Psychometric Properties of the Gastrointestinal Symptom Severity Scale in a Sample of Adolescents and Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Sample Size
2.4. Measures
- -
- Clinical questionnaire of gastro-intestinal symptoms: This is an ad hoc questionnaire that was developed to examine gastro-intestinal disorders according to Rome criteria [1]. The tool consists of a series of questions regarding gastrointestinal disorders (e.g., diarrhea, abdominal pain, dyspepsia, gastroesophageal reflux, etc.) and family history.
- -
- Gastrointestinal Symptom Severity Scale (GSSS): This instrument is based on Rome IV criteria [1] and consists of seven items pertaining to main gastro-intestinal symptoms (constipation, diarrhea, average stool consistency, stool odor, flatulence and gas, and abdominal pain). The instrument comprises an abdominal subscale (abdominal pain, gas, and constipation) and a vomiting and defecation subscale (vomiting, defecation in inappropriate places, diarrhea, and rumination). Items are rated along a four-point Likert scale ranging from 0 (none/nothing or this symptom does not occur) to 3 (very frequent and troublesome symptom). The GSSS presents adequate psychometric properties in individuals with autism and in neurotypical children and adolescents [14,22]. Internal consistency coefficients of 0.73 have been reported in children with typical development [22], whilst coefficients between 0.61 and 0.75 have been reported in individuals with autism [14].Two versions of the instrument are available, namely, a version for caregivers–professionals and a self-report version. The self-report version of the test was administered in the present study (identical to the version for children and adolescents up to 16 years).
- -
- Pain and Sensitivity Reactivity Scale (PSRS): This scale evaluates reactivity to pain and sensory reactivity according to 50 items. It is composed of three dimensions: pain, sensory hypo-reactivity, and sensory hyper-reactivity. Hyposensitivity and hypersensitivity dimensions include tactile, olfactory, visual, gustatory, and auditory items. All items are rated along a four-point Likert scale ranging from 0 (behavior does not occur) to 3 (behavior occurs and is a severe problem). In addition, the PSRS includes a pain reactivity domain that comprises seven items. The PSRS is based on a theory elaborated by Miller et al. [26] that alludes to sensory modulation disorders that are characterized by three different patterns (hyper-response, hypo-response, and sensory seeking) in accordance with identified diagnostic nosology. Two versions of the PSRS are available, specifically, a version for caregivers–professionals and a self-report version. The self-report version was used in the present study. Cronbach’s alpha values were calculated to evaluate the internal consistency of the overall scale, and its subscales showed strong internal consistency in a neurotypical young adult population (PSRS-total = 0.92; pain = 0.79; broad sensory hypo-reactivity = 0.88; broad sensory hyper-reactivity = 0.90) [27]. The caregiver version of the PSRS also demonstrated excellent internal consistency (pain = 0.83; broad sensory hypo-reactivity = 0.90; broad sensory hyper-reactivity = 0.93) in a sample of individuals with autism spectrum disorders (ASD) [14]. The self-report version was used in the present study.
- -
- Sensory Over-Responsivity Scales (SOR-Scales): The SORS assesses sensory hyper-reactivity to auditory, tactile, visual, olfactory, and taste stimuli. This tool was adapted from a measure used with a general community sample in a survey study [28]. It consists of rating scales addressing distress and impairment in relation to both auditory and tactile over-reactivity [29]. Each SORS subscale comprises four questions, with responses being provided along on a scale ranging from 0 to 4. Overall scores range from 0 to 80. Overall scores for each subscale are calculated separately and range from 0 to 16, with higher scores indicating greater severity. Cronbach alpha outcomes evaluating the internal consistency of the SORS overall and of its subscales indicated strong internal consistency when used in a sample from the United States (SOR-total = 0.93; SOR-hearing = 0.89; SOR-touch = 0.88; SOR-smell = 0.90; SOR-sight = 0.94; SOR-taste = 0.88) and in a sample from Spain (hearing = 0.89; touch = 0.86; smell = 0.91; sight = 0.90; taste = 0.86) [30].
