A Systemic Immune Inflammation Index and PD-L1 (SP142) Expression as a Potential Combined Biomarker of the Clinical Benefit of Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PD-L1 (SP142) Immunohistochemistry
2.3. Definition of CBC-IBs
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
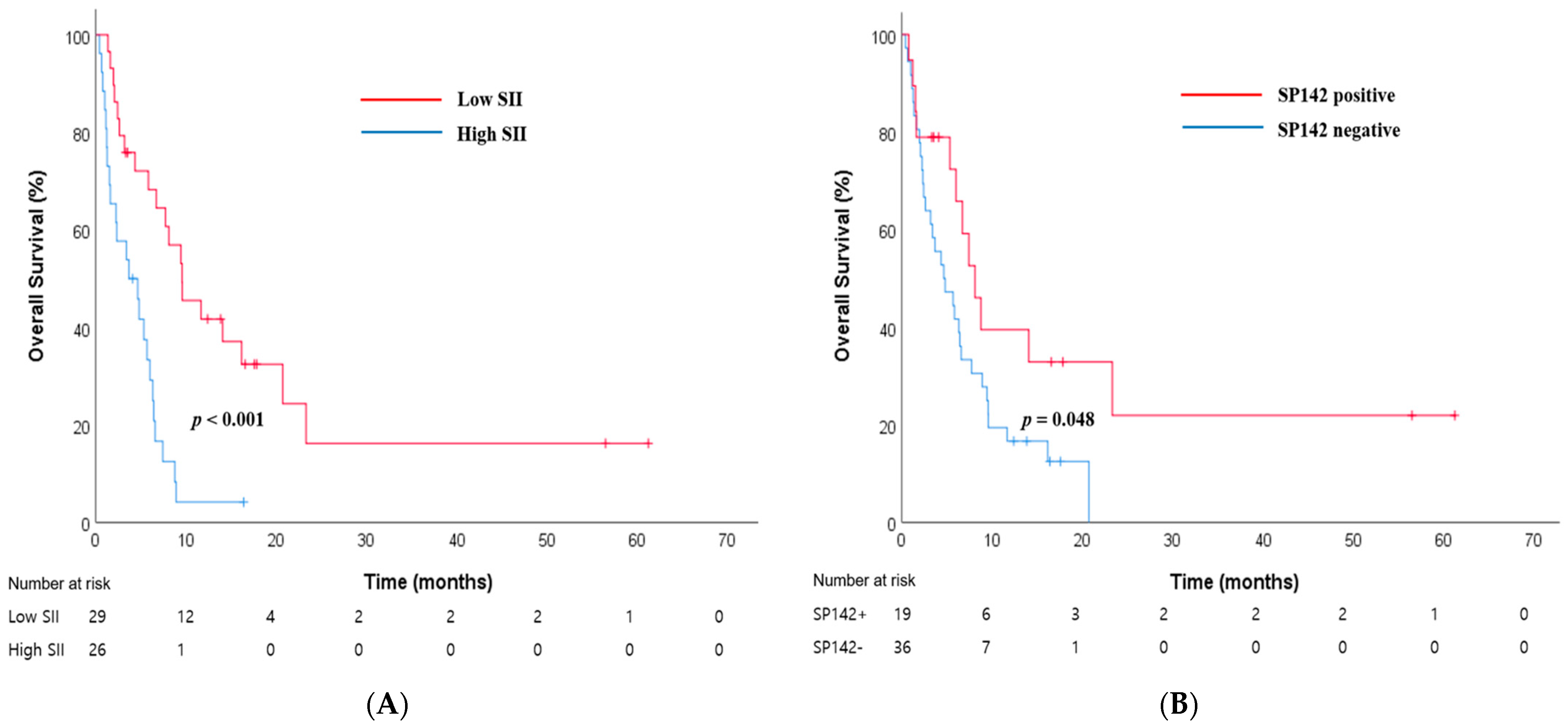
3.2. The Best Independent Prognostic Biomarker among the Four CBC-IBs
3.3. PD-L1 (SP142) Immunohistochemistry Expression
3.4. Prognostic Capacity of the Combined SII-SP142 Biomarker
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.; Chen, H.J.M. SEER Cancer Statistics Review (CSR), 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019.
