Recent Insights and Recommendations for Preventing Excessive Gestational Weight Gain
Abstract
1. Introduction
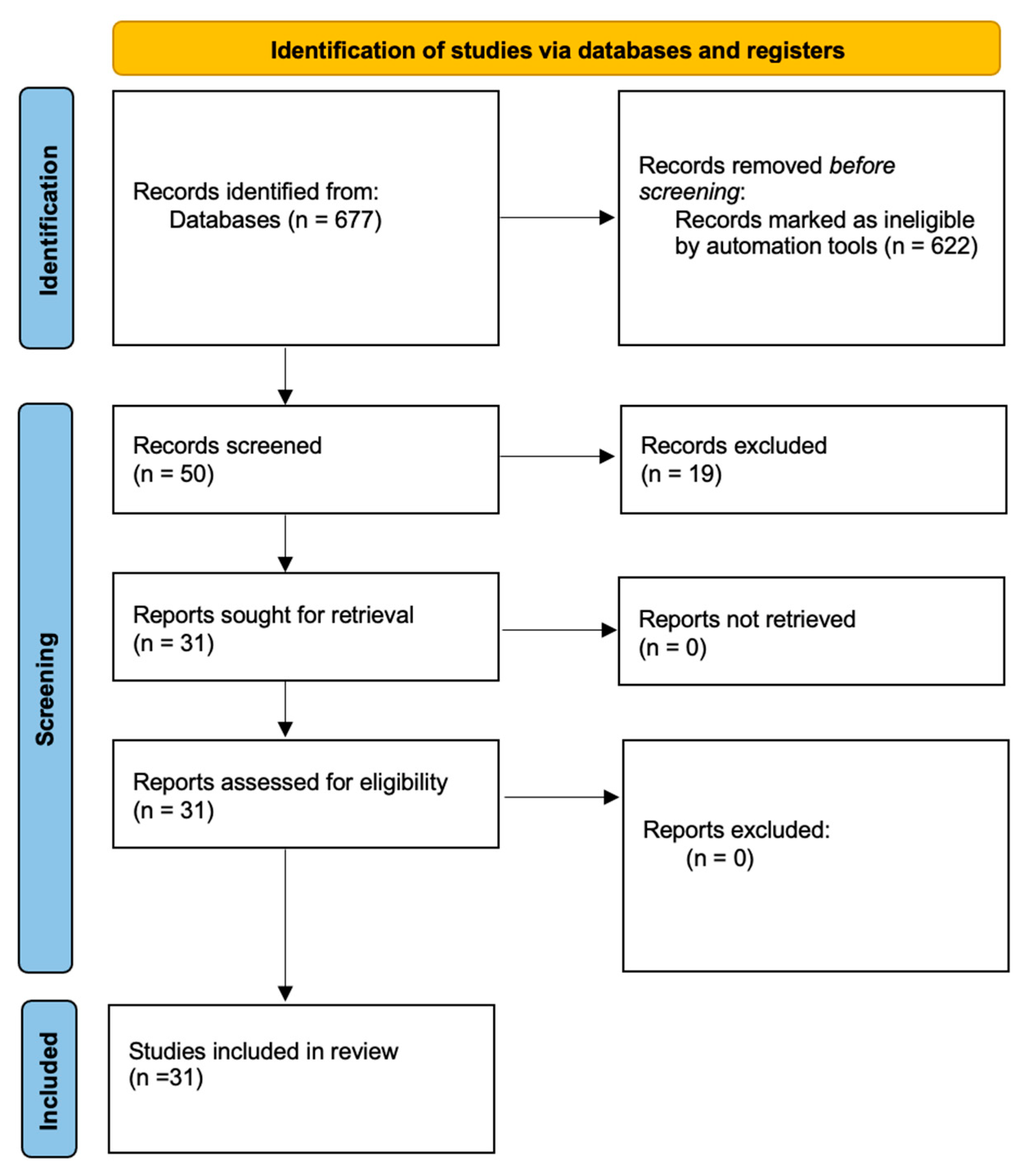
