Microbiota-Derived Short-Chain Fatty Acids Boost Antitumoral Natural Killer Cell Activity
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Lines
2.2. Cell Proliferation
2.3. Extracellular Vesicles Study
2.4. Cytokines Array
2.5. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results
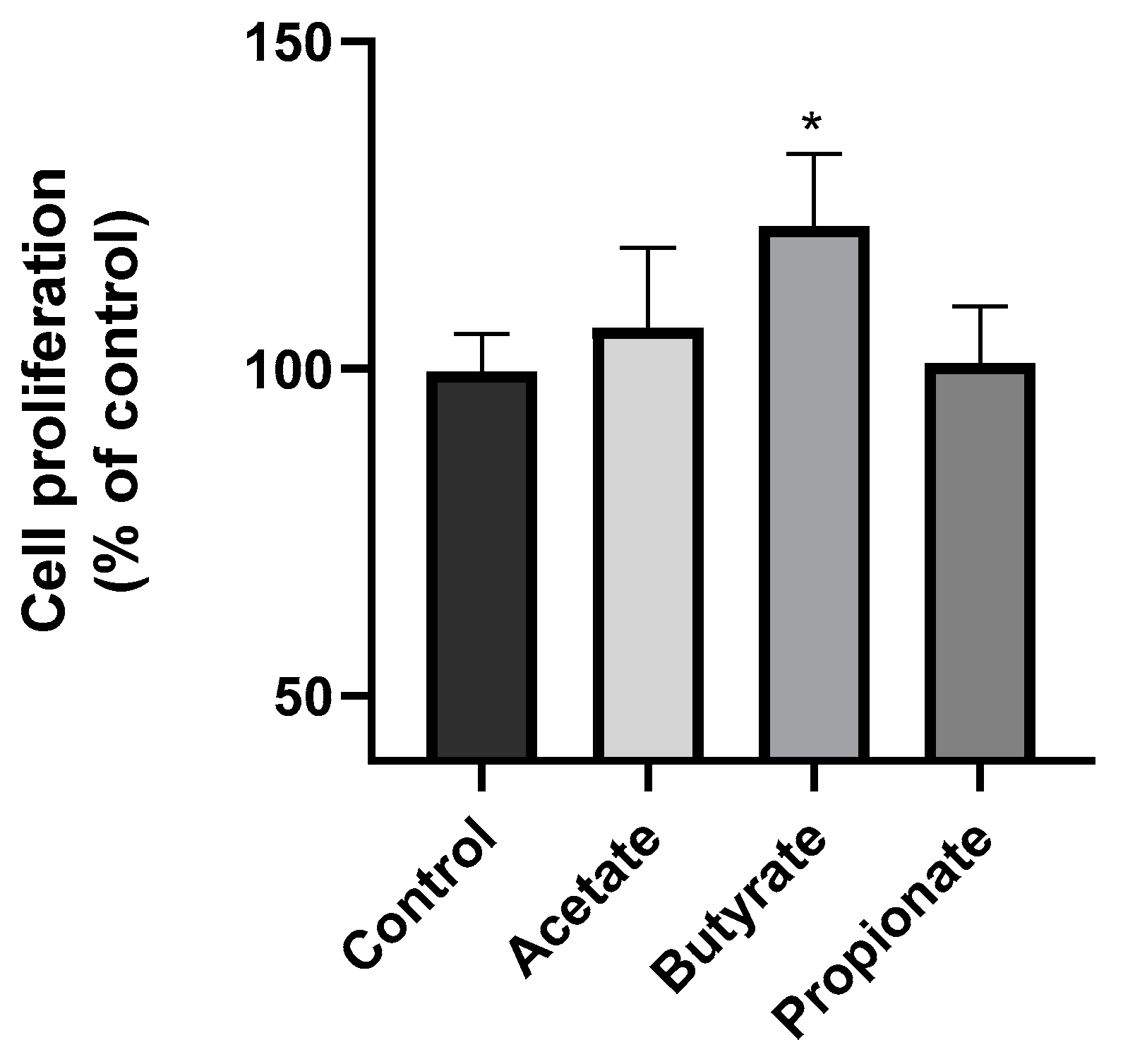
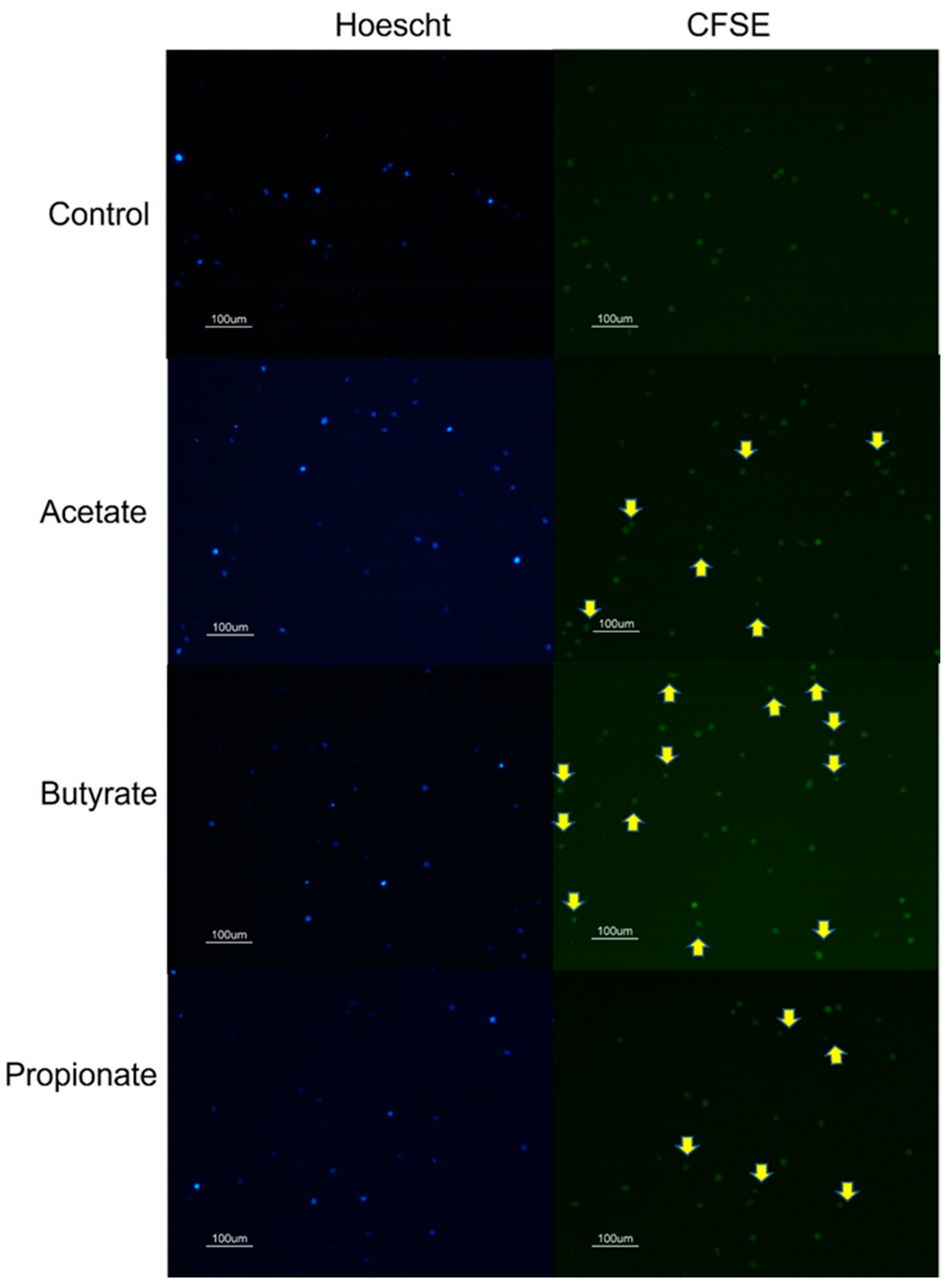
3.1. SCFA Butyrate Increases NK Cell Proliferation
3.2. SCFAs Affect NK Cell Secretome
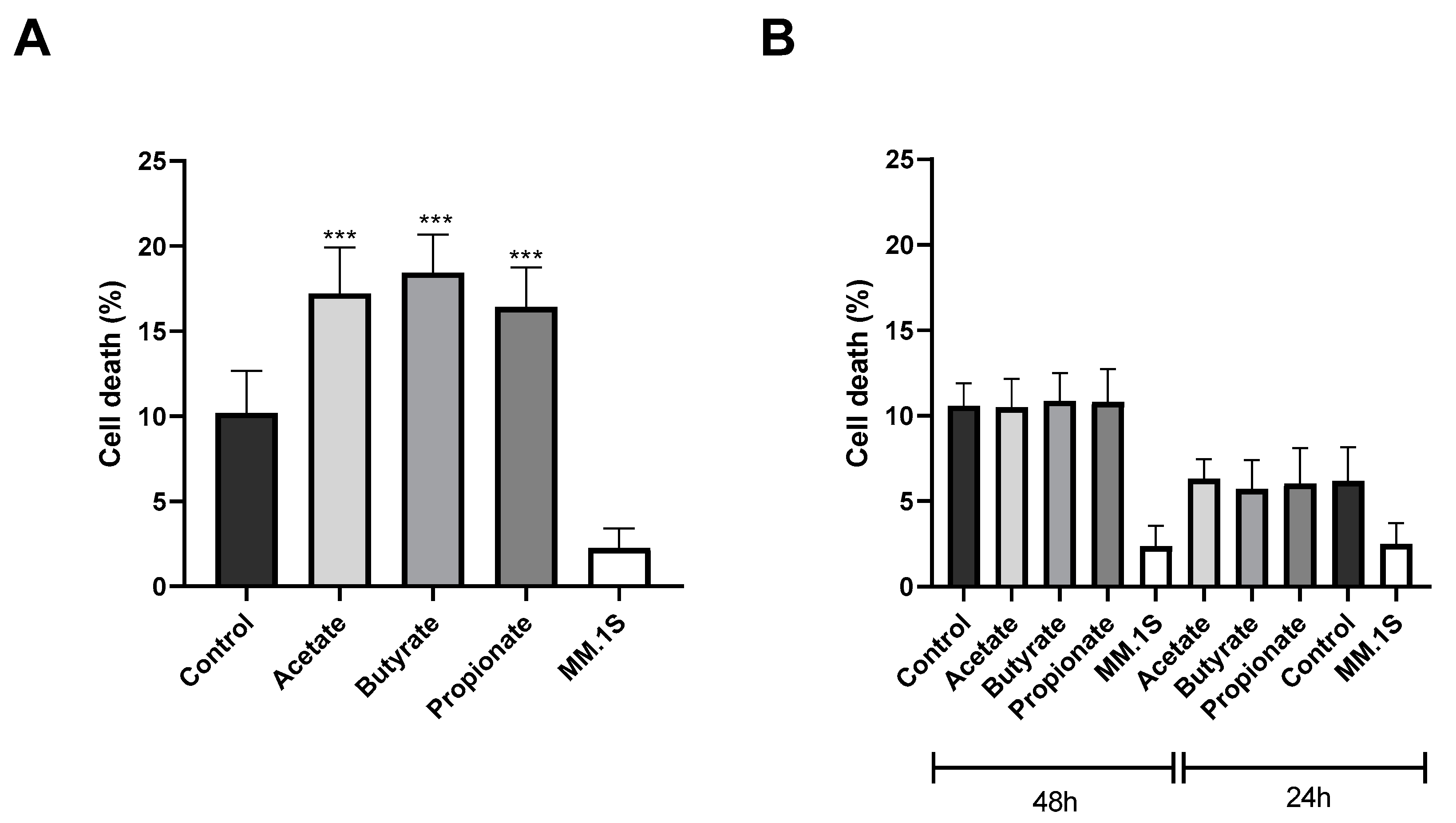
3.3. SCFAs Enhance the Antitumor Cytotoxicity of NK Cells
4. Discussion
Limitations of the Study and Future Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jandhyala, S.M. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Inamura, K. Roles of Microbiota in Response to Cancer Immunotherapy. Semin. Cancer Biol. 2020, 65, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Schütz, B.; Lauth, M.; Visekruna, A. The Impact of Gut Microbiota-Derived Metabolites on the Tumor Immune Microenvironment. Cancers 2023, 15, 1588. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; El Alam, M.B.; Karpinets, T.V.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.; Yoshida-Court, K.; Wu, X.; Biegert, G.W.G.; Delgado Medrano, A.Y.; et al. Gut Microbiome Diversity Is an Independent Predictor of Survival in Cervical Cancer Patients Receiving Chemoradiation. Commun. Biol. 2021, 4, 237. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Weersma, R.K.; Medema, M.H.; Netea, M.G.; et al. Influence of the Microbiome, Diet and Genetics on Inter-Individual Variation in the Human Plasma Metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Chiu, C.J.; Tsai, C.Y.; Lee, Y.R.; Liu, W.L.; Chuang, H.L.; Huang, M.T. Short-Chain Fatty Acids Ameliorate Allergic Airway Inflammation via Sequential Induction of PMN-MDSCs and Treg Cells. J. Allergy Clin. Immunol. Glob. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; MacKay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 Inflammasome Regulates Colonic Microbial Ecology and Risk for Colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Furuta, K.; Han, C.; Uneme, M.; Kano, T.; Ishikawa, K.; Kaito, C. Short-Chain Fatty Acids Stimulate Dendrite Elongation in Dendritic Cells by Inhibiting Histone Deacetylase. FEBS J. 2023, 290, 5794–5810. [Google Scholar] [CrossRef] [PubMed]
