The Prepetrous Segment of the Internal Carotid Artery as a Neglected Site of Symptomatic Atherosclerosis: A Single-Center Series
Abstract
1. Introduction
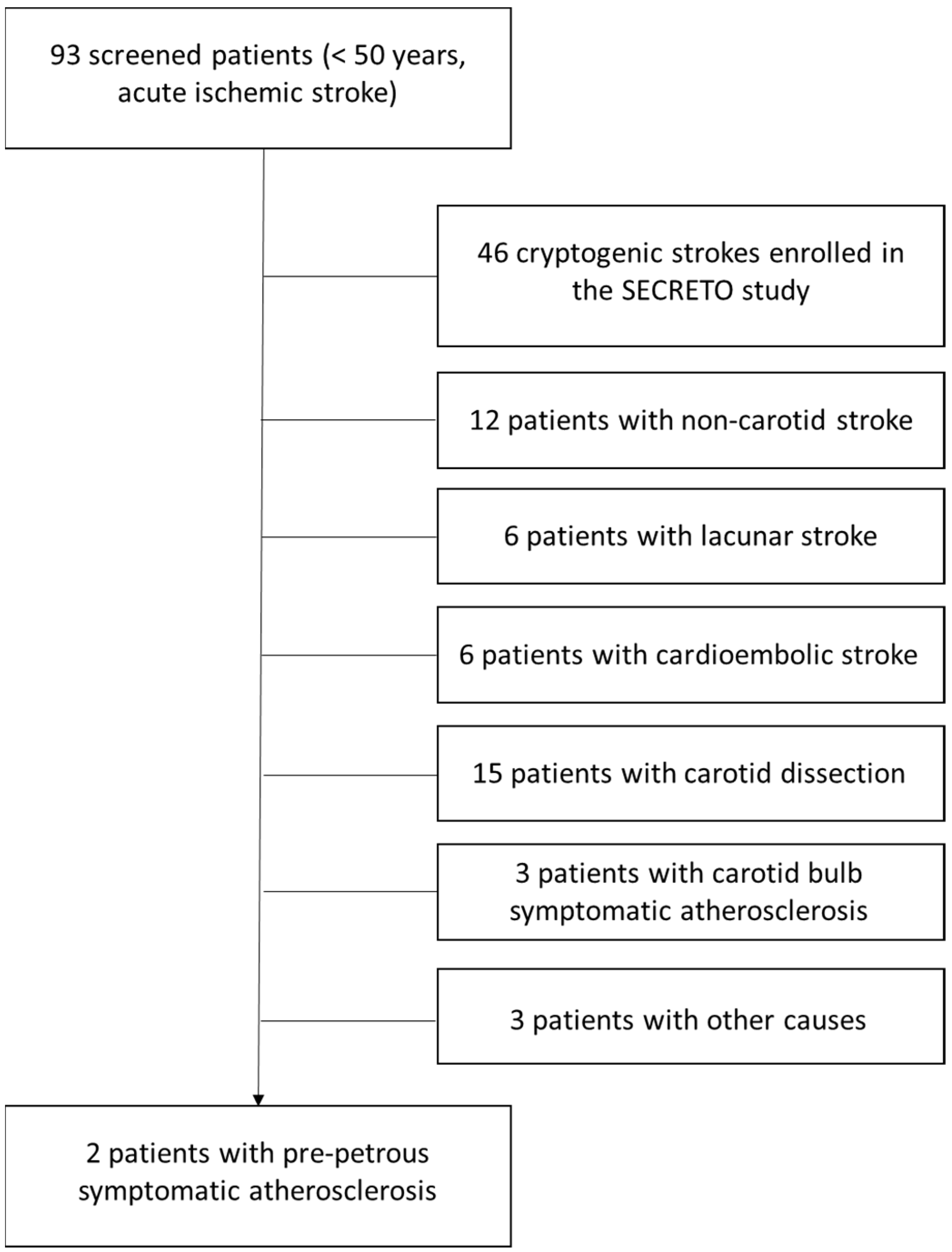
2. Materials and Methods
3. Results
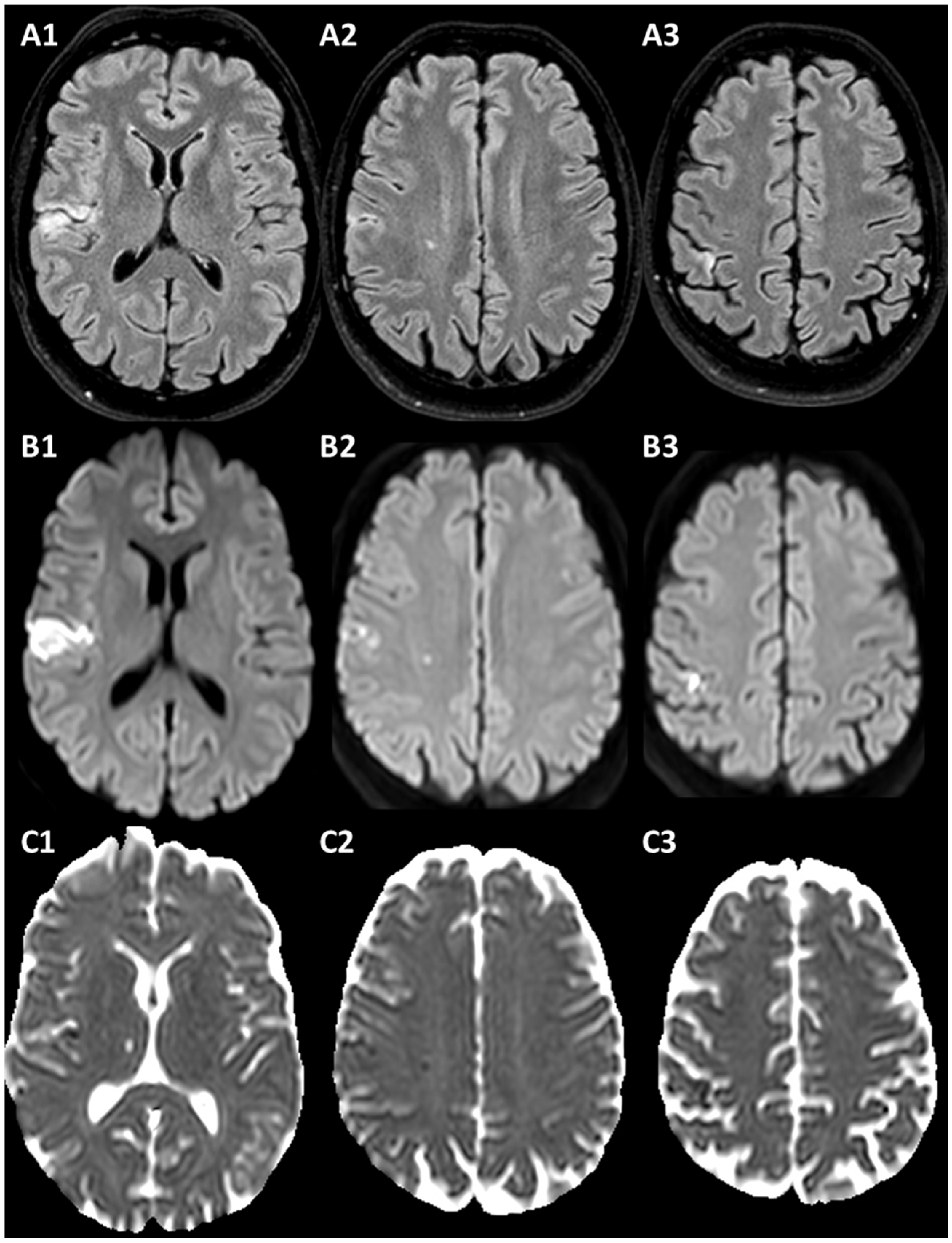
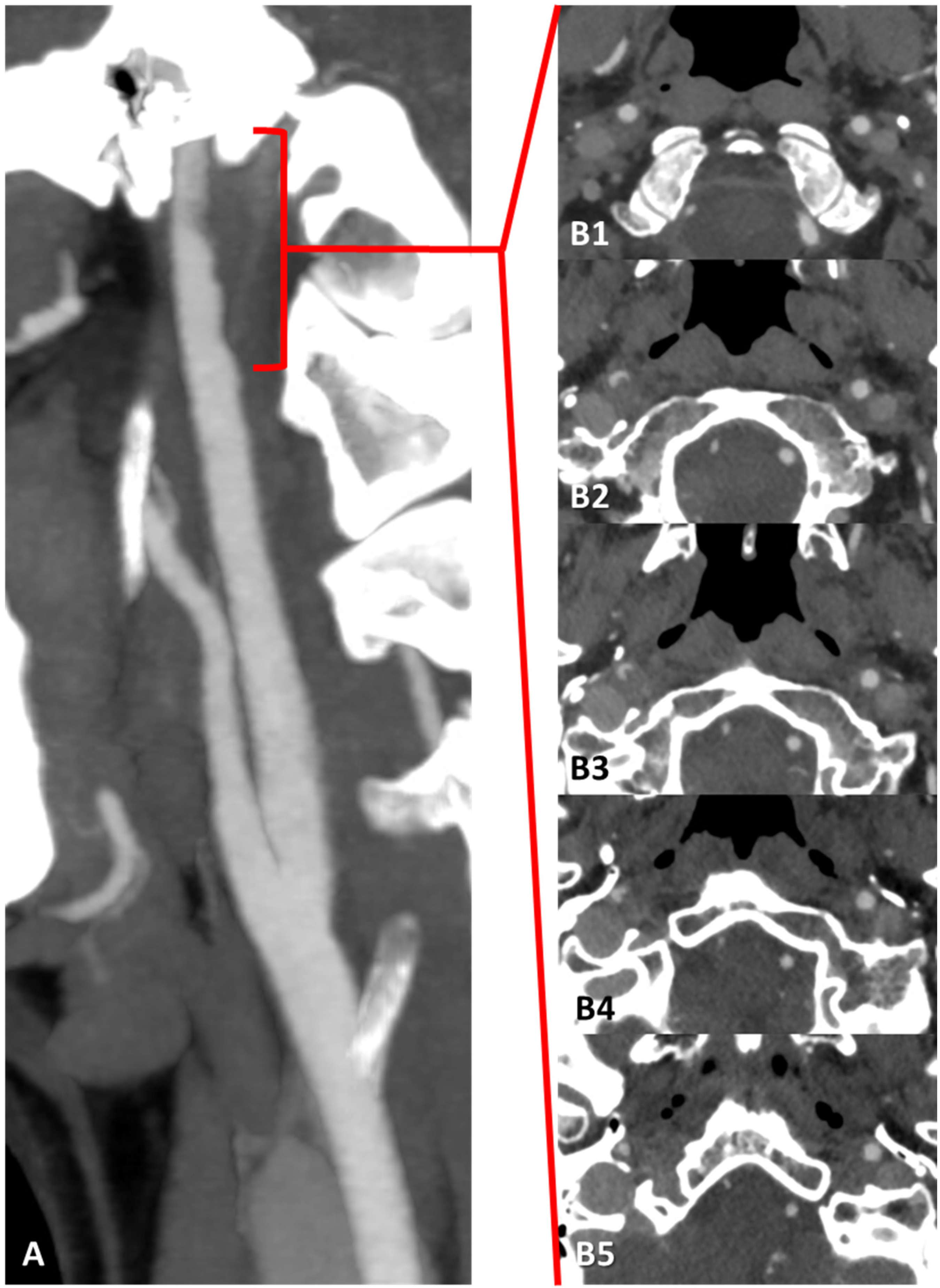
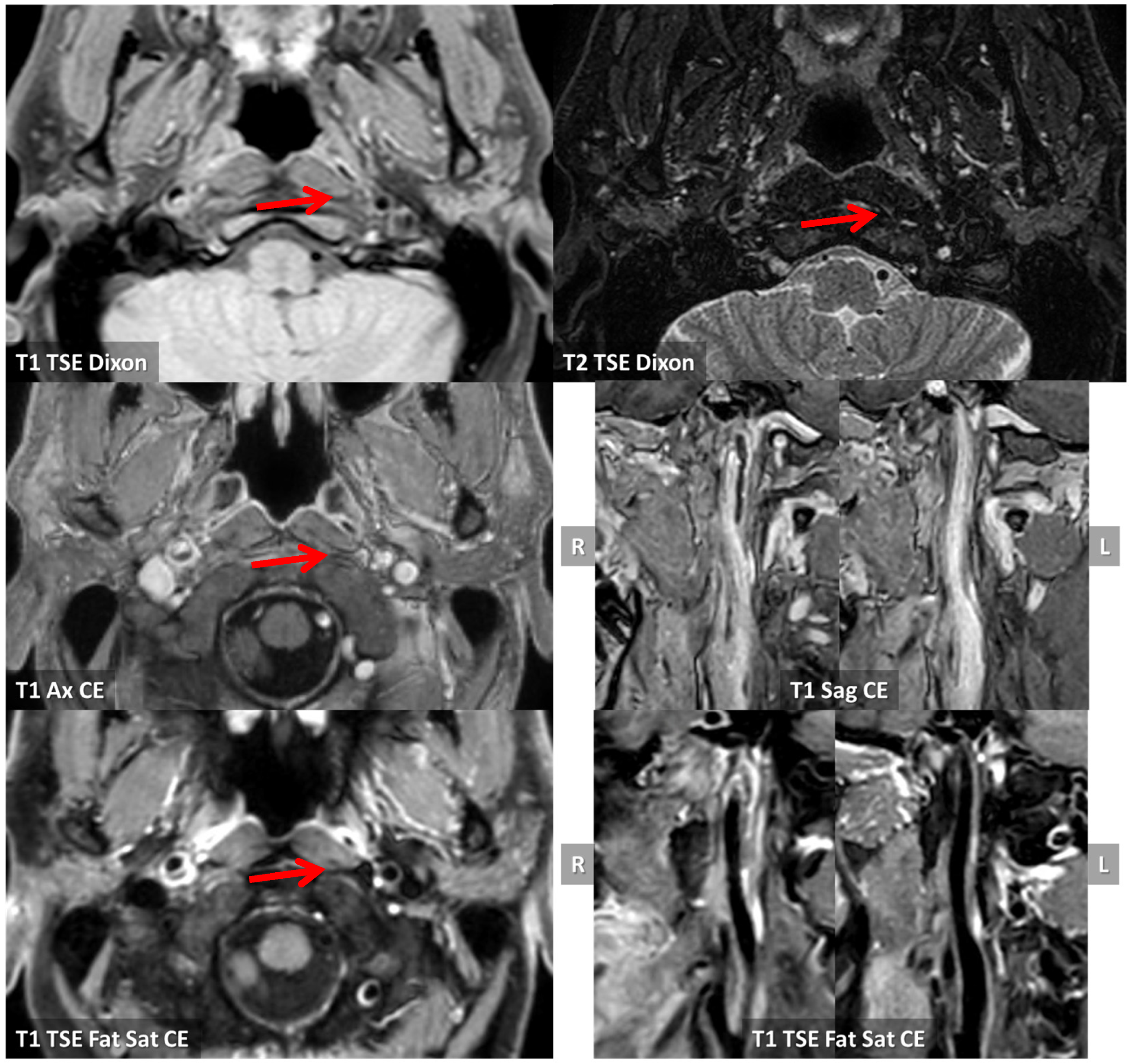
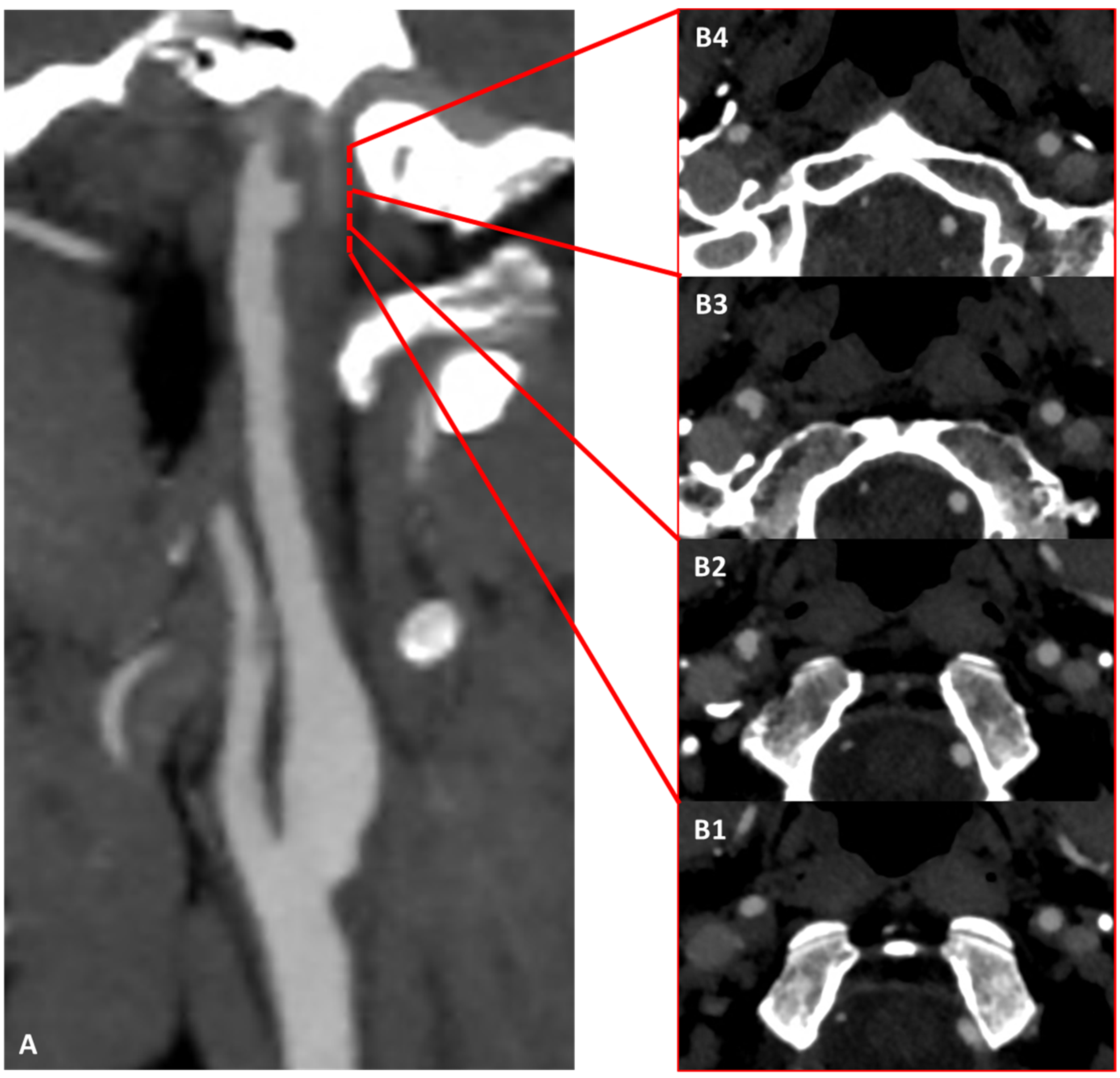
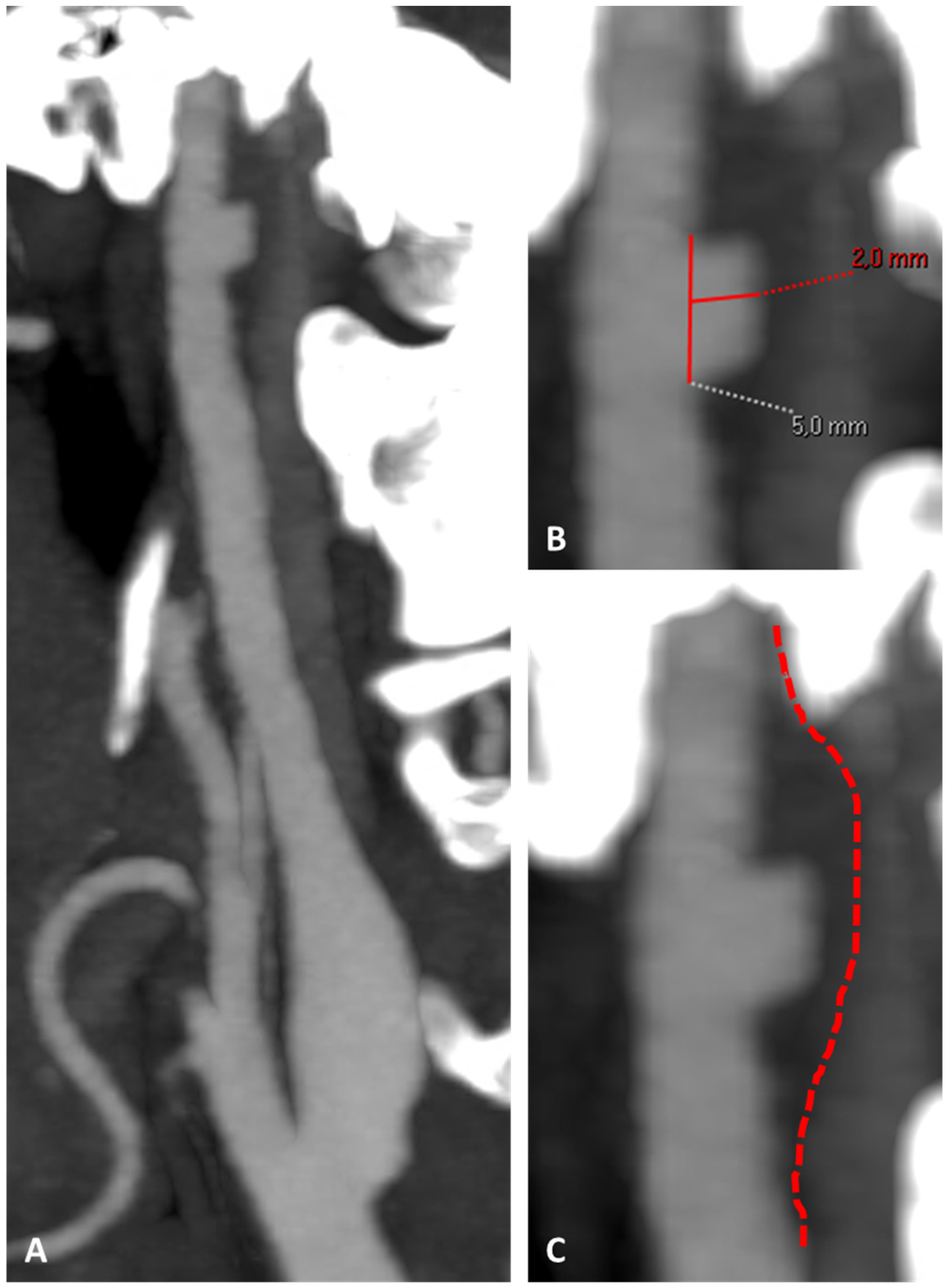
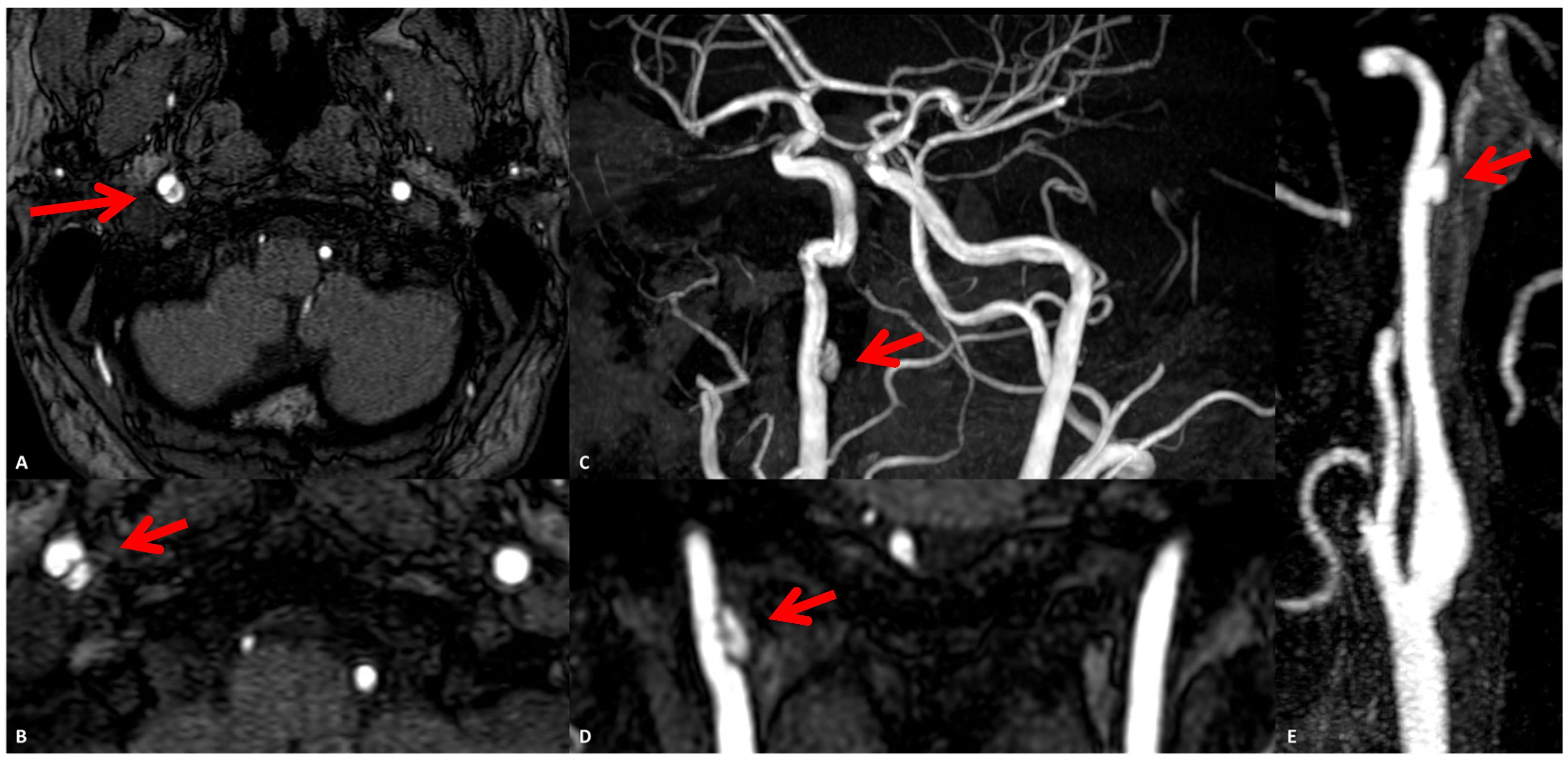
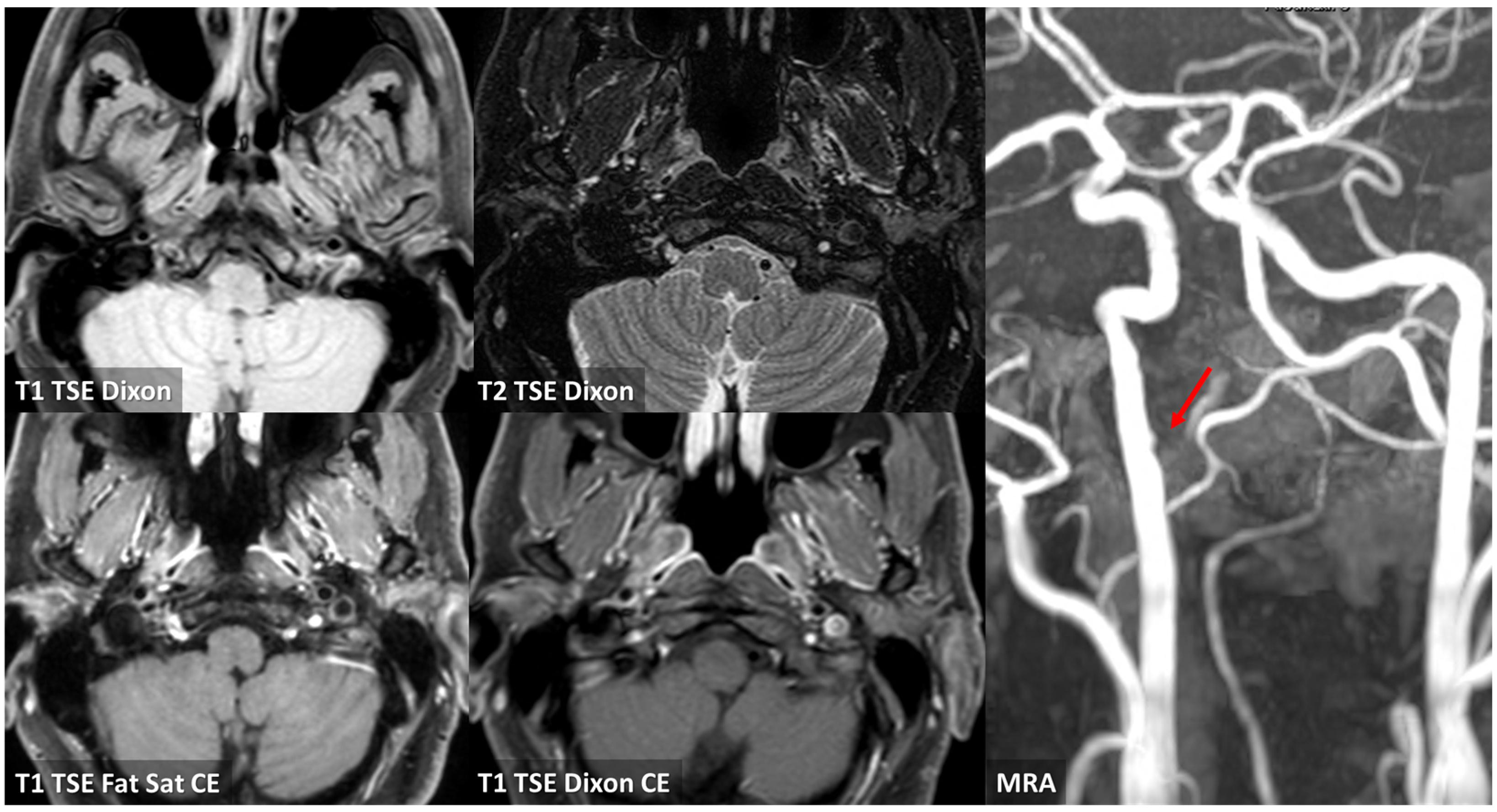
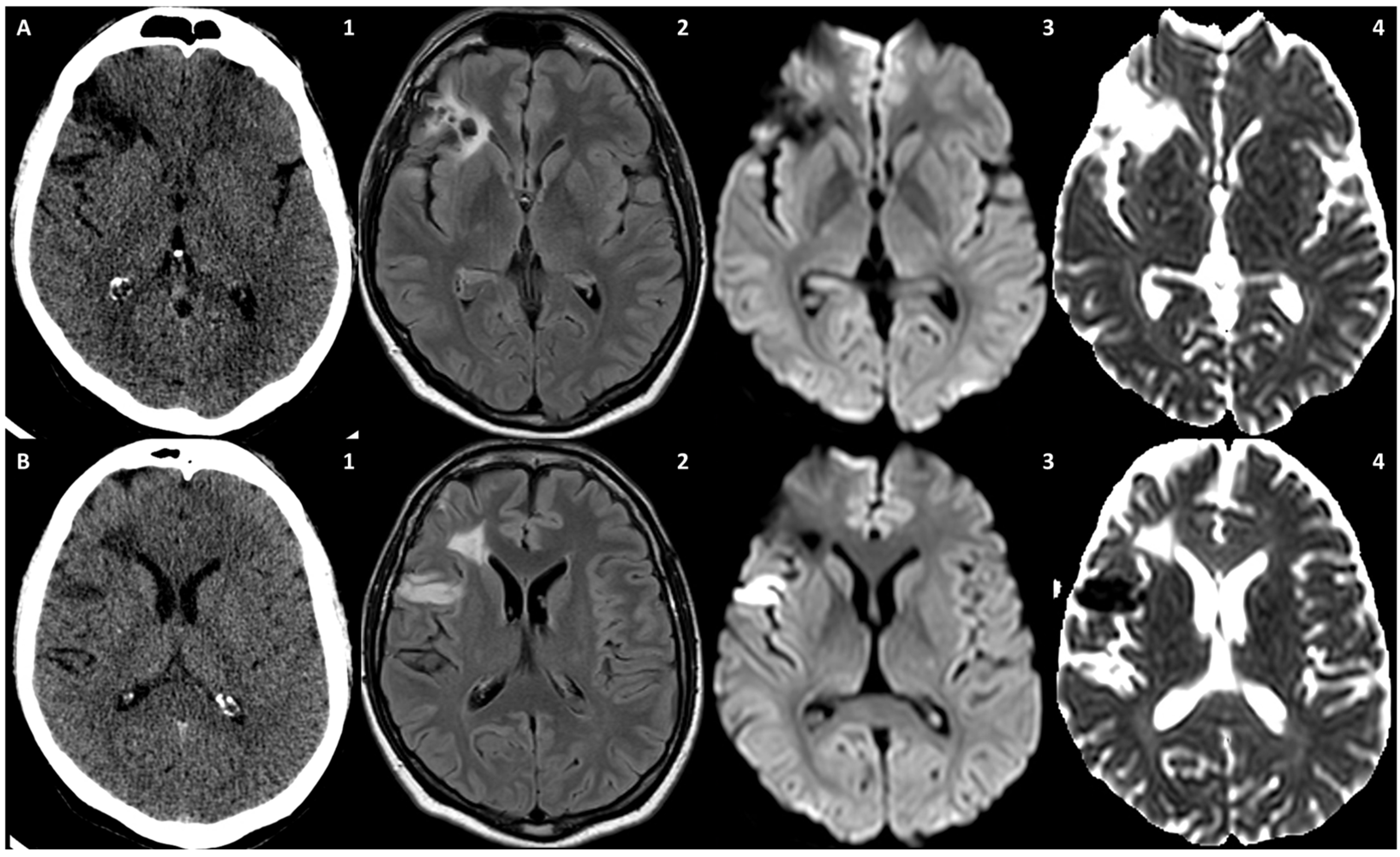
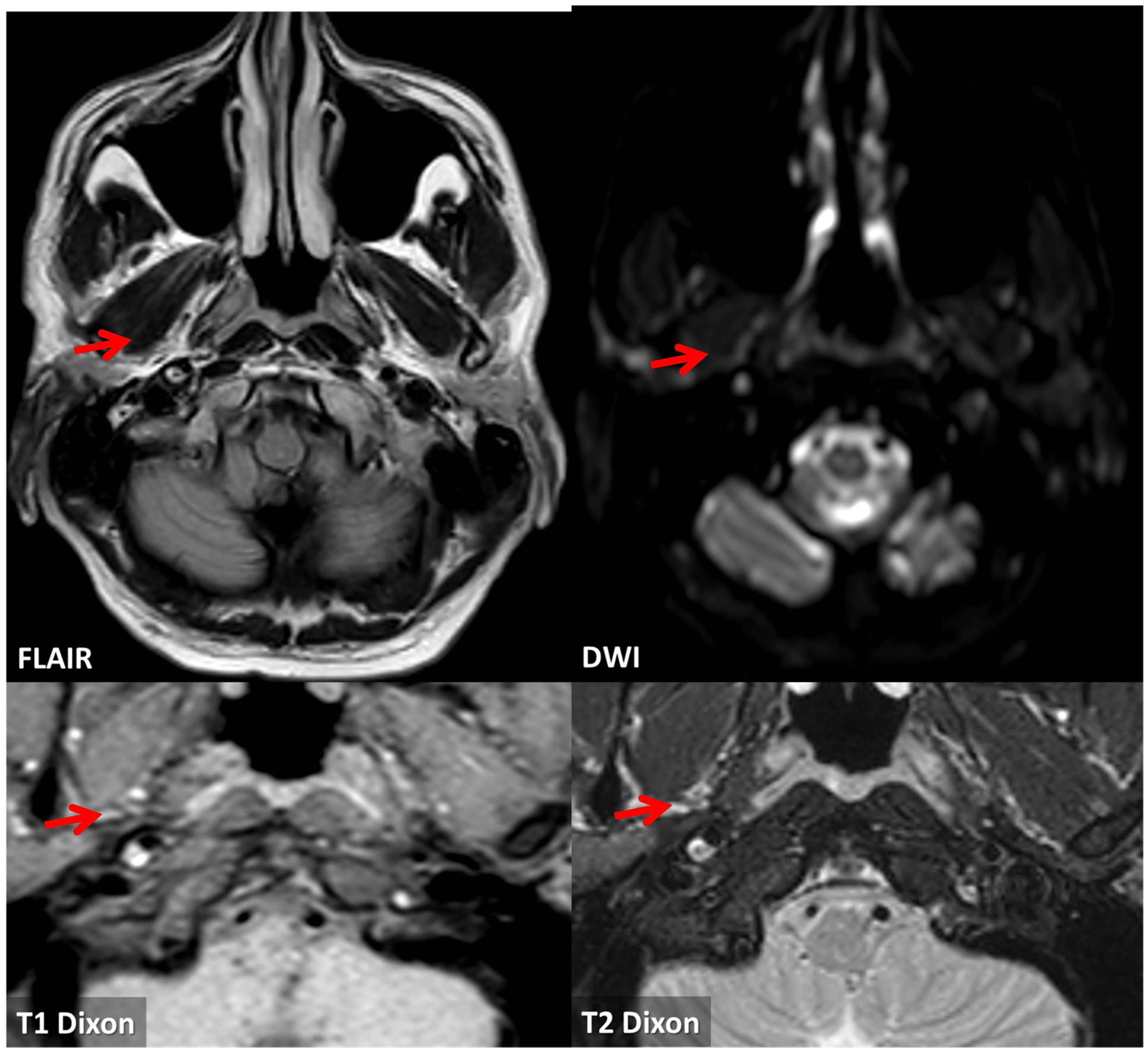
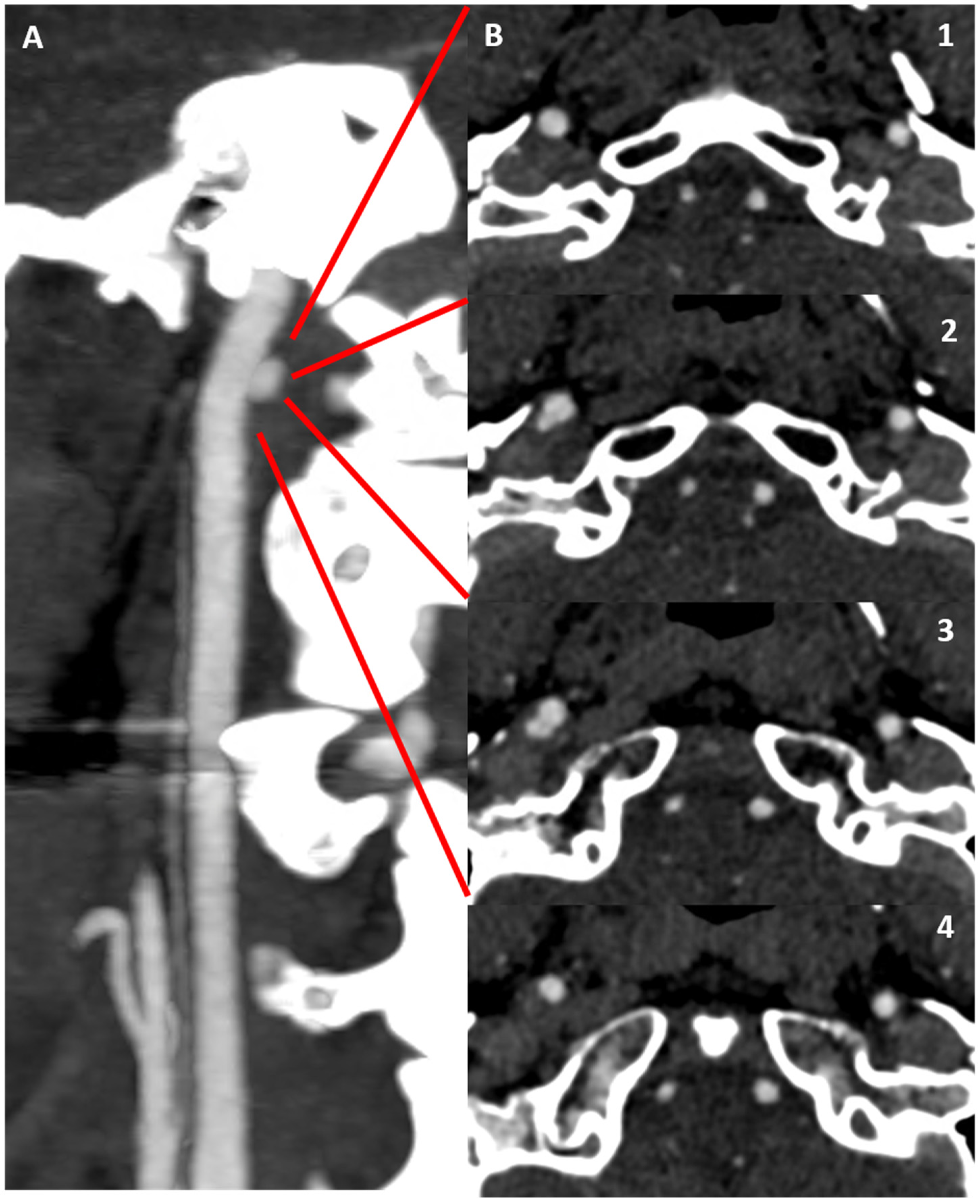
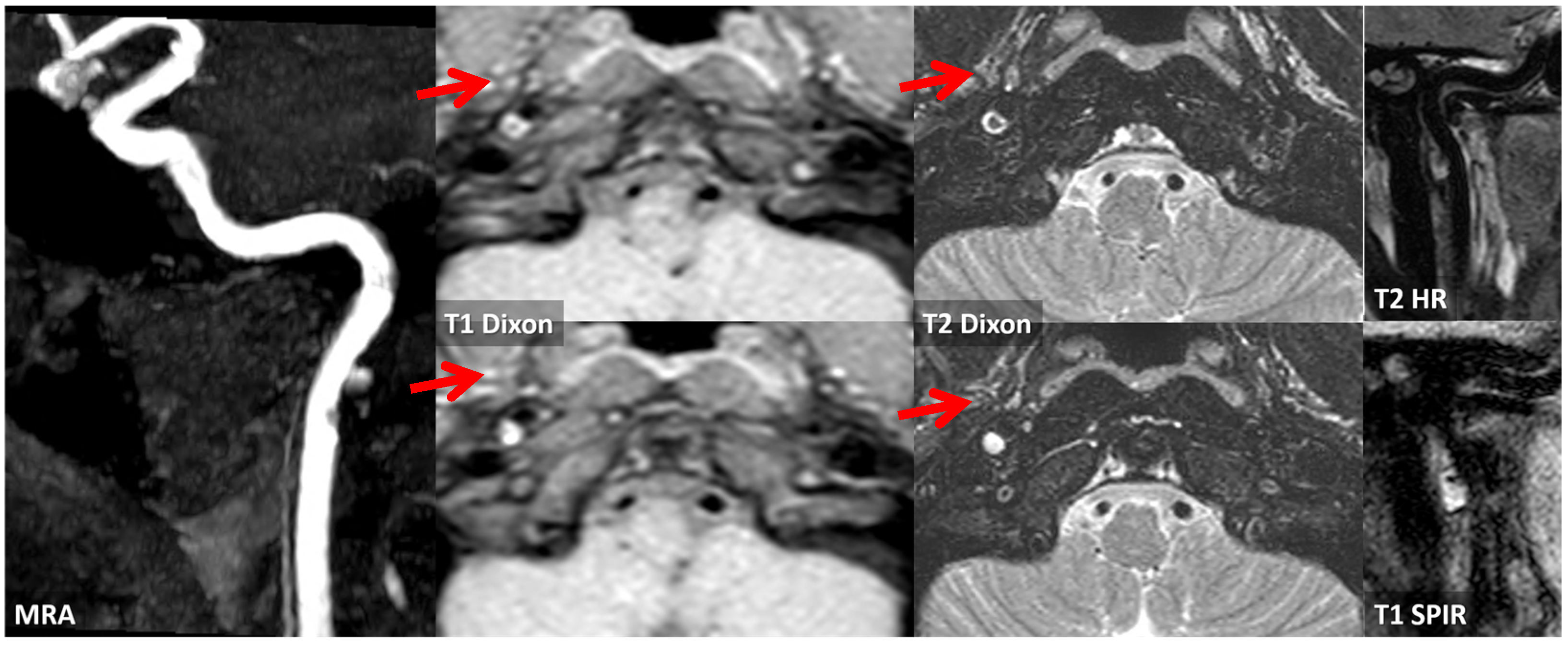
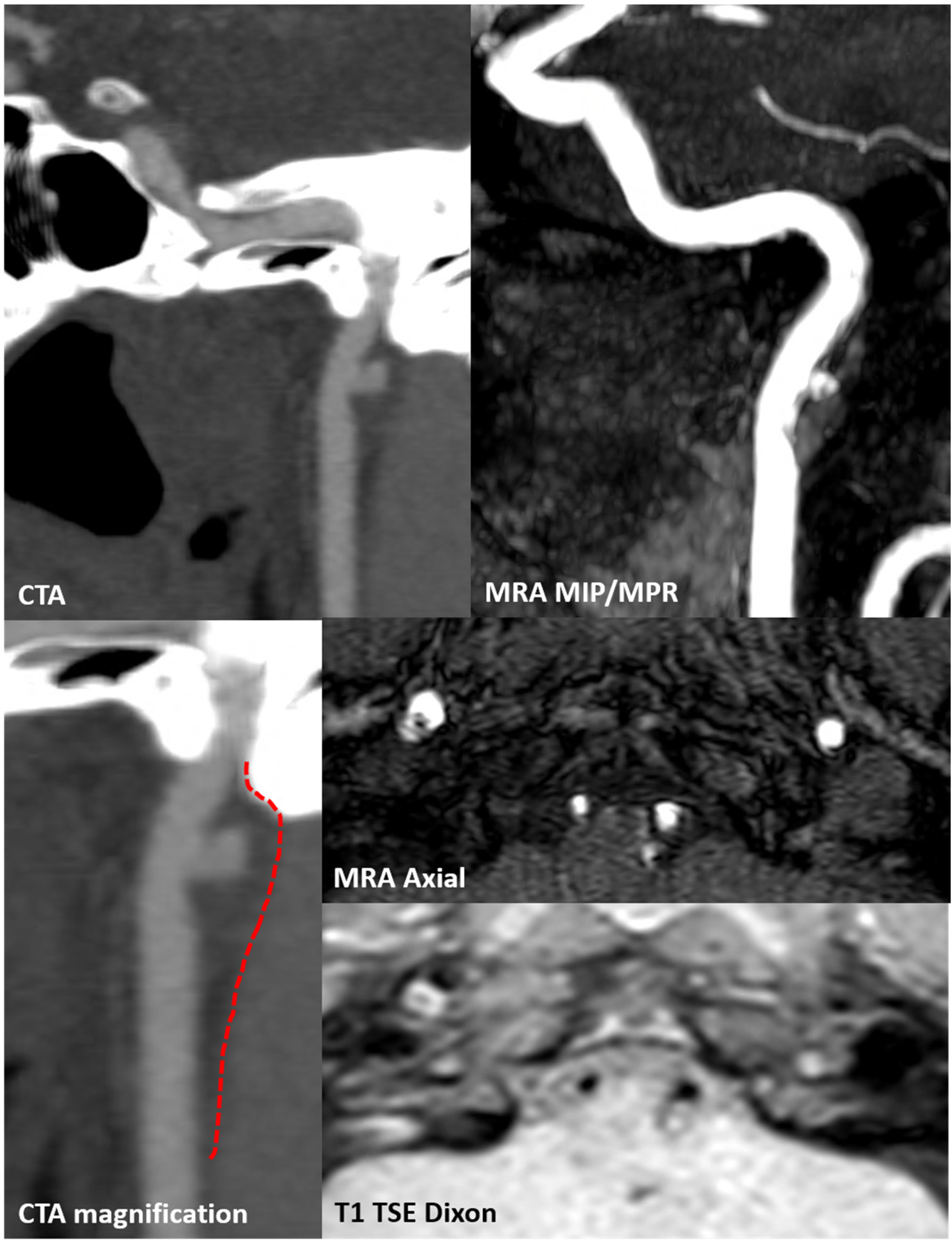
3.1. Case 1
3.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Bouthillier, A.; van Loveren, H.R.; Keller, J.T. Segments of the internal carotid artery: A new classification. Neurosurgery 1996, 38, 425–432, discussion 432–433. [Google Scholar] [CrossRef] [PubMed]
- Lasjaunias, P.; Santoyo-Vazquez, A. Segmental agenesis of the internal carotid artery: Angiographic aspects with embryological discussion. Anat. Clin. 1984, 6, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Leys, D. Cervical-artery dissections: Predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009, 8, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Putaala, J.; Martinez-Majander, N.; Saeed, S.; Yesilot, N.; Jäkälä, P.; Nerg, O.; Tsivgoulis, G.; Numminen, H.; Gordin, D.; von Sarnowski, B.; et al. Searching for Explanations for Cryptogenic Stroke in the Young: Revealing the Triggers, Causes, and Outcome (SECRETO): Rationale and design. Eur. Stroke J. 2017, 2, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Wolf, M.E.; Hennerici, M.G. The