Cardiovascular Risks of Simultaneous Use of Alcohol and Cocaine—A Systematic Review
Abstract
1. Introduction
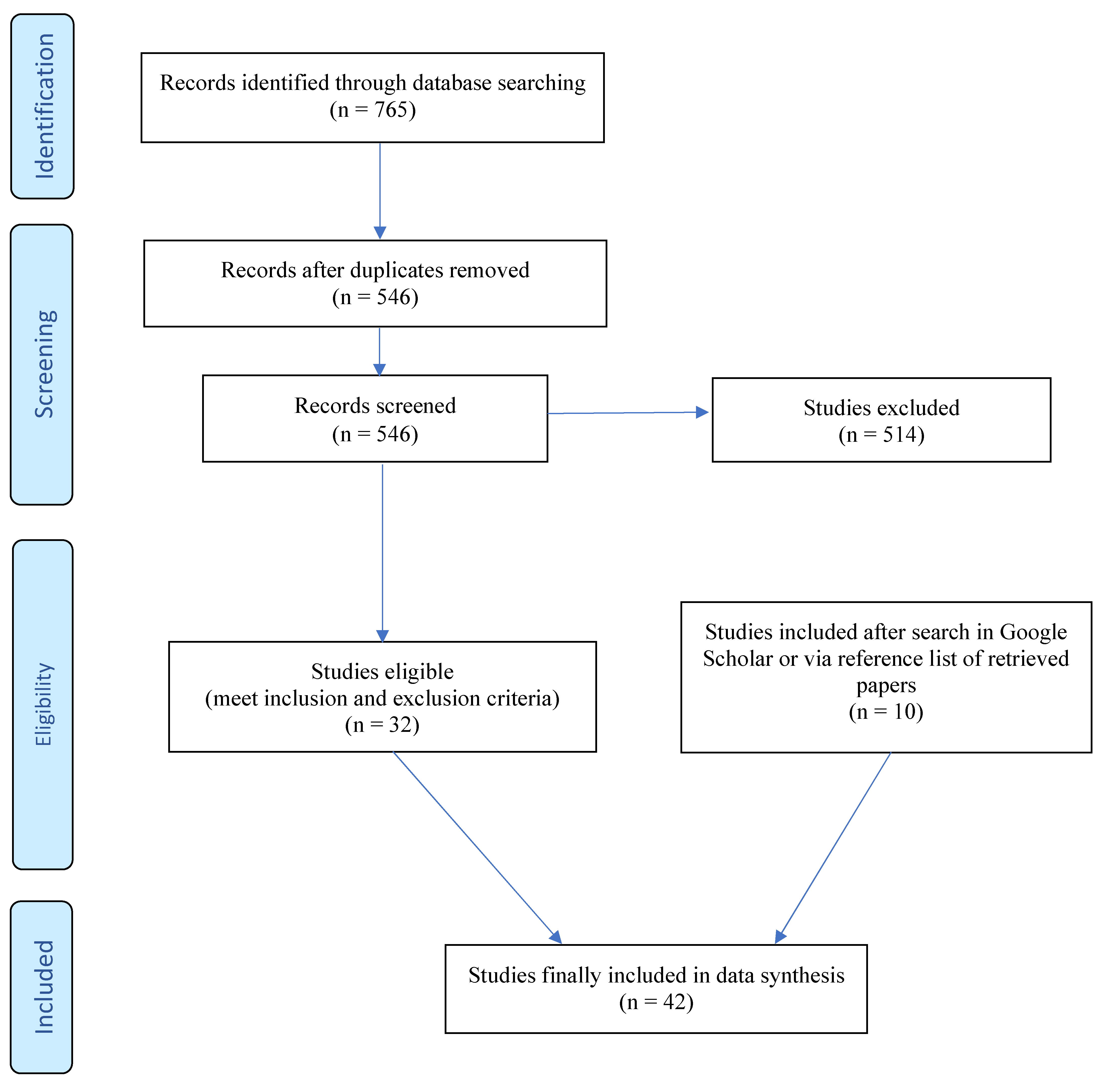
2. Methods
3. Results
3.1. Pharmacodynamic Effects of Cocaine
3.2. Pharmacodynamic Effects of Alcohol
3.3. Pharmacokinetic Interactions of Cocaine and Alcohol in Humans
- (a)
- Increased cocaine plasma levels
- (b)
- Formation of the cardiotoxic metabolite cocaethylene (CE)
3.4. Pharmacodynamic Interactions of Cocaine and Alcohol
3.5. Cocaine and Alcohol Interactions in Cardiac Arrhythmia
3.6. Cocaine-Related ED Presentations and the Role of Alcohol Co-Use
3.7. Cocaine-Related Mortality and the Role of Alcohol Co-Use
4. Discussion
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorelick, D.A. Cocaine Use Disorder in Adults: Epidemiology, Clinical Features, and Diagnosis. Available online: https://medilib.ir/uptodate/show/7802 (accessed on 13 January 2024).
