The Changing Role of Loop Diuretics in Heart Failure Management across the Last Century
Abstract
1. Introduction
2. Loop Diuretic Administration and Application during the Last Century
Route of Administration
3. Loop Diuretic Treatment in Acute Heart Failure
4. The Use of Loop Diuretic in Chronic Heart Failure
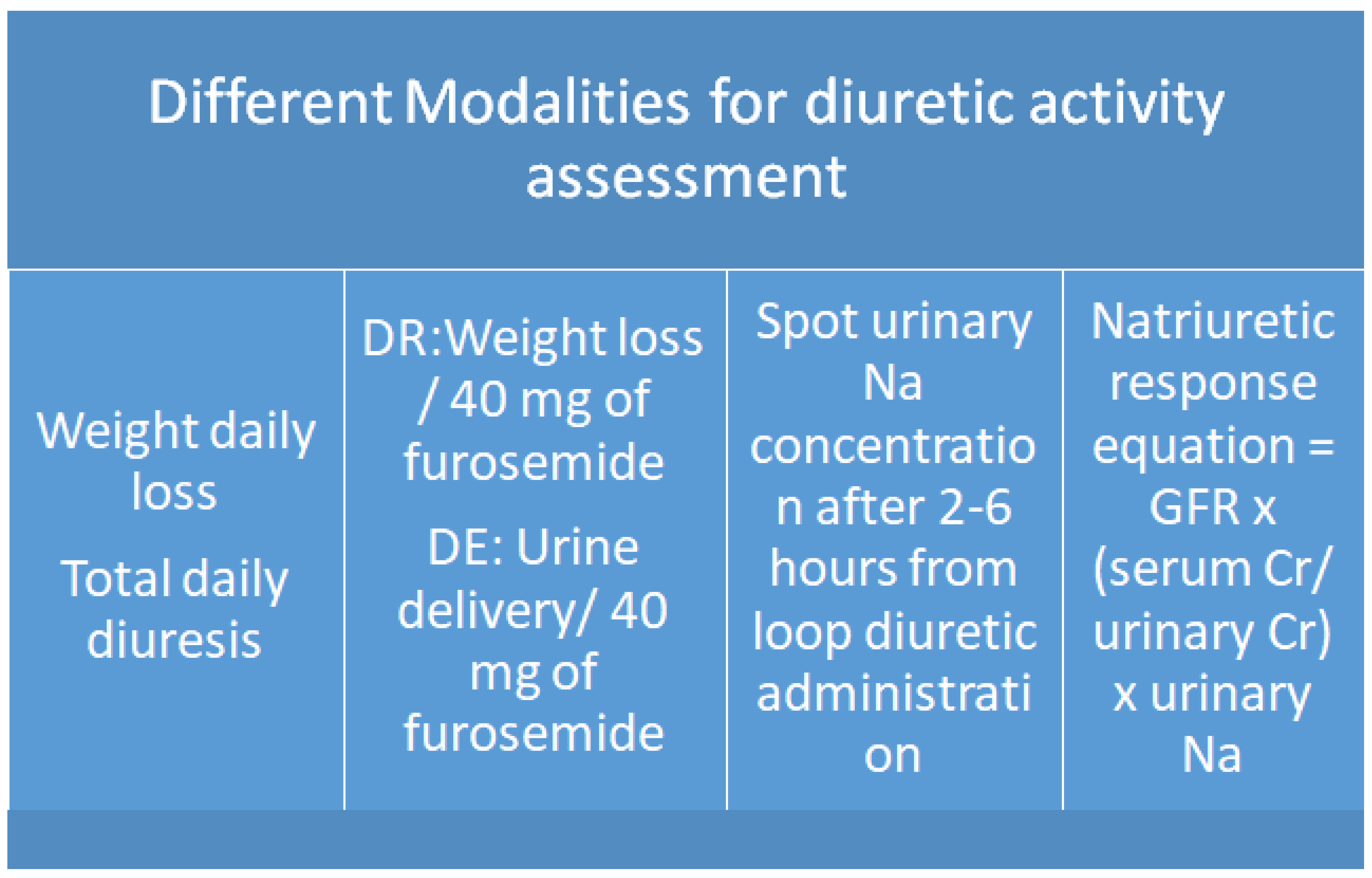
5. How to Evaluate Diuretic Response and Euvolemic Status
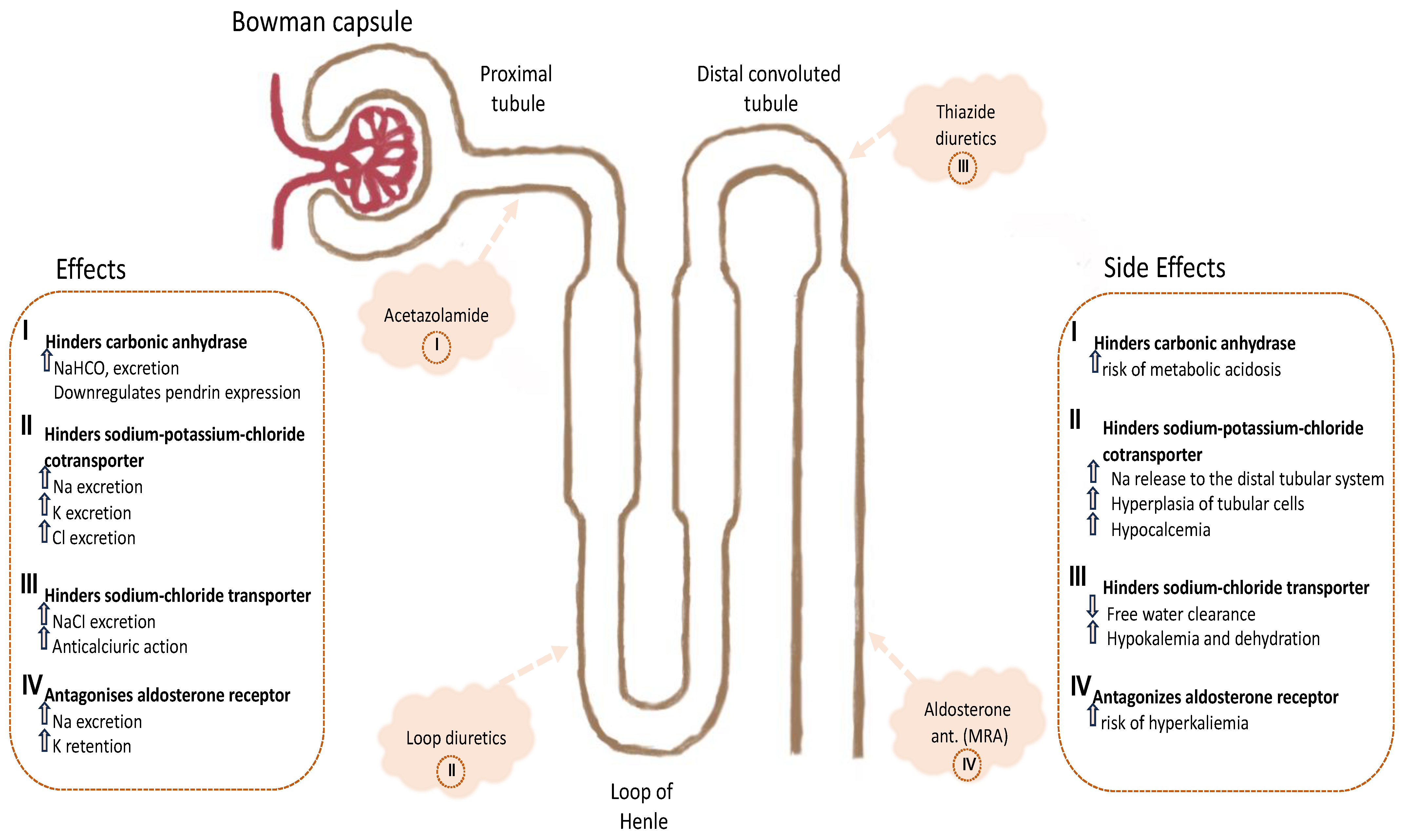
6. How to Avoid Diuretic Resistance by Sequential Nephron Blockade
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Javaloyes, P.; Miró, Ò.; Gil, V.; Martín-Sánchez, F.J.; Jacob, J.; Herrero, P.; Takagi, K.; Alquézar-Arbé, A.; López Díez, M.P.; Martín, E.; et al. Clinical phenotypes of acute heart failure based on signs and symptoms of perfusion and congestion at emergency department presentation and their relationship with patient management and outcomes. Eur. J. Heart Fail. 2019, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Tsang, S.W.; Fang, J.C.; Lewis, E.F.; Jarcho, J.A.; Mudge, G.H.; Stevenson, L.W. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J. Am. Coll. Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R.; et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M. Loop diuretics in heart failure. Heart Fail. Rev. 2012, 17, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Antohi, E.L.; Ambrosy, A.P.; Collins, S.P.; Ahmed, A.; Iliescu, V.A.; Cotter, G.; Pang, P.S.; Butler, J.; Chioncel, O. Therapeutic Advances in the Management of Acute Decompensated Heart Failure. Am. J. Ther. 2019, 26, e222–e233. [Google Scholar] [CrossRef] [PubMed]
- Hasselblad, V.; Stough, W.G.; Shah, M.R.; Lokhnygina, Y.; O’Connor, C.M.; Califf, R.M.; Adams, K.F., Jr. Relation between dose of loop diuretics and outcomes in heart failure population: Results of the ESCAPE trial. Eur. J. Heart Fail. 2007, 9, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Coiro, S.; Girerd, N.; McMurray, J.J.V.; Pitt, B.; Swedberg, K.; van Veldhuisen, D.J.; Lamiral, Z.; Rossignol, P.; Zannad, F. Diuretic therapy as prognostic enrichment factor for clinical trials in patients with heart failure with reduced ejection fraction. Clin. Res. Cardiol. 2021, 110, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Laroche, C.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.S.; Díaz-Molina, B.; Filippatos, G.; Lainscak, M.; Maggioni, A.P.; McDonagh, T.; et al. Association between loop diuretic dose changes and outcomes in chronic heart failure: Observations from the ESC-EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 1424–1437. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Frea, S.; Pidello, S.; Volpe, A.; Canavosio, F.G.; Galluzzo, A.; Bovolo, V.; Camarda, A.; Golzio, P.G.; D’Ascenzo, F.; Bergerone, S.; et al. Diuretic treatment in high-risk acute decompensation of advanced chronic heart failure-bolus intermittent vs. continuous infusion of furosemide: A randomized controlled trial. Clin. Res. Cardiol. 2020, 109, 417–425. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Vescovo, G.; Valle, R.; Di Somma, S.; Nuti, R. Rationale and study design of intravenous loop diuretic administration in acute heart failure: DIUR-AHF. ESC Heart Fail. 2017, 4, 479–486. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic strategies in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M.; Beldhuis, I.E.; van der Meer, P.; Krikken, J.A.; Postmus, D.; Coster, J.E.; Nieuwland, W.; van Veldhuisen, D.J.; Voors, A.A.; Damman, K. Natriuresis-guided diuretic therapy in acute heart failure: A pragmatic randomized trial. Nat. Med. 2023, 29, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Ruocco, G.; Ronco, C.; McCullough, P.A. Loop diuretics in acute heart failure: Beyond the decongestive relief for the kidney. Crit. Care 2015, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Hardin, A.; Grodin, J.L. Diuretic Strategies in Acute Decompensated Heart Failure. Curr. Heart Fail. Rep. 2017, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Bugatti, S.; Bettari, L.; Carubelli, V.; Danesi, R.; Lazzarini, V.; Lombardi, C.; Dei Cas, L. Can we improve the treatment of congestion in heart failure? Expert Opin. Pharmacother 2011, 12, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, J.L.; Testani, J.L. Diuretic Therapy for Patients with Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1178–1195. [Google Scholar] [CrossRef] [PubMed]