- -
- Obsessive–Compulsive Inventory–Revised (OCI-R): The OCI-R is an 18-item self-report questionnaire that assesses obsessive–compulsive symptom severity using a five-point Likert scale ranging from 0 (not at all) to 4 (very much). The OCI-R is comprised of six factors that represent the following symptom domains: checking, ordering, neutralizing, washing, obsessing, and hoarding [31]. Each factor is composed of three items, with possible scores ranging from 0 to 12. Overall, the measure has demonstrated good internal consistency when used in different countries (Cronbach’s α values ranging from 0.81 to 0.95 [32,33,34]).
2.5. Procedure
2.6. Data Analyses
2.7. Ethical Considerations
3. Results
3.1. Socio-Demographic and Clinical Characteristics of the Sample
3.2. Psychometric Assessment
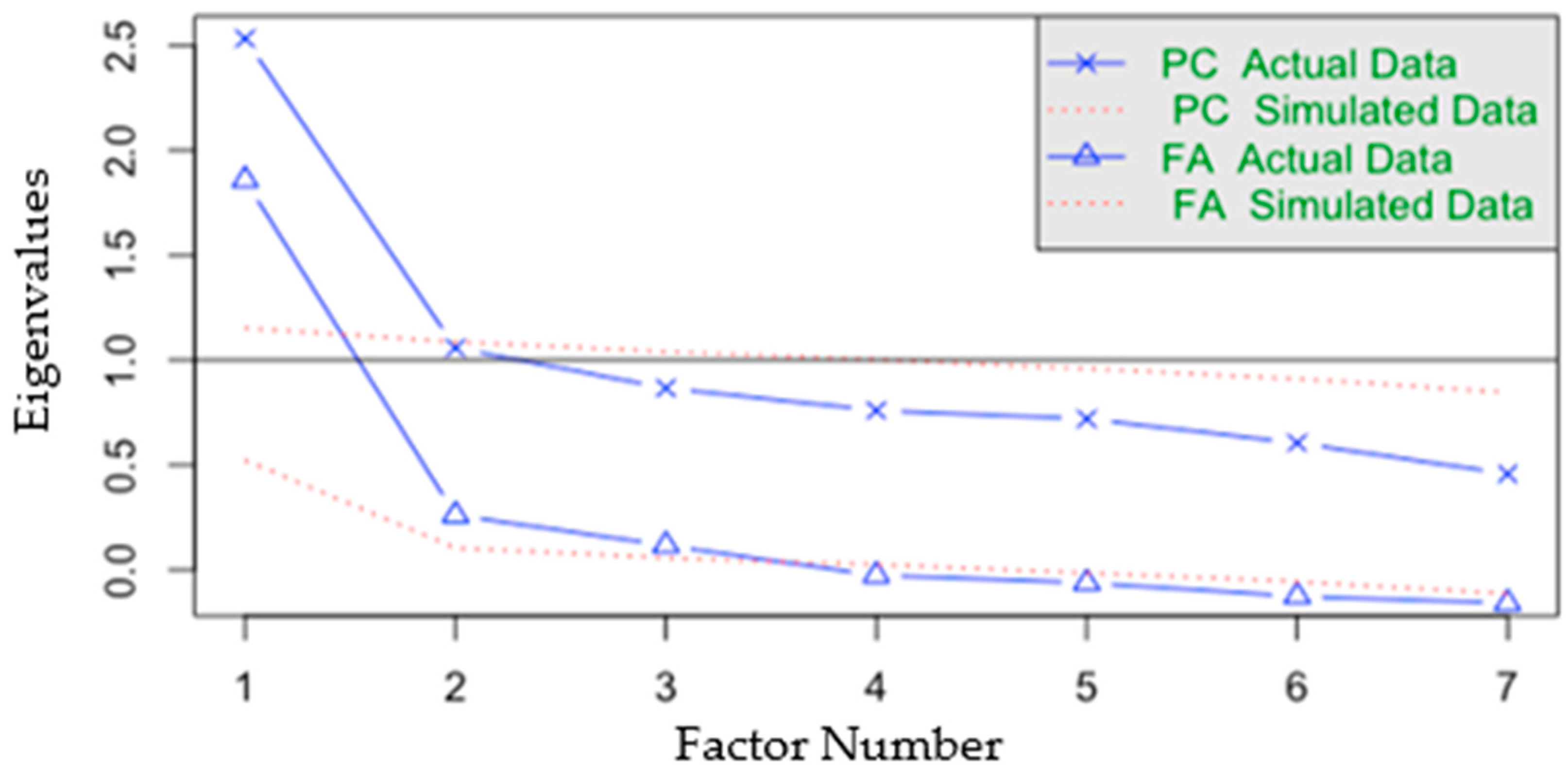
3.2.1. Exploratory Factor Analysis
3.2.2. Confirmatory Factor Analysis
3.2.3. Internal Consistency and Reliability
3.2.4. Measurement Invariance
3.2.5. Hypothesis Testing for Construct Validity
3.2.6. GSSS Descriptive Statistics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, A.E.; Andreo-Martínez, P. The Role of Gut Microbiota in Gastrointestinal Symptoms of Children with ASD. Medicina 2019, 55, 408. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Aziz, I.; Palsson, O.S.; Törnblom, H.; Sperber, A.D.; Whitehead, W.E.; Simrén, M. The Prevalence and Impact of Overlapping Rome IV-Diagnosed Functional Gastrointestinal Disorders on Somatization, Quality of Life, and Healthcare Utilization: A Cross-Sectional General Population Study in Three Countries. Am. Coll. Gastroenterol. 2018, 113, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, U.N.; Ford, A.C. Prevalence of functional gastrointestinal disorders among consecutive new patient referrals to a gastroenterology clinic. Frontline Gastroenterol. 2014, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Baaleman, D.F.; Velasco-Benítez, C.A.; Méndez-Guzmán, L.M.; Benninga, M.A.; Saps, M. Functional gastrointestinal disorders in children: Agreement between Rome III and Rome IV diagnoses. Eur. J. Pediatr. 2021, 180, 2297–2303. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Boronat, A.C.; Ferreira-Maia, A.P.; Matijasevich, A.; Wang, Y.-P. Epidemiology of functional gastrointestinal disorders in children and adolescents: A systematic review. World J. Gastroenterol. 2017, 23, 3915. [Google Scholar] [CrossRef]