- Kolypetri, P.; Weiner, H.L. Monocyte Regulation by Gut Microbial Signals. Trends Microbiol. 2023, 31, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive Effect of Short-Chain Fatty Acids on Production of Proinflammatory Mediators by Neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The Microbial Metabolite Butyrate Regulates Intestinal Macrophage Function via Histone Deacetylase Inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, H.W.J.; Naylor, C.P.E.; MacFarlane, G.T. Short Chain Fatty Acids in Human Large Intestine, Portal, Hepatic and Venous Blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The Luminal Short-Chain Fatty Acid Butyrate Modulates NF-KappaB Activity in a Human Colonic Epithelial Cell Line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.P.; Galmiche, J.P.; Raingeard De La Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M. Butyrate Inhibits Inflammatory Responses through NFkappaB Inhibition: Implications for Crohn’s Disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-Inflammatory Properties of the Short-Chain Fatty Acids Acetate and Propionate: A Study with Relevance to Inflammatory Bowel Disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Gewirtz, A.T.; Chassaing, B. Connecting the Dots: Dietary Fat, Microbiota Dysbiosis, Altered Metabolome, and Colon Cancer. Gastroenterology 2022, 162, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Wong-Rolle, A.; Wei, H.K.; Zhao, C.; Jin, C. Unexpected Guests in the Tumor Microenvironment: Microbiome in Cancer. Protein Cell 2021, 12, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut Microbiome Development along the Colorectal Adenoma–Carcinoma Sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic Bacteria in Colorectal Cancer: Mechanisms and Treatments. Cancer Biol. Med. 2022, 19, 147–162. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Z.; Dong, C.; Luan, Y.; Zhang, A.; Moore, C.; Fu, K.; Peng, J.; Wang, Y.; Ren, Z.; et al. Targeting Tumors with IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis. Cancer Cell 2019, 35, 901–915.