- Liu, Y.; Williamson, V.; Setlow, B.; Cottler, L.B.; Knackstedt, L.A. The importance of considering polysubstance use: Lessons from cocaine research. Drug Alcohol Depend. 2018, 192, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Stinson, F.S.; Grant, B.F.; Dawson, D.A.; Ruan, W.J.; Huang, B.; Saha, T. Comorbidity Between DSM–IV Alcohol and Specific Drug Use Disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res. Health 2006, 29, 94. [Google Scholar] [CrossRef]
- Kim, S.T.; Park, T. Acute and chronic effects of cocaine on cardiovascular health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef] [PubMed]
- Arenas, D.J.; Beltran, S.; Zhou, S.; Goldberg, L.R. Cocaine, cardiomyopathy, and heart failure: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 19795. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Magnusson, P.; LeQuang, J.A.K.; Breve, F.; Varrassi, G.; Pergolizzi, J., Jr. Cocaine and cardiotoxicity: A literature review. Cureus 2021, 13, 14594. [Google Scholar] [CrossRef] [PubMed]
- Knuepfer, M.M. Cardiovascular disorders associated with cocaine use: Myths and truths. Pharmacol. Ther. 2003, 97, 181–222. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.; Luk, A.; Soor, G.S.; Abraham, J.R.; Leong, S.; Butany, J. Cocaine cardiotoxicity: A review of the pathophysiology, pathology, and treatment options. Am. J. Cardiovasc. Drugs 2009, 9, 177–196. [Google Scholar] [CrossRef]
- Gangu, K.; Bobba, A.; Basida, S.D.; Avula, S.; Chela, H.; Singh, S. Trends of Cocaine Use and Manifestations in Hospitalized Patients: A Cross-Sectional Study. Cureus 2022, 14, e22090. [Google Scholar] [CrossRef]
- Winhusen, T.; Theobald, J.; Kaelber, D.C.; Lewis, D. The association between regular cocaine use, with and without tobacco co-use, and adverse cardiovascular and respiratory outcomes. Drug Alcohol Depend. 2020, 214, 108136. [Google Scholar] [CrossRef]
- Havakuk, O.; Rezkalla, S.H.; Kloner, R.A. The cardiovascular effects of cocaine. J. Am. Coll. Cardiol. 2017, 70, 101–113. [Google Scholar] [CrossRef]
- Minor, R.L., Jr.; Scott, B.D.; Brown, D.D.; Winniford, M.D. Cocaine-induced myocardial infarction in patients with normal coronary arteries. Ann. Intern. Med. 1991, 115, 797–806. [Google Scholar] [CrossRef]
- Brody, S.L.; Slovis, C.M.; Wrenn, K.D. Cocaine-related medical problems: Consecutive series of 233 patients. Am. J. Med. 1990, 88, 325–331. [Google Scholar] [CrossRef]
- Hollander, J.E.; Todd, K.H.; Green, G.; Heilpern, K.L.; Karras, D.J.; Singer, A.J.; Brogan, G.X.; Funk, J.P.; Strahan, J.B. Chest pain associated with cocaine: An assessment of prevalence in suburban and urban emergency departments. Ann. Emerg. Med. 1995, 26, 671–676. [Google Scholar] [CrossRef]
- McCord, J.; Jneid, H.; Hollander, J.E.; De Lemos, J.A.; Cercek, B.; Hsue, P.; Gibler, W.B.; Ohman, E.M.; Drew, B.; Philippides, G. Management of cocaine-associated chest pain and myocardial infarction: A scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 2008, 117, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Patel, P.S.; Andhavarapu, S.; Bzihlyanskaya, V.; Friedman, E.; Jeyaraju, M.; Palmer, J.; Raffman, A.; Pourmand, A.; Tran, Q.K. Prevalence of myocardial infarction among patients with chest pain and cocaine use: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021, 50, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Mittleman, M.A.; Mintzer, D.; Maclure, M.; Tofler, G.H.; Sherwood, J.B.; Muller, J.E. Triggering of myocardial infarction by cocaine. Circulation 1999, 99, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Loma-Osorio, P.; Guasch, E.; Nogué, S.; Ortiz, J.T.; Sánchez, M. Prevalence, clinical characteristics and risk of myocardial infarction in patients with cocaine-related chest pain. Rev. Esp. Cardiol. (Engl. Ed.) 2010, 63, 1028–1034. [Google Scholar] [CrossRef]
- Bamberg, F.; Schlett, C.L.; Truong, Q.A.; Rogers, I.S.; Koenig, W.; Nagurney, J.T.; Seneviratne, S.; Lehman, S.J.; Cury, R.C.; Abbara, S. Presence and extent of coronary artery disease by cardiac computed tomography and risk for acute coronary syndrome in cocaine users among patients with chest pain. Am. J. Cardiol. 2009, 103, 620–625. [Google Scholar] [CrossRef]
- SAMHSA. Substance Abuse and Mental Health Services Administration (SAMHSA). Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits; SAMHSA, Rockville, MD, USA. Available online: https://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf (accessed on 13 January 2024).