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global prevalence of functional constipation according to the Rome criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Hansen, R.A.; Morgan, D.R.; Gangarosa, L.M.; Ringel, Y.; Thiny, M.T.; Ruso, M.; Sandler, R.S. The Burden of Gastrointestinal and Liver Diseases. Am. J. Gastroenterol. 2006, 101, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Vasa, R.A.; Kalb, L.G.; Kanne, S.M.; Rosenberg, D.; Keefer, A.; Murray, D.S.; Freedman, B.; Lowery, L.A. Anxiety, Sensory Over-Responsivity, and Gastrointestinal Problems in Children with Autism Spectrum Disorders. J. Abnorm. Child. Psychol. 2013, 41, 165–176. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Keefer, A.; Shui, A.; Vasa, R.A. One-year course and predictors of abdominal pain in children with autism spectrum disorders: The role of anxiety and sensory over-responsivity. Res. Autism. Spectr. Disord. 2014, 8, 1508–1515. [Google Scholar] [CrossRef]
- Martínez-González, A.E.; Cervin, M.; Pérez-Sánchez, S. Prevalence and correlates of gastrointestinal symptoms in people with autism: Applying a new measure based on the Rome IV criteria. Dig. Liver Dis. 2024, in press.
- Treichel, A.J.; Farrugia, G.; Beyder, A. The touchy business of gastrointestinal (GI) mechanosensitivity. Brain Res. 2018, 1693, 197–200. [Google Scholar] [CrossRef]
- Marazziti, D.; Buccianelli, B.; Palermo, S.; Parra, E.; Arone, A.; Beatino, M.F.; Massa, L.; Carpita, B.; Barberi, F.M.; Mucci, F.; et al. The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry? Life 2021, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Turna, J.; Grosman Kaplan, K.; Patterson, B.; Bercik, P.; Anglin, R.; Soreni, N.; Van Ameringen, M. Higher prevalence of irritable bowel syndrome and greater gastrointestinal symptoms in obsessive-compulsive disorder. J. Psychiatr. Res. 2019, 118, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Crowell, M.D.; Umar, S.B.; Lacy, B.E.; Jones, M.P.; DiBaise, J.K.; Talley, N.J. Multi-Dimensional Gastrointestinal Symptom Severity Index: Validation of a Brief GI Symptom Assessment Tool. Dig. Dis. Sci. 2015, 60, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS—A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, A.E.; Rodríguez-Jiménez, T.; Piqueras, J.A.; Infante-Cañete, L.; Hidalgo Berutich, S.; Andreo-Martínez, P.; Ordóñez-Rubio, T.; Belmonte Lillo, V.M.; Cubi, M.A.; Navarro-Soria, I. Cross-disorder comparison of sensory reactivity, pain, gastro-intestinal symptoms and obsessive-compulsive symptoms in adolescents and young adults with autism and other neurodevelopmental disorders. Int. J. Dev. Disabil. 2024, 1–12. [Google Scholar] [CrossRef]
- Montoro-Pérez, N.; Martínez-González, A.E.; Infante-Cañete, L.; Martínez-González, M.A.; Hidalgo-Berutich, S.; Andreo-Martínez, P. Validation of the Gastrointestinal Symptom Severity Scale in children and adolescents. Eur. J. Pediatr. 2024, in press.