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Lin, Y.; Jing, X.; Ye, Y.; Wang, S.; Shen, Z. Innate Tumor Killers in Colorectal Cancer. Cancer Lett. 2022, 527, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural Killer Cells: A Promising Immunotherapy for Cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef]
- Kotzur, R.; Duev-Cohen, A.; Kol, I.; Reches, A.; Mandelboim, O.; Stein, N. NK-92 Cells Retain Vitality and Functionality When Grown in Standard Cell Culture Conditions. PLoS ONE 2022, 17, e0264897. [Google Scholar] [CrossRef]
- Greenstein, S.; Krett, N.L.; Kurosawa, Y.; Ma, C.; Chauhan, D.; Hideshima, T.; Anderson, K.C.; Rosen, S.T. Characterization of the MM.1 Human Multiple Myeloma (MM) Cell Lines: A Model System to Elucidate the Characteristics, Behavior, and Signaling of Steroid-Sensitive and -Resistant MM Cells. Exp. Hematol. 2003, 31, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Wu, F.; Xie, M.; Hun, M.; She, Z.; Li, C.; Luo, S.; Chen, X.; Wan, W.; Wen, C.; Tian, J. Natural Killer Cell-Derived Extracellular Vesicles: Novel Players in Cancer Immunotherapy. Front. Immunol. 2021, 12, 658698. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-T.; Mao, Y.-Q. The Impact of the Microbiome in Cancer: Targeting Metabolism of Cancer Cells and Host. Front. Oncol. 2022, 12, 1029033. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Lin, Z.; Ma, N.; Men, Z.; Zhang, C.; Ma, X.; Zheng, H. Dietary Sodium Butyrate and Forskolin Promote Cell Proliferation to Resist Citrobacter Rodentium Infection by Lysozyme Upregulation. J. Funct. Foods 2024, 112, 105993. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric Short-chain Fatty Acids Promote Proliferation of Human Neural Progenitor Cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Blottiere, H.M.; Buecher, B.; Galmiche, J.-P.; Cherbut, C. Molecular Analysis of the Effect of Short-Chain Fatty Acids on Intestinal Cell Proliferation. Proc. Nutr. Soc. 2003, 62, 101–106. [Google Scholar] [CrossRef]