- Chiong, J.R.; Cheung, R.J. Loop Diuretic Therapy in Heart Failure:The Need for Solid Evidence on a Fluid Issue. Clin. Cardiol. 2010, 33, 345–352. [Google Scholar] [CrossRef]
- Salvador, D.R.; Rey, N.R.; Ramos, G.C.; Punzalan, F.E. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst. Rev. 2004, CD003178. [Google Scholar]
- Trullàs, J.C.; Morales-Rull, J.L.; Formiga, F. Diuretic therapy in acute heart failure. Med. Clin. 2014, 142 (Suppl. S1), 36–41. [Google Scholar]
- Eshaghian, L.; Horwich, T.B.; Fonarow, G. Relation of Loop Diuretic Dose to Mortality in Advanced Heart Failure. Am. J. Cardiol. 2006, 97, 1759–1764. [Google Scholar] [CrossRef]
- Cetin, G.; Corum, O.; Durna Corum, D.; Atik, O.; Turk, E.; Tekeli, I.O.; Uney, K. Pharmacokinetics of furosemide in goats following intravenous, intramuscular, and subcutaneous administration. J. Vet. Pharmacol. Ther. 2021, 44, 961–966. [Google Scholar] [CrossRef]
- Abrams, J. Intramuscular Bumetanide and Furosemide in Congestive Heart Failure. J. Clin. Pharmacol. 1981, 21, 673–679. [Google Scholar] [CrossRef]
- Holazo, A.A.; Colburn, A.; Gustafson, J.H.; Young, R.L.; Parsonnet, M. Pharmacokinetics of bumetanide following intravenous, intramuscular, and oral administrations to normal subjects. Pharm. Sci. 1984, 73, 1108–1113. [Google Scholar] [CrossRef]
- Pentikäinen, P.J.; Neuvonen, P.J.; Kekki, M.; Penttilä, A. Pharmacokinetics of intravenously administered bumetanide in man. J. Pharmacokinet. Biopharm. 1980, 8, 219–228. [Google Scholar] [CrossRef]
- Kido, H.; Ohtaki, Y. Torasemide (LUPRAC): A review of its pharmacological and clinical profile. Nihon Yakurigaku Zasshi 2001, 118, 97–105. [Google Scholar] [CrossRef]
- Cosín, J.; Díez, J.; TORIC investigators. Torasemide in chronic heart failure: Results of the TORIC study. Eur. J. Heart Fail. 2002, 4, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Menon, V. Torsemide comparison with furosemide for management of heart failure (TRANSFORM-HF) trial. Eur. Heart J. Acute Cardiovasc. Care. 2022, 11, 931–932. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Anstrom, K.J.; Eisenstein, E.L.; Sapp, S.; Greene, S.J.; Morgan, S.; Testani, J.M.; Harrington, A.H.; Sachdev, V.; Ketema, F.; et al. Effect of Torsemide vs. Furosemide after Discharge on All-Cause Mortality in Patients Hospitalized with Heart Failure: The TRANSFORM-HF Randomized Clinical Trial. JAMA 2023, 329, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, G.C. Ethacrynic acid. Clin. Ter. 1966, 37, 350–369. [Google Scholar] [PubMed]
- Mosso, H.E.; Lancestremere, R.G.; Califano, J.; Pérgola, F. New diuretic. Ethacrynic acid. Med. Argent. 1966, 53, 1863–1865. [Google Scholar]
- Balduini, M.; Amidani, A. Clinical evaluation of the diuretic effect of ethacrynic acid. Rev. Clin. Esp. 1969, 112, 117–140. [Google Scholar]
- Kim, K.E.; Onesti, G.; Moyer, J.H.; Swartz, C. Ethacrynic acid and furosemide: Diuretic and hemodynamic effects and clinical uses. Am. J. Cardiol. 1971, 27, 407–415. [Google Scholar] [CrossRef]
- Prichard, B.N.; Brogden, R.N. Xipamide. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy. Drugs 1995, 30, 313–332. [Google Scholar] [CrossRef]
- Lahav, M.; Regev, A.; Ra’anami, P.; Theodor, E. Intermittent administration of furosemide vs. continuous infusion preceded by a loading dose for congestive heart failure. Chest 1992, 102, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Pivac, N.; Rumboldt, Z.; Sardelić, S.; Bagatin, J.; Polić, S.; Ljutić, D.; Naranca, M.; Capkun, V. Diuretic effects of furosemide infusion versus bolus injection in congestive heart failure. Int. J. Clin. Pharmacol. Res. 1998, 18, 121–128. [Google Scholar]
- Dormanns, T.P.; Gerlag, P.G.; Russel, F.G.; Smits, P. Combination diuretic therapy in severe congestive heart failure. Drugs 1998, 55, 165–172. [Google Scholar] [CrossRef]
- Kramer, W.G.; Smith, W.B.; Ferguson, J.; Serpas, T.; Grant, A.G., 3rd; Black, P.K.; Brater, D.C. Pharmacodynamics of torsemide administered as an intravenous injection and as a continuous infusion to patients with congestive heart failure. J. Clin. Pharmacol. 1996, 36, 265–270. [Google Scholar] [CrossRef]