- SAMHSA. Substance Abuse and Mental Health Services Administration (SAMHSA). Drug Abuse Warning Network (DAWN). Findings from Drug-Related Emergency Department Visits. 2021. Available online: https://www.drugsandalcohol.ie/37760/1/US_DAWN_Drug_abuse_warning_network_2021.pdf (accessed on 13 January 2024).
- EMCDDA. European Drug Report 2022. Trends and Developments. Available online: https://www.emcdda.europa.eu/system/files/publications/14644/TDAT22001ENN.pdf (accessed on 13 January 2024).
- Supervía, A.; Ibrahim-Achi, D.; Miró, Ò.; Galicia, M.; Ferrando, J.P.; Leciñena, M.A.; de L’Hotellerie, M.J.V.; Bajo, Á.B.; Martín-Pérez, B.; Burillo-Putze, G. Impact of co-ingestion of ethanol on the clinical symptomatology and severity of patients attended in the emergency department for recreational drug toxicity. Am. J. Emerg. Med. 2021, 50, 422–427. [Google Scholar] [CrossRef]
- Helander, A.; Villén, T.; Signell, P. Urine drug tests indicate higher prevalence of combined alcohol and cocaine use compared to alcohol together with cannabis or amphetamine. A possible link to cocaethylene. Alcohol Alcohol 2023, 58, 274–279. [Google Scholar] [CrossRef]
- Teherán, A.A.; Pombo, L.M.; Cadavid, V.; Mejía, M.C.; La Rota, J.F.; Hernández, J.C.; Montoya, N.; López, T.S. Cocaine, ethanol, cannabis and benzodiazepines co-consumption among patients assisted at the emergency room. Open Access Emerg. Med. 2019, 11, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Suen, L.W.; Davy-Mendez, T.; LeSaint, K.T.; Riley, E.D.; Coffin, P.O. Emergency department visits and trends related to cocaine, psychostimulants, and opioids in the United States, 2008–2018. BMC Emerg. Med. 2022, 22, 19. [Google Scholar] [CrossRef]
- Bodmer, M.; Enzler, F.; Liakoni, E.; Bruggisser, M.; Liechti, M.E. Acute cocaine-related health problems in patients presenting to an urban emergency department in Switzerland: A case series. BMC Res. Notes 2014, 7, 173. [Google Scholar] [CrossRef]
- Wallgren, H.; Barry, H. Actions of Alcohol: Biochemical, Physiological and Psychological Aspects; Elsevier Publising Co.: New York, NY, USA, 1970; Volume 2, p. 565. [Google Scholar]
- Mendoza, L.; Hellberg, K.; Rickart, A.; Tillich, G.; Bing, R. The effect of intravenous ethyl alcohol on the coronary circulation and myocardial contractility of the human and canine heart. J. Clin. Pharmacol. New Drugs 1971, 11, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, S.; Tang, C.; Musini, V.M.; Wright, J.M. Effect of alcohol on blood pressure. Cochrane Database System Rev. 2020, 2020, CD012787. [Google Scholar] [CrossRef]
- Jackson, R.; Scragg, R.; Beaglehole, R. Does recent alcohol consumption reduce the risk of acute myocardial infarction and coronary death in regular drinkers? Am. J. Epidemiol. 1992, 136, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Chahal, H.S.; Mukamal, K.J.; Rimm, E.B.; Mittleman, M.A. Alcohol and immediate risk of cardiovascular events: A systematic review and dose–response meta-analysis. Circulation 2016, 133, 979–987. [Google Scholar] [CrossRef]
- Mostofsky, E.; van der Bom, J.G.; Mukamal, K.J.; Maclure, M.; Tofler, G.H.; Muller, J.E.; Mittleman, M.A. Risk of myocardial infarction immediately after alcohol consumption. Epidemiology 2015, 26, 143. [Google Scholar] [CrossRef]
- Farré, M.; de la Torre, R.; Llorente, M.; Lamas, X.; Ugena, B.; Segura, J.; Camí, J. Alcohol and cocaine interactions in humans. J. Pharmacol. Exp. Ther. 1993, 266, 1364–1373. [Google Scholar]