- Meijer, J.J.; Leonetti, A.; Airo, G.; Tiseo, M.; Rolfo, C.; Giovannetti, E.; Vahabi, M. Small cell lung cancer: Novel treatments beyond immunotherapy. Semin. Cancer Biol. 2022, 86, 376–385. [Google Scholar] [CrossRef]
- Demedts, I.K.; Vermaelen, K.Y.; van Meerbeeck, J.P. Treatment of extensive-stage small cell lung carcinoma: Current status and future prospects. Eur. Respir. J. 2010, 35, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gumus, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Aldea, M.; Besse, B.; Planchard, D.; Reck, M.; Giaccone, G.; Soria, J.C. Small cell lung cancer: A slightly less orphan disease after immunotherapy. Ann. Oncol. 2021, 32, 698–709. [Google Scholar] [CrossRef]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Ozguroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczesna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Small Cell Lung Cancer (Version 3.2023). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1462 (accessed on 21 December 2022).
- Belluomini, L.; Calvetti, L.; Inno, A.; Pasello, G.; Roca, E.; Vattemi, E.; Veccia, A.; Menis, J.; Pilotto, S. SCLC Treatment in the Immuno-Oncology Era: Current Evidence and Unmet Needs. Front. Oncol. 2022, 12, 840783. [Google Scholar] [CrossRef]
- Lim, J.U.; Kang, H.S. A narrative review of current and potential prognostic biomarkers for immunotherapy in small-cell lung cancer. Ann. Transl. Med. 2021, 9, 809. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kou, J.; Huang, J.; Li, J.; Wu, Z.; Ni, L. Systemic immune-inflammation index predicts prognosis and responsiveness to immunotherapy in cancer patients: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 3895–3905. [Google Scholar] [CrossRef]
- Nishijima, T.F.; Muss, H.B.; Shachar, S.S.; Tamura, K.; Takamatsu, Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2015, 41, 971–978. [Google Scholar] [CrossRef]
- Jeong, M.J.; Yoon, Y.N.; Kang, Y.K.; Kim, C.J.; Nam, H.S.; Lee, Y.S. A Novel Score Using Lymphocyte-to-Monocyte Ratio in Blood and Malignant Body Fluid for Predicting Prognosis of Patients with Advanced Ovarian Cancer. Cancers 2023, 15, 2328. [Google Scholar] [CrossRef]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.K.; Moon, Y.; Park, M.H.; Lim, J.H.; Kim, Y.S.; Lee, K.H.; Kwak, S.M.; Kim, C.; Nam, H.S. A Preliminary Study on the Prognostic Impact of Neutrophil to Lymphocyte Ratio of the Bronchoalveolar Lavage Fluid in Patients with Lung Cancer. Diagnostics 2021, 11, 2201. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lim, J.H.; Ryu, W.; Park, M.H.; Kim, L.; Kim, K.; Kim, W.Y.; Nam, H.S. The clinical impact of three validated PD-L1 immunohistochemistry assays as a prognostic factor in small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Lausen, B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019, 4, e126908. [Google Scholar] [CrossRef]
- Chung, H.C.; Piha-Paul, S.A.; Lopez-Martin, J.; Schellens, J.H.M.; Kao, S.; Miller, W.H., Jr.; Delord, J.P.; Gao, B.; Planchard, D.; Gottfried, M.; et al. Pembrolizumab after Two or More Lines of Previous Therapy in Patients with Recurrent or Metastatic SCLC: Results from the KEYNOTE-028 and KEYNOTE-158 Studies. J. Thorac. Oncol. 2020, 15, 618–627. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Goldman, J.; Garassino, M.; Dvorkin, M.; Trukhin, D.; Statsenko, G.; Hotta, K.; Ji, J.; Hochmair, M.; Voitko, O.J. PD-L1 expression, patterns of progression and patient-reported outcomes (PROs) with durvalumab plus platinum-etoposide in ES-SCLC: Results from CASPIAN. Ann. Oncol. 2019, 30, v928–v929. [Google Scholar] [CrossRef]