- Aaser, E.; Gullestad, L.; Tølløfsrud, S.; Lundberg, J.; Hall, C.; Djøseland, O.; Kjekshus, J.; Forfang, K. Effect of bolus injection versus continuous infusion of furosemide on diuresis and neurohormonal activation in patients with severe congestive heart failure. Scand. J. Clin. Lab. Investig. 1997, 57, 361–367. [Google Scholar] [CrossRef]
- Schuller, D.; Lynch, J.P.; Fine, D. Protocol-guided diuretic management: Comparison of furosemide by continuous infusion and intermittent bolus. Crit. Care Med. 1997, 25, 1969–1975. [Google Scholar] [CrossRef]
- Wu, M.Y.; Chang, N.C.; Su, C.L.; Hsu, Y.H.; Chen, T.W.; Lin, Y.F.; Wu, C.H.; Tam, K.W. Loop diuretic strategies in patients with acute decompensated heart failure: A meta-analysis of randomized controlled trials. J. Crit. Care 2014, 29, 2–9. [Google Scholar] [CrossRef]
- Licata, G.; Di Pasquale, P.; Parrinello, G.; Cardinale, A.; Scandurra, A.; Follone, G.; Argano, C.; Tuttolomondo, A.; Paterna, S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: Long-term effects. Am. Heart J. 2003, 145, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Khan, M.S.; Patel, K.V.; Butler, J.; Ravichandran, A.K.; Walsh, M.N.; Willett, D.; Fonarow, G.C.; Drazner, M.H.; Mentz, R.J.; et al. A Phenomapping Tool and Clinical Score to Identify Low Diuretic Efficiency in Acute Decompensated Heart Failure. JACC Heart Fail. 2024, 12, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Pellegrini, M.; Ruocco, G.; Martini, G.; Franci, B.; Campagna, M.S.; Gilleman, M.; Nuti, R.; McCullough, P.A.; Ronco, C. Continuous versus bolus intermittent loop diuretic infusion in acutely decompensated heart failure: A prospective randomized trial. Crit. Care. 2014, 18, R134. [Google Scholar] [CrossRef] [PubMed]
- Salvador, D.R.; Punzalan, F.E.; Rey, N.R.; Bernardo, M.R.; Nablo, M.D. Continuous Infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst. Rev. 2005, 2005, CD003178. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Ryan, J. Diuretic dosing in acute decompensated heart failure: Lessons from DOSE. Curr. Heart Fail. Rep. 2012, 9, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Pellegrini, M.; Franci, B.; Beltrami, M.; Ruocco, G.; Gonnelli, S.; Angelini, G.D.; Nuti, R. Short and long-term effects of continuous versus intermittent loop diuretics treatment in acute heart failure with renal dysfunction. Intern. Emerg. Med. 2015, 10, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Pellicori, P.; Incampo, E.; Di Tommaso, C.; Favilli, R.; Evangelista, I.; Nuti, R.; Testani, J.M. The prognostic role of different renal function phenotypes in patients with acute heart failure. Int. J. Cardiol. 2019, 276, 198–203. [Google Scholar] [CrossRef]
- Filippatos, G.; Zannad, F. An introduction to acute heart failure syndromes: Definition and classification. Heart Fail. Rev. 2007, 12, 87–90. [Google Scholar] [CrossRef]
- Harjola, V.P.; Mullens, W.; Banaszewski, M.; Bauersachs, J.; Brunner-La Rocca, H.P.; Chioncel, O.; Collins, S.P.; Doehner, W.; Filippatos, G.S.; Flammer, A.J. Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association(HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2017, 19, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Pang, P.S. Acute heart failure syndromes. J. Am. Coll. Cardiol. 2009, 53, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.; Nunez, E.; Fonarow, G.C.; Sanchis, J.; Bodi, V.; Bertomeu Gonzalez, V.; Minana, G.; Merlos, P.; Bertomeu-Martinez, V.; Redon, J.; et al. Differential prognostic effect of systolic blood pressure on mortality according to left-ventricular function in patients with acute heart failure. Eur. J. Heart Fail. 2010, 12, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.H.; Felker, G.M. Diuretic treatment in heart failure. N. Engl. J. Med. 2017, 377, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Kula, A.J.; Hanberg, J.S.; Wilson, F.P.; Brisco, M.A.; Bellumkonda, L.; Jacoby, D.; Coca, S.G.; Parikh, C.R.; Tang, W.H.; Testani, J.M. Influence of titration of neurohormonal antagonists and blood pressure reduction on renal function and decongestion in decompensated heart failure. Circ. Heart Fail. 2016, 9, e002333. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.N.; Arrigo, M.; Placido, R.; Akiyama, E.; Girerd, N.; Zannad, F.; Manivet, P.; Rossignol, P.; Badoz, M.; Sadoune, M.; et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur. J. Heart Fail. 2018, 20, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Bhatt, A.S.; Gallup, D.; Anstrom, K.J.; Butler, J.; DeVore, A.D.; Felker, G.M.; Fudim, M.; Greene, S.J.; Hernandez, A.F.; et al. Trajectory of congestion metrics by ejection fraction in patients with acute heart failure (from the Heart Failure Network). Am. J. Cardiol. 2017, 120, 98–105. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Mullens, W. Current approach to decongestive therapy in acute heart failure. Curr. Heart Fail. Rep. 2015, 12, 367–378. [Google Scholar] [CrossRef]