- Foltin, R.W.; Fischman, M.W. Ethanol and cocaine interactions in humans: Cardiovascular consequences. Pharmacol. Biochem. Behav. 1988, 31, 877–883. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Jeffcoat, A.R. Ethanol/cocaine interaction: Cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sci. 1992, 51, 553–563. [Google Scholar] [CrossRef]
- Higgins, S.T.; Rush, C.R.; Bickel, W.K.; Hughes, J.R.; Lynn, M.; Capeless, M.A. Acute behavioral and cardiac effects of cocaine and alcohol combinations in humans. Psychopharmacology 1993, 111, 285–294. [Google Scholar] [CrossRef] [PubMed]
- McCance-Katz, E.F.; Kosten, T.R.; Jatlow, P. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone—A multiple-dose study. Biol. Psychiat. 1998, 44, 250–259. [Google Scholar] [CrossRef]
- Cami, J.; Farré, M.; González, M.L.; Segura, J.; de la Torre, R. Cocaine Metabolism in Humans After Use of Alcohol. Clinical and Research Implications. Recent Dev. Alcohol. 1998, 14, 437–455. [Google Scholar] [PubMed]
- Perez-Reyes, M. The order of drug administration: Its effects on the interaction between cocaine and ethanol. Life Sci. 1994, 55, 541–550. [Google Scholar] [CrossRef]
- Jones, A.W. Forensic Drug Profile: Cocaethylene. J. Anal. Toxicol. 2019, 43, 155–160. [Google Scholar] [CrossRef]
- McCance-Katz, E.F.; Price, L.H.; McDougle, C.J.; Kosten, T.R.; Black, J.E.; Jatlow, P.I. Concurrent cocaine-ethanol ingestion in humans: Pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology 1993, 111, 39–46. [Google Scholar] [CrossRef]
- Fowler, J.; Volkow, N.; Logan, J.; MacGregor, R.; Wang, G.J.; Wolf, A. Alcohol inoxication does not change [11C] cocaine pharmacokinetics in human brain and heart. Synapse 1992, 12, 228–235. [Google Scholar] [CrossRef]
- Herbst, E.D.; Harris, D.S.; Everhart, E.T.; Mendelson, J.; Jacob, P.; Jones, R.T. Cocaethylene formation following ethanol and cocaine administration by different routes. Exp. Clin. Psychopharmacol. 2011, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.S.; Everhart, E.T.; Mendelson, J.; Jones, R.T. The pharmacology of cocaethylene in humans following cocaine and ethanol administration. Drug Alcohol Depend. 2003, 72, 169–182. [Google Scholar] [CrossRef]
- Andrews, P. Cocaethylene toxicity. J. Addict. Dis. 1997, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- McCance-Katz, E.F.; Hart, C.L.; Boyarsky, B.; Kosten, T.; Jatlow, P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst. Use Misuse 2005, 40, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Boehrer, J.D.; Moliterno, D.J.; Willard, J.E.; Snyder II, R.W.; Horton, R.P.; Glamann, D.B.; Lange, R.A.; Hillis, L.D. Hemodynamic effects of intranasal cocaine in humans. J. Am. Coll. Cardiol. 1992, 20, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; De La Torre, R.; González, M.L.; Terán, M.T.; Roset, P.N.; Menoyo, E.; Camí, J. Cocaine and alcohol interactions in humans: Neuroendocrine effects and cocaethylene metabolism. J. Pharmacol. Exp. Ther. 1997, 283, 164–176. [Google Scholar]
- Rush, C.R.; Roll, J.M.; Higgins, S.T. Controlled Laboratory Studies on the Effects of Cocaine in Combination with Other Commonly Used Drugs in Humans; Higins, S.T., Katz, J.L., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 239–263. [Google Scholar]
- Lange, R.A.; Cigarroa, R.G.; Yancy, C.W., Jr.; Willard, J.E.; Popma, J.J.; Sills, M.N.; McBride, W.; Kim, A.S.; Hillis, L.D. Cocaine-induced coronary-artery vasoconstriction. N. Engl. J. Med. 1989, 321, 1557–1562. [Google Scholar] [CrossRef]
- Brogan III, W.C.; Lange, R.A.; Glamann, D.B.; Hillis, L.D. Recurrent coronary vasoconstriction caused by intranasal cocaine: Possible role for metabolites. Ann. Intern. Med. 1992, 116, 556–561. [Google Scholar] [CrossRef]