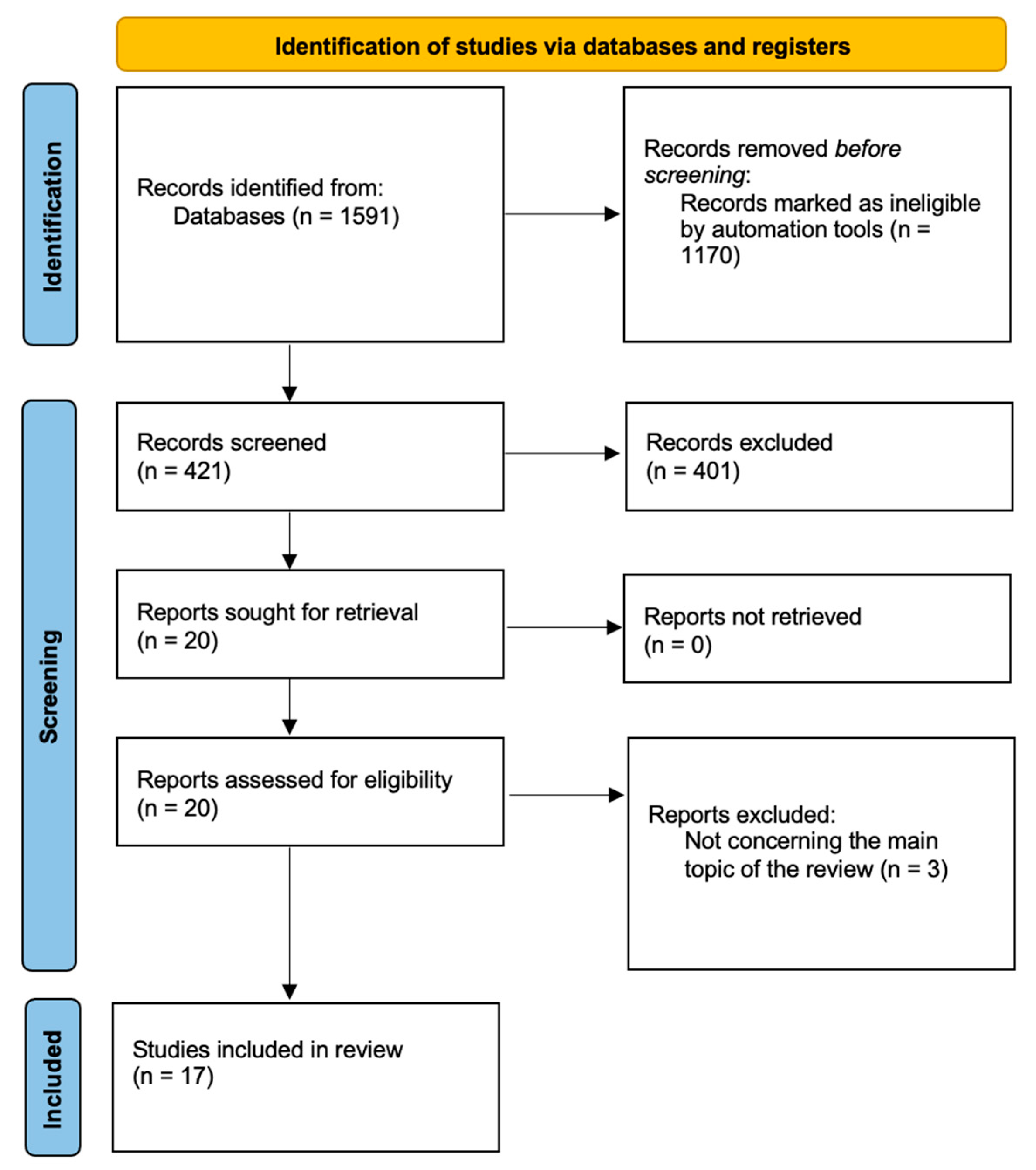
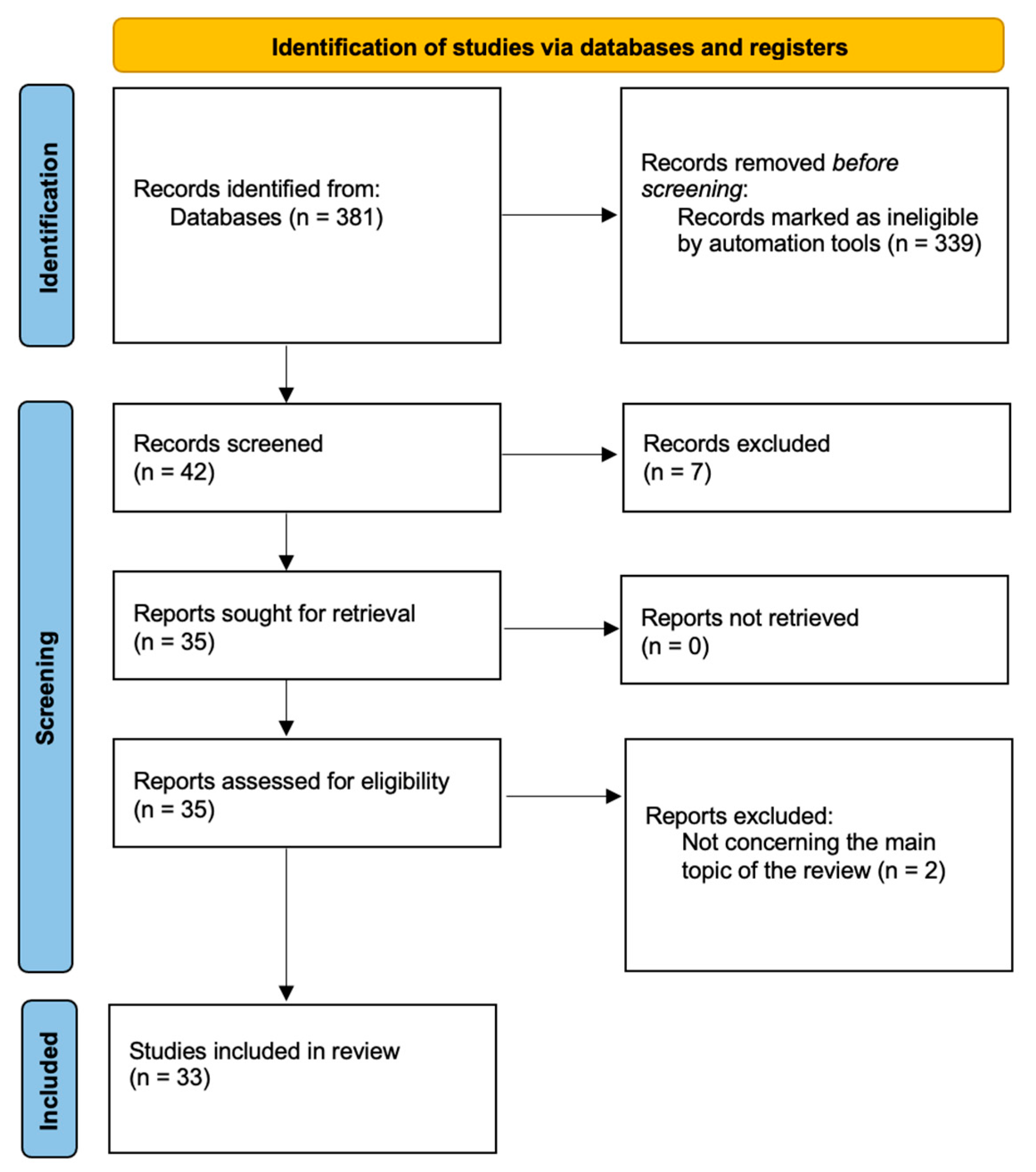