ASCOD phenotyping of ischemic stroke (Updated ASCO Phenotyping). Cerebrovasc. Dis. 2013, 36, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, P.; Godeiro Junior, C.; Moro, E.; Cavallieri, F.; Zedde, M. COVID-19 and Cerebrovascular Diseases: A Systematic Review and Perspectives for Stroke Management. Front. Neurol. 2020, 11, 574694. [Google Scholar] [CrossRef] [PubMed]
- Cavallieri, F.; Marti, A.; Fasano, A.; Dalla Salda, A.; Ghirarduzzi, A.; Moratti, C.; Bonacini, L.; Ghadirpour, R.; Pascarella, R.; Valzania, F.; et al. Prothrombotic state induced by COVID-19 infection as trigger for stroke in young patients: A dangerous association. ENeurologicalsci 2020, 20, 100247. [Google Scholar] [CrossRef]
- Li, L.; Yiin, G.S.; Geraghty, O.C.; Schulz, U.G.; Kuker, W.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: A population-based study. Lancet Neurol. 2015, 14, 903–913. [Google Scholar] [CrossRef]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef]
- Kopczak, A.; Schindler, A.; Bayer-Karpinska, A.; Koch, M.L.; Sepp, D.; Zeller, J.; Strecker, C.; Hempel, J.M.; Yuan, C.; Malik, R.; et al. Complicated Carotid Artery Plaques as a Cause of Cryptogenic Stroke. J. Am. Coll. Cardiol. 2020, 76, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Navi, B.B.; Merkler, A.E.; Baradaran, H.; Díaz, I.; Parikh, N.S.; Kasner, S.E.; Gladstone, D.J.; Iadecola, C.; Gupta, A. Reclassification of Ischemic Stroke Etiological Subtypes on the Basis of High-Risk Nonstenosing Carotid Plaque. Stroke 2020, 51, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, B.; Gutiérrez-Zúñiga, R.; Díez-Tejedor, E. It’s Time to Say Goodbye to the ESUS Construct. Front. Neurol. 