- Pirwitz, M.J.; Willard, J.E.; Landau, C.; Lange, R.A.; Glamann, D.B.; Kessler, D.J.; Foerster, E.H.; Todd, E.; Hillis, L.D. Influence of cocaine, ethanol, or their combination on epicardial coronary arterial dimensions in humans. Arch. Intern. Med. 1995, 155, 1186–1191. [Google Scholar] [CrossRef]
- McCance, E.F.; Price, L.H.; Kosten, T.R.; Jatlow, P.I. Cocaethylene: Pharmacology, physiology and behavioral effects in humans. J. Pharmacol. Exp. Ther. 1995, 274, 215–223. [Google Scholar]
- Perez-Reyes, M. Subjective and cardiovascular effects of cocaethylene in humans. Psychopharmacology 1993, 113, 144–147. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Jeffcoat, A.R.; Myers, M.; Sihler, K.; Cook, C.E. Comparison in humans of the potency and pharmacokinetics of intravenously injected cocaethylene and cocaine. Psychopharmacology 1994, 116, 428–432. [Google Scholar] [CrossRef]
- Erzouki, H.K.; Baum, I.; Goldberg, S.R.; Schindler, C.W. Comparison of the effects of cocaine and its metabolites on cardiovascular function in anesthetized rats. J. Cardiovasc. Pharmacol. 1993, 22, 557–563. [Google Scholar] [CrossRef]
- Kim, S.R. Pharmacokinectic and Pharmacodynamic Aspects of Cocaine and Its Interaction with Ethanol. Chapter 7. Evaluation of the Pharmacokinetic and Pharmacodynamic Relationships of Cocaine and Cocaethylene in Human Subjects. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1997. [Google Scholar]
- Hart, C.L.; Jatlow, P.; Sevarino, K.A.; McCance-Katz, E.F. Comparison of intravenous cocaethylene and cocaine in humans. Psychopharmacology 2000, 149, 153–162. [Google Scholar] [CrossRef]
- Ferreira, S.; Crumb, W.J.; Carlton, C.G.; Clarkson, C.W. Effects of cocaine and its major metabolites on the HERG-encoded potassium channel. J. Pharmacol. Exp. Ther. 2001, 299, 220–226. [Google Scholar]
- Hoffman, R.S. Treatment of patients with cocaine-induced arrhythmias: Bringing the bench to the bedside. Br. J. Clin. Pharmacol. 2010, 69, 448–457. [Google Scholar] [CrossRef]
- Elkattawy, S.; Alyacoub, R.; Al-Nassarei, A.; Younes, I.; Ayad, S.; Habib, M. Cocaine induced heart failure: Report and literature review. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 547–550. [Google Scholar] [CrossRef]
- Taylor, D.; Parish, D.; Thompson, L.; Cavaliere, M. Cocaine induced prolongation of the QT interval. Emerg. Med. J. 2004, 21, 252–253. [Google Scholar] [CrossRef][Green Version]
- Ramirez, F.D.; Femenía, F.; Simpson, C.S.; Redfearn, D.P.; Michael, K.A.; Baranchuk, A. Electrocardiographic findings associated with cocaine use in humans: A systematic review. Expert Rev. Cardiovasc. Ther. 2012, 10, 105–127. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Crumb, W.; Clarkson, C.W. Cocaethylene, a metabolite of cocaine and ethanol, is a potent blocker of cardiac sodium channels. J. Pharmacol. Exp. Ther. 1994, 271, 319–325. [Google Scholar]
- Kupari, M.; Koskinen, P. Alcohol, cardiac arrhythmias and sudden death. Novartis Found. Symp. 1998, 216, 68–79; discussion 79–85. [Google Scholar] [CrossRef]
- Wong, C.X.; Tu, S.J.; Marcus, G.M. Alcohol and Arrhythmias. JACC Clin. Electrophysiol. 2023, 9, 266–279. [Google Scholar] [CrossRef]
- Morentin, B.; Ballesteros, J.; Callado, L.F.; Meana, J.J. Recent cocaine use is a significant risk factor for sudden cardiovascular death in 15–49-year-old subjects: A forensic case–control study. Addiction 2014, 109, 2071–2078. [Google Scholar] [CrossRef]
- Teli, K.J.; Gupta, N.; Parikh, N.; Chang, N.-L.; Kumar, A.; Bajaj, S.; Shamoon, F.; Bikkina, M. Arrhytmogenic Effects of Cocaethylene in Alcohol and Cocaine Users [E730-E30]. Available online: https://www.jacc.org/doi/epdf/10.1016/S0735-1097%2812%2960731-1 (accessed on 13 January 2024).