- Acheampong, E.; Abed, A.; Morici, M.; Bowyer, S.; Amanuel, B.; Lin, W.; Millward, M.; Gray, E.S.J.C. Tumour PD-L1 expression in small-cell lung cancer: A systematic review and meta-analysis. Cells 2020, 9, 2393. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Togashi, Y.; Kamada, T.; Sugiyama, E.; Nishinakamura, H.; Takeuchi, Y.; Vitaly, K.; Itahashi, K.; Maeda, Y.; Matsui, S.; et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020, 21, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T. PD-L1 expression in small cell lung cancer: Should we designate it for assignment? Transl. Lung Cancer Res. 2021, 10, 3697–3700. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.H.; Nam, S.J.; Ock, C.Y.; Moon, J.W.; Yoo, C.W.; Lee, G.K.; Han, J.Y. Association of PD-L1 Expression with Tumor-Infiltrating Immune Cells and Mutation Burden in High-Grade Neuroendocrine Carcinoma of the Lung. J. Thorac. Oncol. 2018, 13, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Azuma, K.; Kawahara, A.; Yamada, K.; Imamura, Y.; Tokito, T.; Kinoshita, T.; Kage, M.; Hoshino, T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J. Thorac. Oncol. 2015, 10, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chen, Z.; Chen, K.; Liao, F.T.; Chung, C.E.; Liu, X.; Lin, Y.C.; Keohavong, P.; Leikauf, G.D.; Di, Y.P. Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Res. 2021, 81, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dai, M.; Zhang, Z. Prognostic Significance of the Systemic Immune-Inflammation Index (SII) in Patients with Small Cell Lung Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 814727. [Google Scholar] [CrossRef]
- Li, W.Q.; Li, L.Y.; Bai, R.L.; Qian, L.; Chen, N.F.; Cui, J.W. Cost-effectiveness of programmed cell death ligand 1 testing and tumor mutational burden testing of immune checkpoint inhibitors for advanced non-small cell lung cancer. Chin. Med. J. 2020, 133, 2630–2632. [Google Scholar] [CrossRef]
- Pavan, A.; Attili, I.; Pasello, G.; Guarneri, V.; Conte, P.F.; Bonanno, L. Immunotherapy in small-cell lung cancer: From molecular promises to clinical challenges. J. Immunother. Cancer 2019, 7, 205. [Google Scholar] [CrossRef]

| Variables | No. (%) n = 55 | SP142 (Positive+/Negative−) +SII (High/Low) | ||||
|---|---|---|---|---|---|---|
| +/Low (n = 9) | −/Low (n = 19) | +/High (n = 10) | −/High (n = 17) | p Value * | ||
| Age | 0.016 | |||||
| <70 | 23 (41.8) | 7 | 8 | 4 | 4 | |
| ≥70 | 32 (58.2) | 2 | 11 | 6 | 13 | |
| Sex | 0.257 | |||||
| Male | 52 (94.5) | 9 | 18 | 10 | 15 | |
| Female | 3 (5.5) | 0 | 1 | 0 | 2 | |
| Smoking | 0.384 | |||||
| Current | 34 (61.8) | 7 | 12 | 5 | 10 | |
| Former + never | 20 (36.4) | 2 | 7 | 4 | 7 | |
| ECOG PS | 0.002 | |||||
| 0–1 | 32 (58.2) | 7 | 15 | 5 | 5 | |
| 2–4 | 23 (41.8) | 2 | 4 | 5 | 12 | |
| CEA (ng/mL) † | 0.777 | |||||
| ≤5.2 | 10 (31.3) | 1 | 4 | 2 | 3 | |
| >5.2 | 22 (68.8) | 3 | 8 | 6 | 5 | |
| LDH (IU/L) † | 0.310 | |||||
| ≤250 | 14 (28.6) | 3 | 5 | 3 | 3 | |
| >250 | 35 (71.4) | 4 | 12 | 7 | 12 | |