2020, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Murata, N.; Chu, B.; Watase, H.; Hippe, D.S.; Balu, N.; Sun, J.; Zhao, X.; Hatsukami, T.S.; Yuan, C.; et al. Characterization of Carotid Atherosclerotic Plaques Using 3-Dimensional MERGE Magnetic Resonance Imaging and Correlation with Stroke Risk Factors. Stroke 2020, 51, 475–480. [Google Scholar] [CrossRef]
- Cai, Y.; He, L.; Yuan, C.; Chen, H.; Zhang, Q.; Li, R.; Li, C.; Zhao, X. Atherosclerotic plaque features and distribution in bilateral carotid arteries of asymptomatic elderly population: A 3D multicontrast MR vessel wall imaging study. Eur. J. Radiol. 2017, 96, 6–11. [Google Scholar] [CrossRef]
- Naim, C.; Douziech, M.; Therasse, É.; Robillard, P.; Giroux, M.F.; Arsenault, F.; Cloutier, G.; Soulez, G. Vulnerable atherosclerotic carotid plaque evaluation by ultrasound, computed tomography angiography, and magnetic resonance imaging: An overview. Can. Assoc. Radiol. J. 2014, 65, 275–286. [Google Scholar] [CrossRef]
- Li, L.; Chai, J.T.; Biasiolli, L.; Robson, M.D.; Choudhury, R.P.; Handa, A.I.; Near, J.; Jezzard, P. Black-blood multicontrast imaging of carotid arteries with DANTE-prepared 2D and 3D MR imaging. Radiology 2014, 273, 560–569. [Google Scholar] [CrossRef]
- Kassem, M.; Florea, A.; Mottaghy, F.M.; van Oostenbrugge, R.; Kooi, M.E. Magnetic resonance imaging of carotid plaques: Current status and clinical perspectives. Ann. Transl. Med. 2020, 8, 1266. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, W.S.; Hatsukami, T.; Yuan, C.; Zhao, X.Q. MRI of carotid atherosclerosis. AJR Am. J. Roentgenol. 2013, 200, W304–W313. [Google Scholar] [CrossRef]
