct- and Predictive Validity of the Cigarette Dependence Scale and the Fagerström Test for Nicotine Dependence. Drug Alcohol. Depend. 2005, 77, 259–268. [Google Scholar] [CrossRef]
- Etter, J.-F. Comparing the Validity of the Cigarette Dependence Scale and the Fagerström Test for Nicotine Dependence. Drug Alcohol. Depend. 2008, 95, 152–159. [Google Scholar] [CrossRef]
- Ising, H.K.; Veling, W.; Loewy, R.L.; Rietveld, M.W.; Rietdijk, J.; Dragt, S.; Klaassen, R.M.C.; Nieman, D.H.; Wunderink, L.; Linszen, D.H.; et al. The Validity of the 16-Item Version of the Prodromal Questionnaire (PQ-16) to Screen for Ultra High Risk of Developing Psychosis in the General Help-Seeking Population. Schizophr. Bull. 2012, 38, 1288–1296. [Google Scholar] [CrossRef]
- Savill, M.; D’Ambrosio, J.; Cannon, T.D.; Loewy, R.L. Psychosis-Risk Screening in Different Populations Using the Prodromal Questionnaire: A Systematic Review. Early Interv. Psychiatry 2018, 12, 3–14. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A. Internal Consistencies of the Original and Revised Beck Depression Inventory. J. Clin. Psychol. 1984, 40, 1365–1367. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and Its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Lemyre, A.; Poliakova, N.; Bélanger, R.E. The Relationship Between Tobacco and Cannabis Use: A Review. Subst. Use Misuse 2019, 54, 130–145. [Google Scholar] [CrossRef]
- Lee, D.K. Data Transformation: A Focus on the Interpretation. Korean J. Anesthesiol. 2020, 73, 503–508. [Google Scholar] [CrossRef]
- Mowbray, F.I.; Fox-Wasylyshyn, S.M.; El-Masri, M.M. Univariate Outliers: A Conceptual Overview for the Nurse Researcher. Can. J. Nurs. Res. 2019, 51, 31–37. [Google Scholar] [CrossRef]
- Lindström, E.; Lewander, T.; Malm, U.; Malt, U.F.; Lublin, H.; Ahlfors, U.G. Patient-Rated versus Clinician-Rated Side Effects of Drug Treatment in Schizophrenia. Clinical Validation of a Self-Rating Version of the UKU Side Effect Rating Scale (UKU-SERS-Pat). Nord. J. Psychiatry 2001, 55 (Suppl. S44), 5–69. [Google Scholar] [CrossRef]
- Kassab, S.E.; Taylor, D.; Hamdy, H. Student Engagement in Health Professions Education: AMEE Guide No. 152. Med. Teach. 2023, 45, 949–965. [Google Scholar] [CrossRef]
- Radua, J.; Ramella-Cravaro, V.; Ioannidis, J.P.A.; Reichenberg, A.; Phiphopthatsanee, N.; Amir, T.; Yenn Thoo, H.; Oliver, D.; Davies, C.; Morgan, C.; et al. What Causes Psychosis? An Umbrella Review of Risk and Protective Factors. World Psychiatry 2018, 17, 49–66. [Google Scholar] [CrossRef]
- Jahrami, H.; AlKaabi, J.; Trabelsi, K.; Pandi-Perumal, S.R.; Saif, Z.; Seeman, M.V.; Vitiello, M.V. The Worldwide Prevalence of Self-Reported Psychological and Behavioral Symptoms in Medical Students: An Umbrella Review and Meta-Analysis of Meta-Analyses. J. Psychosom. Res. 2023, 173, 111479. [Google Scholar] [CrossRef]
- Lee, B.; Krishan, P.; Goodwin, L.; Iduye, D.; de Los Godos, E.F.; Fryer, J.; Gallagher, K.; Hair, K.; O’Connell, E.; Ogarrio, K.; et al. Impact of COVID-19 Mitigations on Anxiety and Depression amongst University Students: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 06035. [Google Scholar] [CrossRef]
- Solmi, M.; De Toffol, M.; Kim, J.Y.; Choi, M.J.; Stubbs, B.; Thompson, T.; Firth, J.; Miola, A.; Croatto, G.; Baggio, F.; et al. Balancing Risks and Benefits of Cannabis Use: Umbrella Review of Meta-Analyses of Randomised Controlled Trials and Observational Studies. BMJ 2023, 382, e072348. [Google Scholar] [CrossRef]
- Pacheco-Colón, I.; Ramirez, A.R.; Gonzalez, R. Effects of Adolescent Cannabis Use on Motivation and Depression: A Systematic Review. Curr. Addict. Rep. 2019, 6, 532–546. [Google Scholar] [CrossRef]