| SP142 | 0.004 | |||||
| negative | 36 (65.5) | 0 | 19 | 0 | 17 | |
| positive | 19 (34.5) | 9 | 0 | 10 | 0 | |
| SII | <0.001 | |||||
| <810 | 29 (52.7) | 9 | 19 | 1 | 0 | |
| ≥810 | 26 (47.3) | 0 | 0 | 9 | 17 | |
| Brain metastasis | 0.411 | |||||
| No | 41 (74.5) | 5 | 15 | 8 | 13 | |
| Yes | 14 (25.5) | 4 | 4 | 2 | 4 | |
| Liver metastasis | 0.002 | |||||
| No | 37 (67.3) | 9 | 14 | 7 | 7 | |
| Yes | 18 (32.7) | 0 | 5 | 3 | 10 | |
| Bone metastasis | 0.024 | |||||
| No | 27 (49.1) | 8 | 9 | 4 | 6 | |
| Yes | 28 (50.9) | 1 | 10 | 6 | 11 | |
| Adrenal metastasis | 0.110 | |||||
| No | 43 (78.2) | 9 | 15 | 7 | 12 | |
| Yes | 12 (21.8) | 0 | 4 | 3 | 5 | |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value * | AHR (95% CI) | p Value * | |
| NLR | 0.001 | 0.978 | ||
| <3.2 (n = 25) | 1.00 (reference) | 1.00 (reference) | ||
| ≥3.2 (n = 30) | 3.06 (1.59–5.90) | 0.98 (0.27–3.52) | ||
| SII | <0.001 | <0.001 | ||
| <810 (n = 29) | 1.00 (reference) | 1.00 (reference) | ||
| ≥810 (n = 26) | 3.46 (1.80–6.65) | 3.46 (1.80–6.65) | ||
| MLR | 0.013 | 0.062 | ||
| <0.2 (n = 9) | 1.00 (reference) | 1.00 (reference) | ||
| ≥0.2 (n = 46) | 3.74 (1.32–1066) | 3.10 (0.95–10.1) | ||
| PLR | 0.034 | 0.201 | ||
| <150 (n = 26) | 1.00 (reference) | 1.00 (reference) | ||
| ≥150 (n = 29) | 1.93 (1.05–3.54) | 0.56 (0.23–1.37) | ||
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value * | AHR (95% CI) | p Value * | |
| Age | 0.031 | 0.559 | ||
| <70 (n = 23) | 1.00 (reference) | 1.00 (reference) | ||
| ≥75 (n = 32) | 2.00 (1.05–3.79) | 1.22 (0.62–2.41) | ||
| Sex | 0.803 | |||
| Male (n = 52) | 1.16 (0.38–3.78) | |||
| Female (n = 3) | 1.00 (reference) | |||
| Smoking habit | 0.143 | |||
| Current (n = 34) | 1.00 (reference) | |||
| Former + never (n = 20) | 1.58 (0.85–2.92) | |||
| ECOG PS | <0.001 | <0.001 | ||
| 0–1 (n = 32) | 1.00 (reference) | 1.00 (reference) | ||
| ≥2 (n = 23) | 3.20 (1.68–6.11) | 2.46 (1.24–4.89) | 0.010 | |
| CEA, ng/nL † | 0.275 | |||
| ≤5.2 (n = 10) | 1.00 (reference) | |||
| >5.2 (n = 22) | 1.60 (0.68–3.75) | |||
| LDH, IU/L † | 0.531 | |||
| ≤250 (n = 14) | 1.00 (reference) | |||
| >250 (n = 35) | 1.26 (0.61–2.61) | |||
| Brain metastasis | 0.361 | |||
| No (n = 41) | 1.00 (reference) | |||
| Yes (n = 14) | 1.37 (0.70–2.67) | |||
| Liver metastasis | 0.002 | 0.340 | ||
| No (n = 37) | 1.00 (reference) | 1.00 (reference) | ||
| Yes (n = 18) | 2.60 (1.39–4.86) | 1.46 (0.67–3.16) | ||
| Bone metastasis | 0.020 | 0.967 | ||
| No (n = 27) | 1.00 (reference) | 1.00 (reference) | ||
| Yes (n = 28) | 2.07 (1.11–3.87) | 0.98 (0.48–2.12) | ||
| Adrenal metastasis | 0.279 | |||
| No (n = 43) | 1.00 (reference) | |||
| Yes (n = 12) | 1.41 (0.72–3.03) | |||
| SP142 + SII | 0.002 | <0.001 | ||
| Positive/low (n = 9) | 1.00 (reference) | 1.00 (reference) | ||
| Negative/low (n = 19) | 3.90 (1.11–13.70) | 3.65 (1.03–12.90) | 0.044 | |
| Positive/high (n = 10) | 6.11 (1.55–24.04) | 5.69 (1.43–22.60) | 0.013 | |
| Negative/high (n = 17) | 8.60 (2.37–31.17) | 5.97 (1.59–22.49) | 0.008 | |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value * | AHR (95% CI) | p Value * | |
| Age | 0.037 | 0.528 | ||
| <70 (n = 20) | 1.00 (reference) | 1.00 (reference) | ||
| ≥75 (n = 22) | 2.10 (1.03–4.26) | 1.29 (0.58–2.87) | ||
| Sex | 0.761 | |||