- Bos, D.; van Dam-Nolen, D.H.K.; Gupta, A.; Saba, L.; Saloner, D.; Wasserman, B.A.; van der Lugt, A. Advances in Multimodality Carotid Plaque Imaging: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2021, 217, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.C.; Saba, L.; Bathla, G.; Brinjikji, W.; Nardi, V.; Lanzino, G. MR Imaging of Carotid Artery Atherosclerosis: Updated Evidence on High-Risk Plaque Features and Emerging Trends. AJNR Am. J. Neuroradiol. 2023, 44, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Agarwal, N.; Cau, R.; Gerosa, C.; Sanfilippo, R.; Porcu, M.; Montisci, R.; Cerrone, G.; Qi, Y.; Balestrieri, A.; et al. Review of imaging biomarkers for the vulnerable carotid plaque. JVS Vasc. Sci. 2021, 2, 149–158. [Google Scholar] [CrossRef]
- Saba, L.; Yuan, C.; Hatsukami, T.S.; Balu, N.; Qiao, Y.; DeMarco, J.K.; Saam, T.; Moody, A.R.; Li, D.; Matouk, C.C.; et al. Vessel Wall Imaging Study Group of the American Society of Neuroradiology. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am. J. Neuroradiol. 2018, 39, E9–E31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, R.; Zhao, X.; He, L.; Wang, X.; Wang, J.; Balu, N.; Yuan, C. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2015, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Balu, N.; Yarnykh, V.L.; Chu, B.; Wang, J.; Hatsukami, T.; Yuan, C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn. Reson. Med. 2011, 65, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guan, M.; Yamada, K.; Hippe, D.S.; Kerwin, W.S.; Yuan, C.; Börnert, P.; Zhao, X. In vivo validation of simultaneous non-Contrast angiography and intraPlaque hemorrhage (SNAP) magnetic resonance angiography: An intracranial artery study. PLoS ONE 2016, 11, e0149130. [Google Scholar] [CrossRef] [PubMed]