- Carretero-Dios, H.; Pérez, C. Normas para el desarrollo y revisión de estudios instrumentales. Int. J. Clin. Health Psychol. 2005, 5, 521–551. [Google Scholar]
- Ferrando, P.J.; Lorenzo-Seva, U.; Hernández-Dorado, A.; Muñiz, J. Decalogue for the factor analysis of test items. Psicothema 2022, 34, 7. [Google Scholar] [CrossRef]
- Lloret-Segura, S.; Ferreres-Traver, A.; Hernández-Baeza, A.; Tomás-Marco, I. El análisis factorial exploratorio de los ítems: Una guía práctica, revisada y actualizada. An. Psicol./Ann. Psychol. 2014, 30, 1151–1169. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept Evolution in Sensory Integration: A Proposed Nosology for Diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Wallace, A.; Infante-Cañete, L.; Martínez-González, A.E.; Piqueras, J.A.; Hidalgo Berutich, S.; Rodríguez-Jiménez, T.; Andreo-Martínez, P.; Moreno-Amador, B.; Veas, A. Validation of the Pain and Sensitivity Reactivity Scale in neurotypical late adolescents. J. Adv. Nurs. 2024, in press.
- Taylor, S.; Conelea, C.A.; McKay, D.; Crowe, K.B.; Abramowitz, J.S. Sensory intolerance: Latent structure and psychopathologic correlates. Compr. Psychiatry 2014, 55, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, M.J.; Conelea, C.A.; Garner, L.E.; Haaga, D.A.F. Sensory over-responsivity in trichotillomania (hair-pulling disorder). Psychiatry Res. 2018, 260, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Amador, B.; Cervin, M.; Martínez-González, A.E.; Piqueras, J.A. Sensory Overresponsivity and Symptoms Across the Obsessive-Compulsive Spectrum: Web-Based Longitudinal Observational Study. J. Med. Internet Res. 2023, 25, e37847. [Google Scholar] [CrossRef] [PubMed]
- Foa, E.B.; Huppert, J.D.; Leiberg, S.; Langner, R.; Kichic, R.; Hajcak, G.; Salkovskis, P.M. The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychol. Assess. 2002, 14, 485. [Google Scholar] [CrossRef]
- Piqueras Rodríguez, J.A.; Martínez González, A.E.; Hidalgo Montesinos, M.D.; Fullana Rivas, M.A.; Mataix Cols, D.; Rosa Alcázar, A.I. Psychometric properties of the Obsessive Compulsive Inventory-revised in a non-clinical sample of late adolescents. Psicol. Conduct. 2009, 17, 561–672. [Google Scholar]
- Martínez-González, A.E.; Piqueras, J.A.; Marzo, J.C. Validación del inventario de obsesiones y compulsiones revisado (OCI-R) para su uso en población adolescente española. An. Psicol./Ann. Psychol. 2011, 27, 763–773. [Google Scholar] [CrossRef]
- Hon, K.S.; Siu, B.W.; Cheng, C.; Wong, W.C.; Foa, E.B. Validation of the Chinese version of obsessive-compulsive inventory-revised. East. Asian Arch. Psychiatry 2019, 29, 103–111. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef]
- Lim, C.R.; Harris, K.; Dawson, J.; Beard, D.J.; Fitzpatrick, R.; Price, A.J. Floor and ceiling effects in the OHS: An analysis of the NHS PROMs data set. BMJ Open 2015, 5, e007765. [Google Scholar] [CrossRef]