- Shastry, S.; Manoochehri, O.; Richardson, L.D.; Manini, A.F. Cocaethylene cardiotoxicity in emergency department patients with acute drug overdose. Acad. Emerg. Med. 2023, 30, 82–88. [Google Scholar] [CrossRef]
- Riley, E.D.; Vittinghoff, E.; Wu, A.H.; Coffin, P.O.; Hsue, P.Y.; Kazi, D.S.; Wade, A.; Braun, C.; Lynch, K.L. Impact of polysubstance use on high-sensitivity cardiac troponin I over time in homeless and unstably housed women. Drug Alcohol Depend. 2020, 217, 108252. [Google Scholar] [CrossRef]
- Wiener, S.E.; Sutijono, D.; Moon, C.H.; Subramanian, R.A.; Calaycay, J.; Rushbrook, J.I.; Zehtabchi, S. Patients with detectable cocaethylene are more likely to require intensive care unit admission after trauma. Am. J. Emerg. Med. 2010, 28, 1051–1055. [Google Scholar] [CrossRef]
- Vanek, V.W.; Dickey-White, H.I.; Signs, S.A.; Schechter, M.D.; Buss, T.; Kulics, A.T. Concurrent use of cocaine and alcohol by patients treated in the emergency department. Ann. Emerg. Med. 1996, 28, 508–514. [Google Scholar] [CrossRef]
- Blaho, K.; Logan, B.; Winbery, S.; Park, L.; Schwilke, E. Blood cocaine and metabolite concentrations, clinical findings. Am. J. Emerg. Med. 2000, 18, 593–598. [Google Scholar] [CrossRef]
- Zucoloto, A.D.; Eller, S.; de Oliveira, T.F.; Wagner, G.A.; Fruchtengarten, L.V.; de Oliveira, C.D.; Yonamine, M. Relationship between cocaine and cocaethylene blood concentration with the severity of clinical manifestations. Am. J. Emerg. Med. 2021, 50, 404–408. [Google Scholar] [CrossRef]
- Signs, S.A.; Dickey-White, H.I.; Vanek, V.W.; Perch, S.; Schechter, M.D.; Kulics, A.T. The formation of cocaethylene and clinical presentation of ED patients testing positive for the use of cocaine and ethanol. Am. J. Emerg. Med. 1996, 14, 665–670. [Google Scholar] [CrossRef]
- Randall, T. Cocaine, alcohol mix in body to form even longer lasting, more lethal drug. JAMA 1992, 267, 1043–1044. [Google Scholar] [CrossRef]
- Pilgrim, J.L.; Woodford, N.; Drummer, O.H. Cocaine in sudden and unexpected death: A review of 49 post-mortem cases. Forensic Sci. Int. 2013, 227, 52–59. [Google Scholar] [CrossRef]
- Molina, D.K.; Hargrove, V.M. Fatal cocaine interactions: A review of cocaine-related deaths in Bexar County, Texas. Am. J. Forensic Med. Pathol. 2011, 32, 71–77. [Google Scholar] [CrossRef]
- Office for National Statistics (ONS). Deaths Related to Drug Poisoning in England and Wales: 2022 Registrations. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2022registrations/pdf (accessed on 13 January 2024).