- Mugler, J.P. Optimized three-dimensional fast-spin-echo MRI. J. Magn. Reson. Imaging 2014, 39, 745–767. [Google Scholar] [CrossRef]
- Zarins, C.K.; Giddens, D.P.; Bharadvaj, B.K.; Sottiurai, V.S.; Mabon, R.F.; Glagov, S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 1983, 53, 502–514. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Bérczi, Á.; Nyárády, B.B.; Szőnyi, Á.; Philippovich, M.; Dósa, E. Short- and Mid-Term Outcomes of Stenting in Patients with Isolated Distal Internal Carotid Artery Stenosis or Post-Surgical Restenosis. J. Clin. Med. 2022, 11, 5640. [Google Scholar] [CrossRef]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American symptomatic carotid endarterectomy trial: Surgical results in 1415 patients. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef]
- Saba, L.; Anzidei, M.; Marincola, B.C.; Piga, M.; Raz, E.; Bassareo, P.P.; Napoli, A.; Mannelli, L.; Catalano, C.; Wintermark, M. Imaging of the carotid artery vulnerable plaque. Cardiovasc. Interv. Radiol. 2014, 37, 572–585. [Google Scholar] [CrossRef]
- Sitzer, M.; Muller, W.; Siebler, M.; Hort, W.; Kniemeyer, H.W.; Jancke, L.; Steinmetz, H. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke 1995, 26, 1231–1233. [Google Scholar] [CrossRef]
- Fellner, C.; Lang, W.; Janka, R.; Wutke, R.; Bautz, W.; Fellner, F.A. Magnetic resonance angiography of the carotid arteries using three different techniques: Accuracy compared with intraarterial x-ray angiography and endarterectomy specimens. J. Magn. Reson. Imaging 2005, 21, 424–431. [Google Scholar] [CrossRef]
- Etesami, M.; Hoi, Y.; Steinman, D.A.; Gujar, S.K.; Nidecker, A.E.; Astor, B.C.; Portanova, A.; Qiao, Y.; Abdalla, W.M.; Wasserman, B.A. Comparison of carotid plaque ulcer detection using contrast enhanced and time-of-flight MRA techniques. Am. J. Neuroradiol. 2013, 34, 177–184. [Google Scholar] [CrossRef]