- Mesiano, G.; Zini, R.; Montagner, G.; Bianchi, N.; Manfredini, R.; Chillemi, A.; Aglietta, M.; Grignani, G.; Lampronti, I.; Fiorino, E.; et al. Analytic and Dynamic Secretory Profile of Patient-Derived Cytokine-Lnduced Killer Cells. Mol. Med. 2017, 23, 235–246. [Google Scholar] [CrossRef]
- Chan, A.M.L.; Cheah, J.M.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Natural Killer Cell-Derived Extracellular Vesicles as a Promising Immunotherapeutic Strategy for Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4026. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune Surveillance Properties of Human NK Cell-Derived Exosomes. J. Immunol. 2012, 189, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kalimuthu, S.; Gangadaran, P.; Oh, J.M.; Lee, H.W.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017, 7, 2732–2745. [Google Scholar] [CrossRef]
- Sheikhpour, E.; Noorbakhsh, P.; Foroughi, E.; Farahnak, S.; Nasiri, R.; Neamatzadeh, H. A Survey on the Role of Interleukin-10 in Breast Cancer: A Narrative. Rep. Biochem. Mol. Biol. 2018, 7, 30–37. [Google Scholar]
- Zhu, X.; Li, K.; Liu, G.; Wu, R.; Zhang, Y.; Wang, S.; Xu, M.; Lu, L.; Li, P. Microbial Metabolite Butyrate Promotes Anti-PD-1 Antitumor Efficacy by Modulating T Cell Receptor Signaling of Cytotoxic CD8 T Cell. Gut Microbes 2023, 15, 2249143. [Google Scholar] [CrossRef]
- Zhi, L.; Wang, X.; Gao, Q.; He, W.; Shang, C.; Guo, C.; Niu, Z.; Zhu, W.; Zhang, X. Intrinsic and Extrinsic Factors Determining Natural Killer Cell Fate: Phenotype and Function. Biomed. Pharmacother. 2023, 165, 115136. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, S.M.; Ashkar, A.A. What defines NK cell functional fate: Phenotype or me-tabolism? Front Immunol. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, L.; Korenfeld, D.; Tung, T.; Klechevsky, E. Dendritic Cell–Derived IL-32α: A Novel Inhibitory Cytokine of NK Cell Function. J. Immunol. 2017, 199, 1290–1300. [Google Scholar] [CrossRef]