- Rhemtulla, M.; Brosseau-Liard, P.É.; Savalei, V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychol. Methods 2012, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. A second generation little jiffy. Psychometrika 1970, 35, 401–415. [Google Scholar] [CrossRef]
- Bartlett, M.S. Tests of significance in factor analysis. Br. J. Stat. Psychol. 1950, 3, 77–85. [Google Scholar] [CrossRef]
- Hayton, J.C.; Allen, D.G.; Scarpello, V. Factor retention decisions in exploratory factor analysis: A tutorial on parallel analysis. Organ. Res. Methods 2004, 7, 191–205. [Google Scholar] [CrossRef]
- Revelle, W. How to: Use the Psych Package for Factor Analysis and Data Reduction; Department of Psychology, Northwestern University: Evanston, IL, USA, 2016. [Google Scholar]
- Yong, A.G.; Pearce, S. A Beginner’s Guide to Factor Analysis: Focusing on Exploratory Factor Analysis. TQMP 2013, 9, 79–94. [Google Scholar] [CrossRef]
- Beauducel, A.; Herzberg, P.Y. On the Performance of Maximum Likelihood Versus Means and Variance Adjusted Weighted Least Squares Estimation in CFA. Struct. Equ. Model. 2006, 13, 186–203. [Google Scholar] [CrossRef]
- Rosseel, Y.; Oberski, D.; Byrnes, J.; Vanbrabant, L.; Savalei, V.; Merkle, E.; Hallquist, M.; Rhemtulla, M.; Katsikatsou, M.; Barendse, M. Package ‘lavaan’. Retrieved June 2017, 17, 2017. [Google Scholar]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Brown, T.A. Confirmatory Factor Analysis for Applied Research, 2nd ed.; The Guilford Press: New York, NY, USA, 2015; p. 462. [Google Scholar]
- Gadermann, A.M.; Guhn, M.; Zumbo, B.D. Estimating ordinal reliability for Likert-type and ordinal item response data: A conceptual, empirical, and practical guide. Pr. Assess. Res. Eval. 2019, 17, 3. [Google Scholar] [CrossRef]
- Zumbo, B.D.; Kroc, E. A Measurement Is a Choice and Stevens’ Scales of Measurement Do Not Help Make It: A Response to Chalmers. Educ. Psychol. Meas. 2019, 79, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Martínez Pérez, J.A.; Pérez Martin, P.S. Coeficiente de correlación intraclase. Med. Fam. Semer. 2023, 49, 101907. [Google Scholar] [CrossRef]
- Wu, H.; Estabrook, R. Identification of Confirmatory Factor Analysis Models of Different Levels of Invariance for Ordered Categorical Outcomes. Psychometrika 2016, 81, 1014–1045. [Google Scholar] [CrossRef]
- Chen, F.F. Sensitivity of Goodness of Fit Indexes to Lack of Measurement Invariance. Struct. Equ. Model. 2007, 14, 464–504. [Google Scholar] [CrossRef]
- Marsh, H.W.; Hau, K.-T.; Grayson, D. Goodness of Fit in Structural Equation Models. In Contemporary Psychometrics: A Festschrift for Roderick P. McDonald; Multivariate Applications Book Series; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2005; pp. 275–340. [Google Scholar]
- Karimian, M.; Ranjbar, R.; Salamati, M.; Adibi, A.; Kazemi, F.; Azami, M. Prevalence of dyspepsia in Iran: A systematic review and meta-analysis. Arch. Iran. Med. 2021, 24, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef] [PubMed]