- Karch, S.; Stephens, B.; Tseng, A. Does ethanol enhance cocaine toxicity? J. Clin. Forensic Med. 1999, 6, 19–23. [Google Scholar] [CrossRef]
- Lucena, J.; Blanco, M.; Jurado, C.; Rico, A.; Salguero, M.; Vazquez, R.; Thiene, G.; Basso, C. Cocaine-related sudden death: A prospective investigation in south-west Spain. Eur. Heart J. 2010, 31, 318–329. [Google Scholar] [CrossRef]
- Merry, A.H.; Boer, J.M.; Schouten, L.J.; Feskens, E.J.; Verschuren, W.M.; Gorgels, A.P.; van den Brandt, P.A. Smoking, alcohol consumption, physical activity, and family history and the risks of acute myocardial infarction and unstable angina pectoris: A prospective cohort study. BMC Cardiovasc. Disord. 2011, 11, 13. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr.; Stampfer, M.J.; Glynn, R.J.; Grodstein, F.; Gaziano, J.M.; Manson, J.E.; Buring, J.E.; Hennekens, C.H. Moderate alcohol consumption and risk for angina pectoris or myocardial infarction in US male physicians. Ann. Intern. Med. 1997, 126, 372–375. [Google Scholar] [CrossRef]
- Ding, C.; O’Neill, D.; Bell, S.; Stamatakis, E.; Britton, A. Association of alcohol consumption with morbidity and mortality in patients with cardiovascular disease: Original data and meta-analysis of 48,423 men and women. BMC Med. 2021, 19, 167. [Google Scholar] [CrossRef]
- Stiltner, B.; Pietrzak, R.H.; Tylee, D.S.; Nunez, Y.Z.; Adhikari, K.; Kranzler, H.R.; Gelernter, J.; Polimanti, R. Polysubstance addiction patterns among 7,989 individuals with cocaine use disorder. iScience 2023, 26, 107336. [Google Scholar] [CrossRef]
- Vroegop, M.; Franssen, E.; Van der Voort, P.; Van den Berg, T.; Langeweg, R.; Kramers, C. The emergency care of cocaine intoxications. Neth. J. Med. 2009, 67, 122–126. [Google Scholar]
- Maceira, A.M.; Ripoll, C.; Cosin-Sales, J.; Igual, B.; Gavilan, M.; Salazar, J.; Belloch, V.; Pennell, D.J. Long term effects of cocaine on the heart assessed by cardiovascular magnetic resonance at 3T. J. Cardiovasc. Magn. Reson. 2014, 16, 26. [Google Scholar] [CrossRef]
- Egred, M.; Davis, G. Cocaine and the heart. Postgrad. Med. J. 2005, 81, 568–571. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef]
- Hollander, J.E.; Hoffman, R.S.; Gennis, P.; Fairweather, P.; DiSano, M.J.; Schumb, D.A.; Feldman, J.A.; Fish, S.S.; Dyer, S.; Wax, P. Prospective multicenter evaluation of cocaine-associated chest pain. Acad. Emerg. Med. 1994, 1, 330–339. [Google Scholar] [CrossRef]
- Afonso, L.; Mohammad, T.; Thatai, D. Crack whips the heart: A review of the cardiovascular toxicity of cocaine. Am. J. Cardiol. 2007, 100, 1040–1043. [Google Scholar] [CrossRef]
- Moliterno, D.J.; Willard, J.E.; Lange, R.A.; Negus, B.H.; Boehrer, J.D.; Glamann, D.B.; Landau, C.; Rossen, J.D.; Winniford, M.D.; Hillis, L.D. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N. Engl. J. Med. 1994, 330, 454–459. [Google Scholar] [CrossRef]
- Lange, R.A.; Hillis, L.D. Cardiovascular complications of cocaine use. N. Engl. J. Med. 2001, 345, 351–358. [Google Scholar] [CrossRef]
- Hollander, J.E.; Hoffman, R.S. Cocaine-induced myocardial infarction: An analysis and review of the literature. J. Emerg. Med. 1992, 10, 169–177. [Google Scholar] [CrossRef]
- Brookoff, D.; Rotondo, M.F.; Shaw, L.M.; Campbell, E.A.; Fields, L. Coacaethylene levels in patients who test positive for cocaine. Ann. Emerg. Med. 1996, 27, 316–320. [Google Scholar] [CrossRef]
- Bailey, D.N. Comprehensive review of cocaethylene and cocaine concentrations in patients. Am. J. Clin. Pathol. 1996, 106, 701–704. [Google Scholar] [CrossRef]

| Sample Characteristics | Sample Size | Main Findings | Reference |
|---|---|---|---|