- Piñeiro Fernández, J.; Luddy, K.A.; Harmon, C.; O’Farrelly, C. Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. Int. J. Mol. Sci. 2019, 20, 4131. [Google Scholar] [CrossRef]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The Paradoxical Role of IL-10 in Immunity and Cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.X. Microbiota-Associated Metabolites and Related Immunoregulation in Colorectal Cancer. Cancers 2021, 13, 4054. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad-S, S.; Casolaro, V.; Lv, Z.; Li, D. Potential Links between the Microbiota and T Cell Immunity Determine the Tumor Cell Fate. Cell Death Dis. 2023, 14, 154. [Google Scholar] [CrossRef]
- Martín-Antonio, B.; Suñe, G.; Najjar, A.; Perez-Amill, L.; Antoñana-Vildosola, A.; Castella, M.; León, S.; Velasco-de Andrés, M.; Lozano, F.; Lozano, E.; et al. Extracellular NK Histones Promote Immune Cell Anti-Tumor Activity by Inducing Cell Clusters through Binding to CD138 Receptor. J. Immunother. Cancer 2019, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; Arroyo, A.; García-Vicente, R.; Morales, M.L.; Gómez-Gordo, R.; Justo, P.; Cuéllar, C.; Sánchez-Pina, J.; López, N.; Alonso, R.; et al. Short-Chain Fatty Acid Production by Gut Microbiota Predicts Treatment Response in Multiple Myeloma. Clin. Cancer Res. 2024, OF1–OF14. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Ye, F.; Yang, C.; Jiang, H. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Prostate Cancer Progression via Inducing Cancer Cell Autophagy and M2 Macrophage Polarization. Neoplasia 2023, 43, 100928. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, W.; Wang, R.; Chen, Y.; Liu, J.; Guo, X.; Can, C.; Yang, X.; Wang, D.; Hu, X.; et al. Propionate Promotes Ferroptosis and Apoptosis through Mitophagy and ACSL4-Mediated Ferroptosis Elicits Anti-Leukemia Immunity. Free Radic. Biol. Med. 2024, 213, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Dhatt, J.; Bhatti, I.; Hamie, A.; Baker, K. Short Chain Fatty Acids Prime Colorectal Cancer Cells to Activate Antitumor Immunity. Front. Immunol. 2023, 14, 1190810. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef]
- Hezaveh, K.; Shinde, R.S.; Klötgen, A.; Halaby, M.J.; Lamorte, S.; Ciudad, M.T.; Quevedo, R.; Neufeld, L.; Liu, Z.Q.; Jin, R.; et al. Tryptophan-Derived Microbial Metabolites Activate the Aryl Hydrocarbon Receptor in Tumor-Associated Macrophages to Suppress Anti-Tumor Immunity. Immunity 2022, 55, 324–340.e8. [Google Scholar] [CrossRef]