| Male (n = 40) | 1.00 (reference) | |||
| Female (n = 2) | 1.25 (0.30–5.28) | |||
| Smoking habit | 0.261 | |||
| Current (n = 29) | 1.00 (reference) | |||
| Former + never (n = 12) | 1.51 (0.73–3.12) | |||
| ECOG PS | 0.305 | |||
| 0–1 (n = 29) | 1.00 (reference) | |||
| ≥2 (n = 13) | 1.46 (0.71–3.00) | |||
| CEA, ng/nL † | 0.676 | |||
| ≤5.2 (n = 8) | 1.00 (reference) | |||
| >5.2 (n = 17) | 1.22 (0.49–3.05) | |||
| LDH, IU/L † | 0.967 | |||
| ≤250 (n = 12) | 1.00 (reference) | |||
| >250 (n = 25) | 1.02 (0.48–2.17) | |||
| SP142 | 0.024 | 0.052 | ||
| Negative (n = 26) | 2.41 (1.10–5.26) | 4.47 (1.14–20.5) | ||
| Positive (n = 16) | 1.00 (reference) | 1.00 (reference) | ||
| SII | 0.096 | |||
| <810 (n = 26) | 1.00 (reference) | |||
| ≥810 (n = 16) | 1.80 (0.89–3.61) | |||
| NLR | 0.084 | |||
| <3.2 (n = 23) | 1.00 (reference) | |||
| ≥3.2 (n = 19) | 1.84 (0.91–3.70) | |||
| MLR | 0.164 | |||
| <0.202 (n = 9) | 1.00 (reference) | |||
| ≥0.202 (n = 33) | 1.79 (0.78–4.12) | |||
| PLR | 0.252 | |||
| <150 (n = 24) | 1.00 (reference) | |||
| ≥150 (n = 18) | 1.48 (0.75–2.90) | |||
| Brain metastasis | 0.679 | |||
| No (n = 31) | 1.00 (reference) | |||
| Yes (n = 11) | 1.19 (053–2.66) | |||
| Liver metastasis | 0.559 | |||
| No (n = 30) | 1.00 (reference) | |||
| Yes (n = 12) | 1.24 (0.60–2.56) | |||
| Bone metastasis | 0.052 | |||
| No (n = 22) | 1.00 (reference) | |||
| Yes (n = 20) | 1.96 (0.98–390) | |||
| Adrenal metastasis | 0.105 | |||
| No (n = 32) | 1.00 (reference) | |||
| Yes (n = 10) | 1.88 (0.87–4.09) | |||
| SP142 + SII | 0.019 | 0.019 | ||
| Positive/low (n = 9) | 1.00 (reference) | 1.00 (reference) | ||
| Negative/low (n = 16) | 6.19 (1.69–22.65) | 6.19 (1.69–22.65) | 0.06 | |
| Positive/high (n = 7) | 6.74 (1.55–29.33) | 6.74 (1.55–29.33) | 0.011 | |
| Negative/high (n = 10) | 5.82 (1.55–22.87) | 5.82 (1.55–22.87) | 0.009 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.-M.; Cha, H.; Moon, Y.; Kim, L.; Kwak, S.M.; Park, E.S.; Nam, H.-S. A Systemic Immune Inflammation Index and PD-L1 (SP142) Expression as a Potential Combined Biomarker of the Clinical Benefit of Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Med. 2024, 13, 1521. https://doi.org/10.3390/jcm13051521
Baek J-M, Cha H, Moon Y, Kim L, Kwak SM, Park ES, Nam H-S. A Systemic Immune Inflammation Index and PD-L1 (SP142) Expression as a Potential Combined Biomarker of the Clinical Benefit of Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. Journal of Clinical Medicine. 2024; 13(5):1521. https://doi.org/10.3390/jcm13051521
Chicago/Turabian StyleBaek, Jong-Min, Hyungkeun Cha, Yeonsook Moon, Lucia Kim, Seung Min Kwak, Eun Sun Park, and Hae-Seong Nam. 2024. "A Systemic Immune Inflammation Index and PD-L1 (SP142) Expression as a Potential Combined Biomarker of the Clinical Benefit of Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer" Journal of Clinical Medicine 13, no. 5: 1521. https://doi.org/10.3390/jcm13051521
APA StyleBaek, J.-M., Cha, H., Moon, Y., Kim, L., Kwak, S. M., Park, E. S., & Nam, H.-S. (2024). A Systemic Immune Inflammation Index and PD-L1 (SP142) Expression as a Potential Combined Biomarker of the Clinical Benefit of Chemo-Immunotherapy in Extensive-Stage Small-Cell Lung Cancer. Journal of Clinical Medicine, 13(5), 1521. https://doi.org/10.3390/jcm13051521