- Felker, G.M.; Mentz, R.J. Diuretics and ultrafiltration in acute decompensated heart failure. J. Am. Coll. Cardiol. 2012, 59, 2145–2153. [Google Scholar] [CrossRef]
- Bikdeli, B.; Strait, K.M.; Dharmarajan, K.; Partovian, C.; Coca, S.G.; Kim, N.; Li, S.X.; Testani, J.M.; Khan, U.; Krumholz, H.M. Dominance of furosemide for loop diuretic therapy in heart failure: Time to revisit the alternatives? J. Am. Coll. Cardiol. 2013, 61, 1549–1550. [Google Scholar] [CrossRef]
- Damman, K.; Kjekshus, J.; Wikstrand, J.; Cleland, J.G.; Komajda, M.; Wedel, H.; Waagstein, F.; McMurray, J.J. Loop diuretics, renal function and clinical out come in patients with heart failure and reduced ejection fraction. Eur. J. Heart Fail. 2016, 18, 328–336. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Martens, P.; Damman, K.; Dickstein, K.; Ponikowski, P.; Lang, C.C.; Ng, L.L.; Anker, S.D.; Samani, N.J.; Filippatos, G.; et al. Higher doses of loop diuretics limit uptitration of angiotensin-converting enzyme inhibitors in patients with heart failure and reduced ejection fraction. Clin. Res. Cardiol. 2020, 109, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in acute decompensated heart failure with volume overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Testani, J.M.; Brisco, M.A.; Kociol, R.D.; Jacoby, D.; Bellumkonda, L.; Parikh, C.R.; Coca, S.G.; Tang, W.W. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am. J. Med. 2015, 128, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Albert, N.M.; Boehmer, J.P.; Collins, S.P.; Ezekowitz, J.A.; Givertz, M.M.; Katz, S.D.; Klapholz, M.; Moser, D.K.; Rogers, J.G.; et al. HFSA 2010 comprehensive heart failure practice guideline. J. Card. Fail. 2010, 16, e1–e194. [Google Scholar]
- Honda, S.; Nagai, T.; Nishimura, K.; Nakai, M.; Honda, Y.; Nakano, H.; Iwakami, N.; Sugano, Y.; Asaumi, Y.; Aiba, T.; et al. Longterm prognostic significance of urinary sodium concentration in patients with acute heart failure. Int. J. Cardiol. 2018, 254, 189–194. [Google Scholar] [CrossRef]
- Luk, A.; Groarke, J.D.; Desai, A.S.; Mahmood, S.S.; Gopal, D.M.; Joyce, E.; Shah, S.P.; Lindenfeld, J.; Stevenson, L.; Lakdawala, N.K. First spot urine sodium after initial diuretic identifies patients at high risk for adverse outcome after heart failure hospitalization. Am. Heart J. 2018, 203, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Zymlinski, R.; Sokolski, M.; Todd, J.; Cotter, G.; Metra, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur. J. Heart Fail. 2019, 21, 624–633. [Google Scholar] [CrossRef]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ. Heart Fail. 2016, 9, e002370. [Google Scholar] [CrossRef]
- Mullens, W.; Verbrugge, F.H.; Nijst, P.; Martens, P.; Tartaglia, K.; Theunissen, E.; Bruckers, L.; Droogne, W.; Troisfontaines, P.; Damman, K.; et al. Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur. J. Heart Fail. 2018, 20, 1591–1600. [Google Scholar] [CrossRef]
- Cox, Z.L.; Hung, R.; Lenihan, D.J.; Testani, J.M. Diuretic strategies for loop diuretic resistance in acute heart failure: The 3T trial. JACC Heart Fail. 2020, 8, 157–168. [Google Scholar] [CrossRef]
- Pellicori, P.; Platz, E.; Dauw, J.; Maaten, J.M.; Martens, P.; Pivetta, E.; Cleland, J.G.; McMurray, J.J.; Mullens, W.; Solomon, S.D.; et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur. J. Heart Fail. 2021, 23, 703–712. [Google Scholar] [CrossRef]