- Sabe, M.; Zhao, N.; Kaiser, S. Cannabis, Nicotine and the Negative Symptoms of Schizophrenia: Systematic Review and Meta-Analysis of Observational Studies. Neurosci. Biobehav. Rev. 2020, 116, 415–425. [Google Scholar] [CrossRef]
- Maia, T.V.; Frank, M.J. An Integrative Perspective on the Role of Dopamine in Schizophrenia. Biol. Psychiatry 2017, 81, 52–66. [Google Scholar] [CrossRef]
| Variable | N = 2128 |
|---|---|
| Sociodemographic | |
| Age, years | 19.80 (2.25); [18–34]; (95% CI [19.70–19.90]) |
| Gender, % men | 29.1%; (95% CI [27.2–31.0]) |
| Clinical | |
| SNS emotional expression score | 4.25 (3.27); [0–16]; (95% CI [4.11–4.39]) |
| SNS motivational score | 6.07 (4.47); [0–24]; (95% CI [5.88–6.26]) |
| SNS total score | 10.33 (6.77); [0–30]; (95% CI [10.00–10.60]) |
| PQ-16 distress score | 3.69 (5.10); [0–46]; (95% CI [3.47–3.90]) |
| BDI | 6.74 (6.05); [0–39]; (95% CI [6.48–7.00]) |
| Toxic consumption | |
| CAST scores | 0.58 (2.32); [0–24]; (95% CI [0.48–0.68]) |
| CDS | 11.9 (4.75); [5–24]; (95% CI [11.4–12.4]) |
| SNS Cut-Off | Sensibility (%) | Specificity (%) | Youden’s Index | AUC |
|---|---|---|---|---|
| 10 | 90.74 | 52.07 | 0.428 | 0.823 |
| 11 | 86.92 | 58.83 | 0.458 | 0.823 |
| 12 | 82.83 | 64.91 | 0.477 | 0.823 |
| 13 | 79.84 | 70.58 | 0.504 | 0.823 |
| 14 | 75.20 | 75.01 | 0.502 | 0.823 |
| 15 | 70.84 | 79.27 | 0.501 | 0.823 |
| 16 | 64.85 | 82.91 | 0.478 | 0.823 |
| 17 | 58.86 | 86.54 | 0.454 | 0.823 |
| NS Dimension | SNS Subscores | SNS Item | Mean (SD); [Minimum–Maximum] | Percentage of Affirmative Responses (Score = 2) |
|---|---|---|---|---|
| Motivational dimension | Social withdrawal | 1 | 0.786 (0.682); [0–2] | 14.8%, (95% CI [13.3–16.3]) |
| 2 | 0.319 (0.578); [0–2] | 5.9%, (95% CI [4.7–6.8]) | ||
| 3 | 0.221 (0.491); [0–2] | 3.4%, (95% CI [2.7–4.2]) | ||
| 4 | 0.541 (0.696); [0–2] | 11.8%, (95% CI [10.4–13.1]) | ||
| Avolition | 13 | 0.721 (0.696); [0–2] | 14.2%, (95% CI [12.7–15.6]) | |
| 14 | 0.953 (0.730); [0–2] | 24.4%, (95% CI [22.6–26.3]) | ||
| 15 | 1.011 (0.762); [0–2] | 29.6%, (95% CI [27.6–31.5]) | ||
| 16 | 0.540 (0.708); [0–2] | 12.6%, (95% CI [11.2–14.1]) | ||
| Anhedonia | 17 | 0.236 (0.493); [0–2] | 3.1%, (95% CI [2.4–3.9]) | |
| 18 | 0.248 (0.513); [0–2] | 3.8%, (95% CI [3.0–4.64]) | ||
| 19 | 0.246 (0.499); [0–2] | 3.2%, (95% CI [2.4–3.9]) | ||
| 20 | 0.279 (0.564); [0–2] | 5.8%, (95% CI [4.8–6.8]) | ||
| Emotional expression dimension | Reduced emotional range | 5 | 0.539 (0.703); [0–2] | 12.3%, (95% CI [10.9–13.7]) |
| 6 | 0.428 (0.630); [0–2] | 7.6%, (95% CI [6.4–8.7]) | ||
| 7 | 0.296 (0.581); [0–2] | 6.5%, (95% CI [5.4–7.5]) | ||
| 8 | 0.824 (0.761); [0–2] | 21.7%, (95% CI [19.9–23.4]) | ||
| Alogia | 9 | 0.712 (0.777); [0–2] | 19.9%, (95% CI [18.2–21.6]) | |
| 10 | 0.427 (0.655); [0–2] | 9.2%, (95% CI [7.9–10.4]) | ||
| 11 | 0.555 (0.734); [0–2] | 14.6%, (95% CI [13.1–16.1]) | ||
| 12 | 0.492 (0.693); [0–2] | 11.5%, (95% CI [10.1–12.9]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Métivier, L.; Mauduy, M.; Beaunieux, H.; Dollfus, S. Revealing the Unseen: Detecting Negative Symptoms in Students. J. Clin. Med. 2024, 13, 1709. https://doi.org/10.3390/jcm13061709
Métivier L, Mauduy M, Beaunieux H, Dollfus S. Revealing the Unseen: Detecting Negative Symptoms in Students. Journal of Clinical Medicine. 2024; 13(6):1709. https://doi.org/10.3390/jcm13061709
Chicago/Turabian StyleMétivier, Lucie, Maxime Mauduy, Hélène Beaunieux, and Sonia Dollfus. 2024. "Revealing the Unseen: Detecting Negative Symptoms in Students" Journal of Clinical Medicine 13, no. 6: 1709. https://doi.org/10.3390/jcm13061709
APA StyleMétivier, L., Mauduy, M., Beaunieux, H., & Dollfus, S. (2024). Revealing the Unseen: Detecting Negative Symptoms in Students. Journal of Clinical Medicine, 13(6), 1709. https://doi.org/10.3390/jcm13061709