- Dawoodi, S.; Dawoodi, I.; Dixit, P. Gastrointestinal problem among Indian adults: Evidence from longitudinal aging study in India 2017–2018. Front. Public Health 2022, 10, 911354. [Google Scholar] [CrossRef] [PubMed]
- Saigo, T.; Tayama, J.; Hamaguchi, T.; Nakaya, N.; Tomiie, T.; Bernick, P.J.; Kanazawa, M.; Labus, J.S.; Naliboff, B.D.; Shirabe, S.; et al. Gastrointestinal specific anxiety in irritable bowel syndrome: Validation of the Japanese version of the visceral sensitivity index for university students. BioPsychoSocial Med. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed]


| Variables | Total (n = 1247) |
|---|---|
| Age | |
| 22.17 (7.19) * | |
| Sex n (%) | |
| Female | 898 (72.0) |
| Male | 337 (27.0) |
| Other | 12 (1.2) |
| Country/region of origin n (%) | |
| Spain | 1190 (95.0) |
| Rest of Europe | 14 (1.2) |
| America | 29 (2.5) |
| Africa | 12 (1.1) |
| Asia | 2 (0.2) |
| Items | Min | Max | M (SD) | Skewness | Kurtosis | F.E (%) | C.E (%) |
|---|---|---|---|---|---|---|---|
| 1. Regurgitation or rumination | 0 | 3 | 0.18 (0.42) | 2.39 | 5.64 | 1051 (83.9) | 1 (0.1) |
| 2. Vomiting | 0 | 3 | 0.19 (0.46) | 2.71 | 8.26 | 1051 (83.9) | 5 (0.4) |
| 3. Gas | 0 | 3 | 0.65 (0.76) | 1.04 | 0.66 | 625 (49.9) | 32 (2.6) |
| 4. Abdominal pain | 0 | 3 | 0.54 (0.79) | 1.40 | 1.28 | 766 (61.1) | 40 (3.2) |
| 5. Constipation | 0 | 3 | 0.45 (0.74) | 1.74 | 2.53 | 847 (67.6) | 39 (3.1) |
| 6. Diarrhea | 0 | 3 | 0.30 (0.59) | 2.24 | 5.30 | 956 (76.3) | 17 (1.4) |
| 7. Defecation in inappropriate places | 0 | 3 | 0.07 (0.32) | 5.03 | 27.54 | 1183 (94.4) | 2 (0.2) |
| Items | Factor 1 | Factor 2 |
|---|---|---|
| 1. Regurgitation or rumination | 0.387 | |
| 2. Vomiting | 0.515 | |
| 3. Gas | 0.565 | |
| 4. Abdominal pain | 0.801 | |
| 5. Constipation | 0.415 | |
| 6. Diarrhea | 0.619 | |
| 7. Defecation in inappropriate places | 0.517 | |
| Explained variance % | 36.19 | 15.11 |
| Factor Correlations | ||
| Factor 1 | 1 | |
| Factor 2 | 0.628 | 1 |
| Models | χ2 | df | RMSEA (90% CI) | CFI | TLI | |
|---|---|---|---|---|---|---|
| 2-Factors Model after EFA | TM | 102.394 | 18 | 0.085 (0.069–0.102) | 0.824 | 0.795 |
| CM | 28.052 | 13 | 0.007 (0.000–0.041) | 0.999 | 0.999 | |
| 1-Factor Model | TM | 202.978 | 20 | 0.126 (0.111–0.142) | 0.570 | 0.549 |
| CM | 41.497 | 14 | 0.027 (0.000–0.051) | 0.986 | 0.979 |
| 2-Factors Model | X2 | gl | CFI | ΔCFI | RMSEA (90% CI) | ΔRMSEA |
|---|---|---|---|---|---|---|
| Configurational | 52.283 | 26 | 0.942 | - | 0.040 (0.024–0.056) | - |
| Metric | 39.535 | 31 | 0.981 | - | 0.021 (0.000–0.039) | - |
| Scalar | 62.468 | 36 | 0.942 | −0.039 | 0.035 (0.019–0.049) | 0.014 |
| Strict | 93.854 | 43 | 0.888 | −0.054 | 0.044 (0.032–0.056) | 0.009 |
| 1-Factor Model | ||||||
| Configurational | 72.806 | 28 | 0.902 | - | 0.037 (0.027–0.048) | - |
| Metric | 53.200 | 34 | 0.958 | - | 0.029 (0.012–0.044) | - |