- Brisco, M.A.; Zile, M.R.; Hanberg, J.S.; Wilson, F.P.; Parikh, C.R.; Coca, S.G.; Tang, W.H.; Testani, J.M. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: Insights from the DOSE trial. J. Card. Fail. 2016, 22, 753–760. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Swennen, Q.; Tang, W.H.; Mullens, W. The kidney in congestive heart failure: ‘Are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur. J. Heart Fail. 2014, 16, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; De Luca, L.; Fonarow, G.C.; Filippatos, G.; Metra, M.; Francis, G.S. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am. J. Cardiol. 2005, 96, 11G–17G. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and grading congestion in acute heart failure: A scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Weingert, M.E.; Fisher, R.H.; Gryfe, C.I.; Schulman, H.S. Unnecessary diuretic therapy in the elderly. Age Ageing 1982, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.G.; Liang, C.S.; Baumgardner, S.; Charuzi, Y.; Chardo, F.; Kim, C.S. Peritanide, a potent diuretic with potassiumsparing properties, for treatment of congestive heart failure. Clin. Pharmacol. Ther. 1986, 40, 587–594. [Google Scholar] [CrossRef]
- De Jong, J.W.; Knottnerus, J.A.; van Zutphen, W.M.; De Bruijne, G.A.; Struijker Boudier, H.A. Short-term effect of withdrawal of diuretic drugs prescribed for ankle oedema. BMJ 1994, 308, 511–513. [Google Scholar] [CrossRef]
- Boccanelli, A.; Zachara, E.; Liberatore, S.M.; Carboni, G.P.; Prati, P.L. Addition of captopril versus increasing diuretics in moderate but deteriorating heart failure: A double-blind comparative trial. Postgrad. Med. J. 1986, 62 (Suppl. S1), 184–187. [Google Scholar] [PubMed]
- Haerer, W.; Hetzel, M.; Fehske, K.L.; Fehske, K.L.; Stauch, M. The impact of digoxin and diuretics on exercise tolerance in congestive heart failure: A placebo controlled randomised double-blind trial. Circulation 1989, 80, 1698A. [Google Scholar]
- Verel, D.; Rahman, F.; Stentiford, N.H.; Saynor, R. A Clinical Trial of Frusemide. Lancet 1964, 284, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Galve, E.; Mallol, A.; Catalan, R.; Palet, J.; Mendez, S.; Nieto, E.; Diaz, A.; Soler-Soler, J. Clinical and neurohumoral consequences of diuretic withdrawal in patients with chronic, stabilized heart failure and systolic dysfunction. Eur. J. Heart Fail. 2005, 7, 892–898. [Google Scholar] [CrossRef]
- Grinstead, W.C.; Francis, M.J.; Marks, G.F.; Tawa, C.B.; Zoghbi, W.A.; Young, J.B. Discontinuation of chronic diuretic therapy in stable congestive heart failure secondary to coronary artery disease or to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1994, 73, 881–888. [Google Scholar] [CrossRef]
- Beygui, F.; Anguita, M.; Tebbe, U.; Comin-Colet, J.; Galinier, M.; Bramlage, P.; Turgonyi, E.; Lins, K.; Imekraz, L.; de Frutos, T.; et al. A real-world perspective on the prevalence and treatment of heart failure with a reduced ejection fraction but no specific or only mild symptoms. Heart Fail. Rev. 2015, 20, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Mathieu, C.; Verbrugge, F.H. Promise of SGLT2 inhibitors in heart failure: Diabetes and beyond. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 23. [Google Scholar] [CrossRef]
- Martens, P.; Belien, H.; Dupont, M.; Mullens, W. Insights into implementation of sacubitril/valsartan into clinical practice. ESC Heart Fail. 2018, 5, 275–283. [Google Scholar] [CrossRef]
- Cotter, G.; Metra, M.; Milo-Cotter, O.; Dittrich, H.C.; Gheorghiade, M. Fluid overload in acute heart failure--re-distribution and other mechanisms beyond fluid accumulation. Eur. J. Heart Fail. 2008, 10, 165–169. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Nuti, R. Congestion occurrence and evaluation in acute heart failure scenario: Time to reconsider different pathways of volume overload. Heart Fail. Rev. 2020, 25, 119–131. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Testani, J.M.; Pitt, B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension 2020, 76, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.A.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Spatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.H. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail. 2014, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Cox, Z.L.; Rao, V.S.; Testani, J.M. Classic and Novel Mechanisms of Diuretic Resistance in Cardiorenal Syndrome. Kidney360 2022, 3, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Mullens, W.; Tang, W.H. Management of Cardio-Renal Syndrome and Diuretic Resistance. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Biegus, J.; Zymlinski, R.; Testani, J.; Marciniak, D.; Zdanowicz, A.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high-risk acute heart failure patients. Eur. J. Heart Fail. 2021, 23, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Ivey-Miranda, J.B.; Cox, Z.L.; Riello, R.; Griffin, M.; Fleming, J.; Soucier, R.; Sangkachand, P.; O’Brien, M.; LoRusso, F.; et al. Natriuretic Equation to Predict Loop Diuretic Response in Patients with Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Verbrugge, F.H.; Nijst, P.; Tang, W.H.W. Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. Eur. Heart J. 2017, 38, 1872–1882. [Google Scholar] [CrossRef]