- Schindler, A.; Schinner, R.; Altaf, N.; Hosseini, A.A.; Simpson, R.J.; Esposito-Bauer, L.; Singh, N.; Kwee, R.M.; Kurosaki, Y.; Yamagata, S.; et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: Meta-analysis of individual patient data. JACC Cardiovasc. Imaging 2020, 13, 395–406. [Google Scholar] [CrossRef]
- Gupta, A.; Baradaran, H.; Schweitzer, A.D.; Kamel, H.; Pandya, A.; Delgado, D.; Dunning, A.; Mushlin, A.I.; Sanelli, P.C. Carotid plaque MRI and stroke risk: A systematic review and meta-analysis. Stroke 2013, 44, 3071–3077. [Google Scholar] [CrossRef]
- Brinjikji, W.; Iii, J.H.; Rabinstein, A.A.; Kim, G.; Lerman, A.; Lanzino, G. Plaque vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef]
- Saba, L.; Moody, A.R.; Saam, T.; Kooi, M.E.; Wasserman, B.A.; Staub, D.; van der Lugt, A.; DeMarco, J.K.; Saloner, D.; Wintermark, M.; et al. Vessel walleimaging biomarkers of carotid plaque vulnerability in stroke prevention trials: A viewpoint from the Carotid Imaging Consensus Group. JACC Cardiovasc. Imaging 2020, 13, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Porcu, M.; Anzidei, M.; Suri, J.S.; Wasserman, B.A.; Anzalone, N.; Lucatelli, P.; Loi, F.; Montisci, R.; Sanfilippo, R.; Rafailidis, V.; et al. Carotid artery imaging: The study of intra-plaque vascularization and hemorrhage in the era of the “vulnerable” plaque. J. Neuroradiol. 2020, 47, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Micheletti, G.; Brinjikji, W.; Garofalo, P.; Montisci, R.; Balestrieri, A.; Suri, J.S.; DeMarco, J.K.; Lanzino, G.; Sanfilippo, R. Carotid intraplaque-hemorrhage volume and its association with cerebrovascular events. AJNR Am. J. Neuroradiol. 