| Scalar | 86.725 | 40 | 0.898 | −0.06 | 0.044 (0.031–0.056) | 0.015 |
| Strict | 118.250 | 47 | 0.844 | −0.054 | 0.053 (0.041–0.065) | 0.009 |
| Factor 1 | Factor 2 | Total GSSS | ||
|---|---|---|---|---|
| PSRS | Pain | 0.30 ** | 0.22 ** | 0.22 ** |
| Total Hypo | 0.26 ** | 0.27 ** | 0.30 ** | |
| Hypo-Tactile | 0.93 ** | 0.69 ** | 0.99 ** | |
| Hypo-Olfactory | 0.21 ** | 0.21 ** | 0.24 ** | |
| Hypo-Visual | 0.18 ** | 0.18 ** | 0.21 ** | |
| Hypo-Taste | 0.19 ** | 0.24 ** | 0.24 ** | |
| Hypo-Auditory | 0.24 ** | 0.22 ** | 0.22 ** | |
| Total Hyper | 0.30 ** | 0.26 ** | 0.33 ** | |
| Hyper-Tactile | 0.26 ** | 0.23 ** | 0.23 ** | |
| Hyper-Olfactory | 0.24 ** | 0.22 ** | 0.27 ** | |
| Hyper-Visual | 0.19 ** | 0.20 ** | 0.22 ** | |
| Hyper-Taste | 0.18 ** | 0.20 ** | 0.21 ** | |
| Hyper-Auditory | 0.25 ** | 0.18 ** | 0.26 ** | |
| SOR | Touch | 0.19 ** | 0.19 ** | 0.22 ** |
| Smell | 0.19 ** | 0.18 ** | 0.21 ** | |
| Sight | 0.17 ** | 0.19 ** | 0.21 ** | |
| Taste | 0.15 ** | 0.18 ** | 0.18 ** | |
| Hearing | 0.24 ** | 0.20 ** | 0.20 ** | |
| OCI-R | Hoarding | 0.18 ** | 0.22 ** | 0.23 ** |
| Checking | 0.17 ** | 0.19 ** | 0.20 ** | |
| Ordering | 0.18 ** | 0.12 ** | 0.18 ** | |
| Neutralizing | 0.18 ** | 0.15 ** | 0.19 ** | |
| Washing | 0.18 ** | 0.19 ** | 0.22 ** | |
| Obsessing | 0.23 ** | 0.19 ** | 0.25 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-González, A.E.; Montoro-Pérez, N.; Wallace, A.; Pérez-Sánchez, S.; Piqueras, J.A.; Infante-Cañete, L.; Hidalgo-Berutich, S.; Rodríguez-Jiménez, T.; Andreo-Martínez, P. Psychometric Properties of the Gastrointestinal Symptom Severity Scale in a Sample of Adolescents and Young Adults. J. Clin. Med. 2024, 13, 1662. https://doi.org/10.3390/jcm13061662
Martínez-González AE, Montoro-Pérez N, Wallace A, Pérez-Sánchez S, Piqueras JA, Infante-Cañete L, Hidalgo-Berutich S, Rodríguez-Jiménez T, Andreo-Martínez P. Psychometric Properties of the Gastrointestinal Symptom Severity Scale in a Sample of Adolescents and Young Adults. Journal of Clinical Medicine. 2024; 13(6):1662. https://doi.org/10.3390/jcm13061662
Chicago/Turabian StyleMartínez-González, Agustín Ernesto, Néstor Montoro-Pérez, Agustín Wallace, Susana Pérez-Sánchez, José A. Piqueras, Lidia Infante-Cañete, Silvia Hidalgo-Berutich, Tíscar Rodríguez-Jiménez, and Pedro Andreo-Martínez. 2024. "Psychometric Properties of the Gastrointestinal Symptom Severity Scale in a Sample of Adolescents and Young Adults" Journal of Clinical Medicine 13, no. 6: 1662. https://doi.org/10.3390/jcm13061662
APA StyleMartínez-González, A. E., Montoro-Pérez, N., Wallace, A., Pérez-Sánchez, S., Piqueras, J. A., Infante-Cañete, L., Hidalgo-Berutich, S., Rodríguez-Jiménez, T., & Andreo-Martínez, P. (2024). Psychometric Properties of the Gastrointestinal Symptom Severity Scale in a Sample of Adolescents and Young Adults. Journal of Clinical Medicine, 13(6), 1662. https://doi.org/10.3390/jcm13061662