- Ellison, D.H. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001, 96, 132–143. [Google Scholar] [CrossRef]
- Knauf, H.; Mutschler, E. Diuretic effectiveness of hydrochlorothiazide and furosemide alone and in combination in chronic renal failure. J. Cardiovasc. Pharmacol. 1995, 26, 394–400. [Google Scholar] [CrossRef]
- Sica, D.A. Metolazone and its role in edema management. Congest. Heart Fail. 2003, 9, 100–105. [Google Scholar] [CrossRef]
- Shulenberger, C.E.; Jiang, A.; Devabhakthuni, S.; Ivaturi, V.; Liu, T.; Reed, B.N. Efficacy and Safety of Intravenous Chlorothiazide versus Oral Metolazone in Patients with Acute Decompensated Heart Failure and Loop Diuretic Resistance. Pharmacotherapy 2016, 36, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, T.; Pan, I.Z.; Biskupiak, J.; Shah, K.S.; Fang, J.C.; Jacobs, J.A. Metolazone versus intravenous chlorothiazide for decompensated heart failure sequential nephron blockade: A retrospective cohort study. J. Cardiac Fail. 2022, 28, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Ruocco, G.; Severino, P.; Gennari, L.; Pirrotta, F.; Stefanini, A.; Tramonte, F.; Feola, M.; Mancone, M.; Fedele, F. Effects of Metolazone Administration on Congestion, Diuretic Response and Renal Function in Patients with Advanced Heart Failure. J. Clin. Med. 2021, 10, 4207. [Google Scholar] [CrossRef] [PubMed]
- Brisco-Bacik, M.A.; Ter Maaten, J.M.; Houser, S.R.; Vedage, N.A.; Rao, V.; Ahmad, T.; Wilson, F.P.; Testani, J.M. Outcomes Associated with a Strategy of Adjuvant Metolazone or High-Dose Loop Diuretics in Acute Decompensated Heart Failure: A Propensity Analysis. J. Am. Heart Assoc. 2018, 7, e009149. [Google Scholar] [CrossRef]
- Asare, K. Management of loop diuretic resistance in the intensivecare unit. Am. J. Health Syst. Pharm. 2009, 66, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Knauf, H.; Mutschler, E. Sequential nephron blockade breaks resistance to diuretics in edematous states. J. Cardiovasc. Pharmacol. 1997, 29, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Stevens, S.R.; De Las Fuentes, L.; Kiernan, M.; Birati, E.Y.; Gupta, D.; Bart, B.A.; Felker, G.M.; Chen, H.H.; Butler, J.; et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J. Card. Fail. 2016, 22, 26–32. [Google Scholar] [CrossRef]
- Zahedi, K.; Barone, S.; Xu, J.; Soleimani, M. Potentiation of the effect of thiazide derivatives by carbonic anhydrase inhibitors: Molecular mechanisms and potential clinical implications. PLoS ONE 2013, 8, e79327. [Google Scholar] [CrossRef]
- Amlal, H.; Soleimani, M. Pendrin as a novel target for diuretic therapy. Cell Physiol. Biochem. 2011, 28, 521–526. [Google Scholar] [CrossRef]
- Soleimani, M.; Barone, S.; Xu, J.; Shull, G.E.; Siddiqui, F.; Zahedi, K.; Amlal, H. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc. Natl. Acad. Sci. USA 2012, 109, 13368–13373. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Martens, P.; Ameloot, K.; Haemels, V.; Penders, J.; Dupont, M.; Tang, W.H.W.; Droogné, W.; Mullens, W. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur. J. Heart Fail. 2019, 21, 1415–1422. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J. Efficacy and safety of spironolactone in acute heart failure: The ATHENA-HF randomized clinical trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Llàcer, P.; Núñez, J.; García, M.; Ruiz, R.; López, G.; Fabregate, M.; Fernández, C.; Croset, F.; Del Hoyo, B.; Gomis, A.; et al. Comparison of chlorthalidone and spironolactone as additional diuretic therapy in patients with acute heart failure and preserved ejection fraction. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Munoz, K.; Brune, S.; Bailey, S.; Prasad, A.; Velagapudi, C. High-Dose Spironolactone When Patients with Acute Decompensated Heart Failure Are Resistant to Loop Diuretics: A Pilot Study. Ann. Intern. Med. 2019, 171, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Pitt, B.; Palmer, B.F.; Kovesdy, C.P.; Burgess, E.; Filippatos, G.; Małyszko, J.; Ruilope, L.M.; Rossignol, P.; Rossing, P.; et al. A comparative post hoc analysis of finerenone and spironolactone in resistant hypertension in moderate-to-advanced chronic kidney disease. Clin. Kidney J. 2022, 16, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Pitt, B.; Agarwal, R.; Farmakis, D.; Ruilope, L.M.; Rossing, P.; Bauersachs, J.; Mentz, R.J.; Kolkhof, P.; Scott, C.; et al. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: A prespecified subgroup analysis of the FIDELIO-DKD trial. Eur. J. Heart Fail. 2022, 24, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Anker, S.D.; Agarwal, R.; Ruilope, L.M.; Rossing, P.; Bakris, G.L.; Tasto, C.; Joseph, A.; Kolkhof, P.; Lage, A.; et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation 2022, 145, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Angermann, C.E.; Collins, S.P.; Teerlink, J.R.; Ponikowski, P.; Biegus, J.; Comin-Colet, J.; Ferreira, J.P.; Mentz, R.J.; Nassif, M.E.; et al. Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results from the EMPULSE Trial. Circulation 2022, 146, 279–288. [Google Scholar] [CrossRef]
- Biegus, J.; Voors, A.A.; Collins, S.P.; Kosiborod, M.N.; Teerlink, J.R.; Angermann, C.E.; Tromp, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; et al. Impact of empagliflozin on decongestion in acute heart failure: The EMPULSE trial. Eur. Heart J. 2023, 44, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]


| First Author, Publication Year, Study Type | Loop Diuretic Type | Patient Characteristics (n), NYHA Class Age (Years) Sex (M/F) | Administration Modality | Main Endpoints and Findings |
|---|---|---|---|---|
| Lahav, 1992 [36] prospective, randomized, cross-over, intention to treat | Furosemide | 9 III–IV 74 (68–80) 5/4 | Continuous furosemide: 30–40 mg of loading dose IV followed by 2.5–3.3 mg/h (60–80 mg/day × 48 h; total dose: 90–120 mg/day) Bolus: 30–40 mg every 8 h × 48 h (total dose: 90–120 mg/day) | Major natriuresis and diuresis in continuous infusion group No significant differences in side effects between two groups |
| Bagatin, 1998 [37] randomized cross-over, single-blind; intention to treat | Furosemide | 12 70.9 ± 6.9 5/7 | Continuous: 40 mg IV in 125 cc of 5% dextrose in 1 h × 2 doses Bolus: 40 mg IV × 2 doses | Greater natriuresis and 24 h urinary output in continuous infusion LD vs. bolus LD group |
| Dormanns, 1996 [38] randomized, cross-over, intention to treat | Furosemide | 20 III–IV 71 (51–89) 13/7 | Continuous furosemide: 20% of the total dose as loading dose; then, infusion of 10% of the total dose in 8 h Bolus: 1 dose injected in 5 min of a mean of 690 mg (250–2000 mg) | Mean daily urinary volume and sodium excretion were significantly higher after treatment with continuous infusion than with bolus injection |
| Kramer, 1996 [39] open label, randomized cross-over, intention to treat | Torasemide | 8 II–III 44–65 7/1 | Continuous torasemide: 25 mg as loading dose, followed by 75 mg in 24 h (3.125 mg/h) Bolus: 100 mg | Continuous vs. bolus torsemide administration resulted in greater 24 h diuresis and natriuresis |
| Aaser, 1997 [40] prospective randomized cross-over, intention to treat; | Furosemide | 8 54 ± 3 6/2 | Continuous furosemide: 145 ± 80 mg in 100cc 5% dextrose (range 80–320 mg) × 24 h Bolus: 145 ± 80 mg (range 80–320 mg) given morning (8 am) and afternoon (3 pm) | Bolus LD administration in HF is equally effective as continuous intravenous, which may be related to maximal neurohormonal activation which could not be further activated by bolus administration |
| Schuller, 1997 [41] prospective, randomized, comparative, intention to treat; method of blinding: not stated | Furosemide | 33 18–85 30/3 | Furosemide 40 mg then continuous 250 mg in 250 cc of 5% dextrose started at 0.1 mg/kg/h, increased until hourly urine output ≥ 1 cc/kg Bolus: Repeat or double previous dose increased until a net hourly urine output ≥ 1 mg/kg | A tailored patient strategy of diuretic treatment in intensive care is safe and effective |