- Song, X.; An, Y.; Chen, D.; Zhang, W.; Wu, X.; Li, C.; Wang, S.; Dong, W.; Wang, B.; Liu, T.; et al. Microbial Metabolite Deoxycholic Acid Promotes Vasculogenic Mimicry Formation in Intestinal Carcinogenesis. Cancer Sci. 2022, 113, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal Microbiota Transplant Overcomes Resistance to Anti–PD-1 Therapy in Melanoma Patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Son, M.-Y.; Cho, H.-S. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J. Microbiol. Biotechnol. 2023, 33, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Karimi, Z.; Taymouri, S.; Minaiyan, M.; Mirian, M. Evaluation of Thermosensitive Chitosan Hydrogel Containing Gefitinib Loaded Cellulose Acetate Butyrate Nanoparticles in a Subcutaneous Breast Cancer Model. Int. J. Pharm. 2022, 624, 122036. [Google Scholar] [CrossRef]
- Pham, C.H.; Lee, J.-E.; Yu, J.; Lee, S.H.; Yu, K.-R.; Hong, J.; Cho, N.; Kim, S.; Kang, D.; Lee, S.; et al. Anticancer Effects of Propionic Acid Inducing Cell Death in Cervical Cancer Cells. Molecules 2021, 26, 4951. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, M.; Buey, B.; Corral, P.; Giraldos, D.; Latorre, E. Microbiota-Derived Short-Chain Fatty Acids Boost Antitumoral Natural Killer Cell Activity. J. Clin. Med. 2024, 13, 3885. https://doi.org/10.3390/jcm13133885
Pérez M, Buey B, Corral P, Giraldos D, Latorre E. Microbiota-Derived Short-Chain Fatty Acids Boost Antitumoral Natural Killer Cell Activity. Journal of Clinical Medicine. 2024; 13(13):3885. https://doi.org/10.3390/jcm13133885
Chicago/Turabian StylePérez, Marina, Berta Buey, Pilar Corral, David Giraldos, and Eva Latorre. 2024. "Microbiota-Derived Short-Chain Fatty Acids Boost Antitumoral Natural Killer Cell Activity" Journal of Clinical Medicine 13, no. 13: 3885. https://doi.org/10.3390/jcm13133885
APA StylePérez, M., Buey, B., Corral, P., Giraldos, D., & Latorre, E. (2024). Microbiota-Derived Short-Chain Fatty Acids Boost Antitumoral Natural Killer Cell Activity. Journal of Clinical Medicine, 13(13), 3885. https://doi.org/10.3390/jcm13133885


_Amit_Rahat.jpg)