2019, 40, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Francone, M.; Bassareo, P.P.; Lai, L.; Sanfilippo, R.; Montisci, R.; Suri, J.S.; De Cecco, C.N.; Faa, G. CT attenuation analysis of carotid intraplaque hemorrhage. Am. J. Neuroradiol. 2018, 39, 131–137. [Google Scholar] [CrossRef]
- Cai, J.; Hatsukami, T.S.; Ferguson, M.S.; Kerwin, W.S.; Saam, T.; Chu, B.; Takaya, N.; Polissar, N.L.; Yuan, C. In vivo quantitative measurement of intact fibrous cap and lipid rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005, 112, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Aarnio, K.; Siegerink, B.; Pirinen, J.; Sinisalo, J.; Lehto, M.; Haapaniemi, E.; Nave, A.H.; Kaste, M.; Tatlisumak, T.; Putaala, J. Cardiovascular events after ischemic stroke in young adults: A prospective follow-up study. Neurology 2016, 86, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Lavallée, P.C.; Charles, H.; Albers, G.W.; Caplan, L.R.; Donnan, G.A.; Ferro, J.M.; Hennerici, M.G.; Labreuche, J.; Molina, C.; Rothwell, P.M.; et al. Effect of atherosclerosis on 5-year risk of major vascular events in patients with transient ischaemic attack or minor ischaemic stroke: An international prospective cohort study. Lancet Neurol. 2023, 22, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Fridman, S.; Lownie, S.P.; Mandzia, J. Diagnosis and management of carotid free-floating thrombus: A systematic literature review. Int. J. Stroke 2019, 14, 247–256. [Google Scholar] [CrossRef]
- Dowlatshahi, D.; Lum, C.; Menon, B.K.; Bharatha, A.; Dave, P.; Puac-Polanco, P.; Blacquiere, D.; Stotts, G.; Shamy, M.; Momoli, F.; et al. Aetiology of extracranial carotid free-floating thrombus in a prospective multicentre cohort. Stroke Vasc. Neurol. 2023, 8, 194–196. [Google Scholar] [CrossRef]