| ED patients | 199 | CE-positive serum (cocaine–alcohol) compared with CE-negative serum (cocaine alone): higher rates of cardiac arrest (6.1% vs. 0.7%, p = 0.048) and hyperlactatemia (4.1 mM vs. 2.9 mM; p = 0.038) but lower rate of myocardial injury (mean initial troponin 0.01 ng/mL vs. 0.16 ng/mL; p = 0.02) | [70] |
| Homeless drug users | 245 | Serum levels of CE were associated with serum troponin levels, a sensitive marker of myocardial ischemia (adjusted effect: 1.12; 95% CI:1.02–1.22) | [71] |
| ED patients | 417 | CE-positivity associated with risk of requiring ICU assistance (OR = 5.9; 95% CI: 1.6–22) | [72] |
| ED patients | 212 | Drug screen: Cocaine–alcohol vs. cocaine alone: more often confusion (28% vs. 9.5%), higher ICU admission (48% vs. 31%), no significant difference in the rate of chest pain | [73] |
| ED patients | 3925 | No difference in chest pain between cocaine–alcohol use and cocaine-alone use (OR = 0.96; 95% CI: 0.64–1.45) | [23] |
| ED patients | 111 | No relation between serum levels of CE and cocaine metabolites and severity of intoxication, clinical symptoms, admission to ICU, or need for treatment | [74] |
| ED patients | 81 | No relation between the blood levels of cocaine CE with severity of stimulant intoxication | [75] |
| ED patients | 228 | No significant differences in cardiac and neurological complaints between cocaine and cocaine–alcohol intoxication | [76] |
| Sample Characteristics | Sample Size | Main Findings | Reference |
|---|---|---|---|
| Postmortem evidence | 49 | Cases of sudden and unexpected death between 2000 and 2011 in Australia: cocaine–alcohol in 17 of 49 cases (35%) | [78] |
| Postmortem evidence | 461 | Ethanol was detected in 194 of 461 cocaine-related deaths (42%) | [79] |
| Postmortem evidence | 857 | Ethanol was detected in 189 of 857 cocaine-related deaths (22%) in England/Wales in 2022 | [80] |
| Postmortem evidence | 72 | Ethanol was detected in 25 of 72 cocaine-related deaths (35%) | [81] |
| Postmortem evidence | Not reported | Lower cocaine blood level in patients who had also used alcohol (0.9 versus 2.8 mg/L; p = 0.06) | [13] |
| Postmortem evidence | Not reported | Cocaine–alcohol increased the risk of sudden death 18-fold | [77] |
| Postmortem evidence | 21 | Cocaine-related sudden deaths: cocaine–alcohol in 76% and cigarette smoking in 81% of cases | [82] |
| Postmortem evidence | Not reported | Cocaethylene was associated with an 18 to 25-fold higher risk of acute cardiac death compared to cases using cocaine alone | [46] |
| In-hospital mortality | 2,368,886 | Non-significantly lower in-hospital mortality rate for cocaine–alcohol than for cocaine alone: 1.07% vs. 1.34% (aOR = 0.99; p = 0.87) | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Amsterdam, J.; Gresnigt, F.; van den Brink, W. Cardiovascular Risks of Simultaneous Use of Alcohol and Cocaine—A Systematic Review. J. Clin. Med. 2024, 13, 1475. https://doi.org/10.3390/jcm13051475
van Amsterdam J, Gresnigt F, van den Brink W. Cardiovascular Risks of Simultaneous Use of Alcohol and Cocaine—A Systematic Review. Journal of Clinical Medicine. 2024; 13(5):1475. https://doi.org/10.3390/jcm13051475
Chicago/Turabian Stylevan Amsterdam, Jan, Femke Gresnigt, and Wim van den Brink. 2024. "Cardiovascular Risks of Simultaneous Use of Alcohol and Cocaine—A Systematic Review" Journal of Clinical Medicine 13, no. 5: 1475. https://doi.org/10.3390/jcm13051475
APA Stylevan Amsterdam, J., Gresnigt, F., & van den Brink, W. (2024). Cardiovascular Risks of Simultaneous Use of Alcohol and Cocaine—A Systematic Review. Journal of Clinical Medicine, 13(5), 1475. https://doi.org/10.3390/jcm13051475