| Wu, 2014 [42] randomized single-blind cross-over, intention to treat | Furosemide | 20 III–IV 35–75 9/11 | Continuous furosemide: 40 mg in 116 cc saline × four h, 2 × day Bolus: 40 mg × three minutes, twice a day | In the treatment of refractory edema in patients with congestive heart failure, continuous intravenous infusion of furosemide is superior to the conventional intermittent bolus injection, especially if it is administered at the very beginning of the hospital treatment, |
| TORIC STUDY, 2002 [28] open-label prospective cohort | Torasemide | 1377 II–III | Oral | The study documented torsemide safety and efficacy in HF treatment and suggested a lower mortality rate in comparison with furosemide |
| Licata, 2003 [43] randomized, single-blind, intention to treat | Furosemide | 107 IV 65–90 39/21 | Continuous furosemide: hypertonic saline solution (150 cc of 1.4–4.6% sodium chloride) with 500–1000 mg of furosemide twice a day in 30 min Bolus: 500–1000 mg twice a day without hypertonic saline solution Treatment duration: 6–12 days | Urine output at 24 h Length of hospitalization All-cause mortality, cardiac mortality |
| DOSE (diuretic optimization strategies evaluation), 2011 [12], prospective-double-blind, randomized trial | Furosemide | 308 | Continuous infusion and low dose versus double bolus and high dose | The combination of high-dose furosemide and small-volume hypertonic saline solution (HSS) infusion is effective and well tolerated, improves quality of life, and may delay more aggressive HF treatments |
| DIUR- HF, 2019 [45] single-center, retrospective, observational study | Furosemide | 247 | Intravenous continuous versus intermittent infusion | Cardiovascular rehospitalization rate was higher in high dose furosemide group (>125 mg median dose) vs. low-dose group (<125 mg median dose) |
| Frea, 2020 [10] single-center, double-blind, double-dummy, randomized | Furosemide | 80 | Bolus intermittent vs. continuous infusion | Among patients with acute decompensation of ACHF and high risk of diuretic resistance, continuous infusion of intravenous furosemide was associated with better decongestion |
| Diuretic | Site of Action | Starting Dose | Usual Dose | Half-Life | Onset | Oral Bioavailability | |
|---|---|---|---|---|---|---|---|
| Loop diuretics | Furosemide | Ascending loop of Henle | 20–40 mg | 40–240 mg | 1.5–3.0 h | 0.5–1 | 10–100% |
| Bumetanide | Ascending loop of Henle | 0.5–1 mg | 1–5 mg | 1–1.5 h | 0.5–1 h | 80–100% | |
| Torasemide | Ascending loop of Henle | 5–10 mg | 10–20 mg | 3–6 h | 0.5–1 h | 80–100% | |
| Thiazides/thiazide-like diuretics | Bendroflumethiazide | Early distal convoluted tubule | 2.5 mg | 2.5–10 mg | 6–12 h | 1–2.5 h | 100% |
| Hydrochlorothiazide | Early distal convoluted tubule | 25 mg | 12.5–100 mg | 6–15 h | 1–2.5 h | 65–75% | |
| Metolazone | Early distal convoluted tubule | 2.5 mg | 2.5–10 mg | 6–20 h | 1–2.5 h | 60–65% | |
| Non-thiazide sulfonamide | Indapamide | Early distal convoluted tubule | 2.5 mg | 2.5–5 mg | 14–18 h | 1–2.5 h | 100% |
| How to use |
| ||||||
| Problem solving | Low blood pressure, asymptomatic |
| |||||
| Symptomatic hypotension |
| ||||||
| Hypokalemia/hypomagnesemia |
| ||||||
| Hyponatremia (<135 mmol/L) |
| ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzuoli, A.; Mazzeo, P.; Fortunato, M.; Cadeddu Dessalvi, C.; Mariano, E.; Salzano, A.; Severino, P.; Fedele, F. The Changing Role of Loop Diuretics in Heart Failure Management across the Last Century. J. Clin. Med. 2024, 13, 1674. https://doi.org/10.3390/jcm13061674
Palazzuoli A, Mazzeo P, Fortunato M, Cadeddu Dessalvi C, Mariano E, Salzano A, Severino P, Fedele F. The Changing Role of Loop Diuretics in Heart Failure Management across the Last Century. Journal of Clinical Medicine. 2024; 13(6):1674. https://doi.org/10.3390/jcm13061674
Chicago/Turabian StylePalazzuoli, Alberto, Pietro Mazzeo, Martino Fortunato, Christian Cadeddu Dessalvi, Enrica Mariano, Andrea Salzano, Paolo Severino, and Francesco Fedele. 2024. "The Changing Role of Loop Diuretics in Heart Failure Management across the Last Century" Journal of Clinical Medicine 13, no. 6: 1674. https://doi.org/10.3390/jcm13061674
APA StylePalazzuoli, A., Mazzeo, P., Fortunato, M., Cadeddu Dessalvi, C., Mariano, E., Salzano, A., Severino, P., & Fedele, F. (2024). The Changing Role of Loop Diuretics in Heart Failure Management across the Last Century. Journal of Clinical Medicine, 13(6), 1674. https://doi.org/10.3390/jcm13061674