- Zedde, M.; Moratti, C.; Napoli, M.; Valzania, F.; Pascarella, R. A vertebral artery “donut sign”. Acta Neurol. Belg. 2023. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Grisendi, I.; Assenza, F.; Napoli, M.; Moratti, C.; Bonacini, L.; Di Cecco, G.; D’Aniello, S.; Pavone, C.; Merlino, G.; et al. The Prepetrous Segment of the Internal Carotid Artery as a Neglected Site of Symptomatic Atherosclerosis: A Single-Center Series. J. Clin. Med. 2024, 13, 1696. https://doi.org/10.3390/jcm13061696
Zedde M, Grisendi I, Assenza F, Napoli M, Moratti C, Bonacini L, Di Cecco G, D’Aniello S, Pavone C, Merlino G, et al. The Prepetrous Segment of the Internal Carotid Artery as a Neglected Site of Symptomatic Atherosclerosis: A Single-Center Series. Journal of Clinical Medicine. 2024; 13(6):1696. https://doi.org/10.3390/jcm13061696
Chicago/Turabian StyleZedde, Marialuisa, Ilaria Grisendi, Federica Assenza, Manuela Napoli, Claudio Moratti, Lara Bonacini, Giovanna Di Cecco, Serena D’Aniello, Claudio Pavone, Giovanni Merlino, and et al. 2024. "The Prepetrous Segment of the Internal Carotid Artery as a Neglected Site of Symptomatic Atherosclerosis: A Single-Center Series" Journal of Clinical Medicine 13, no. 6: 1696. https://doi.org/10.3390/jcm13061696
APA StyleZedde, M., Grisendi, I., Assenza, F., Napoli, M., Moratti, C., Bonacini, L., Di Cecco, G., D’Aniello, S., Pavone, C., Merlino, G., Putaala, J., Valzania, F., & Pascarella, R. (2024). The Prepetrous Segment of the Internal Carotid Artery as a Neglected Site of Symptomatic Atherosclerosis: A Single-Center Series. Journal of Clinical Medicine, 13(6), 1696. https://doi.org/10.3390/jcm13061696