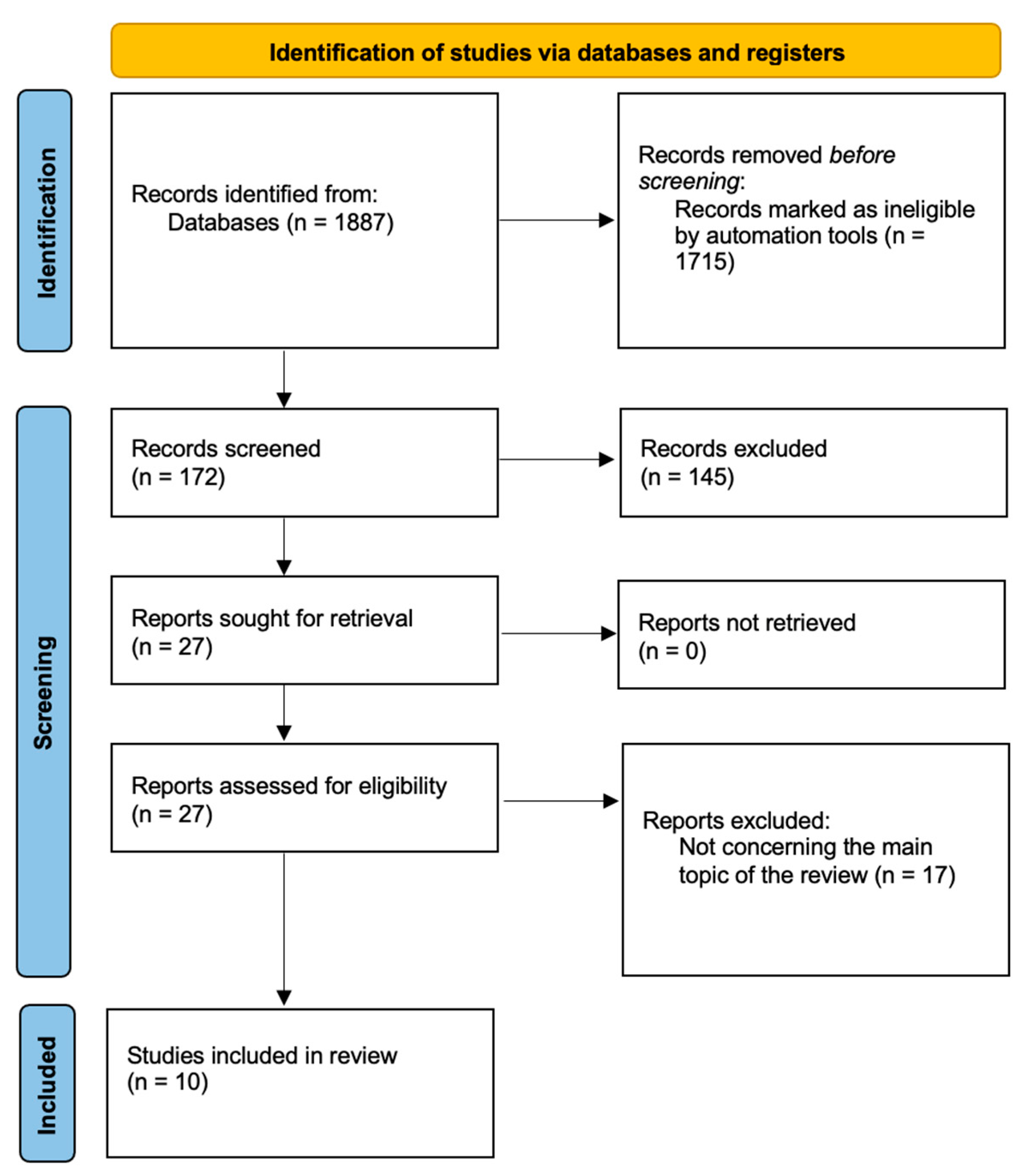
2. Material and Methods
3. Results
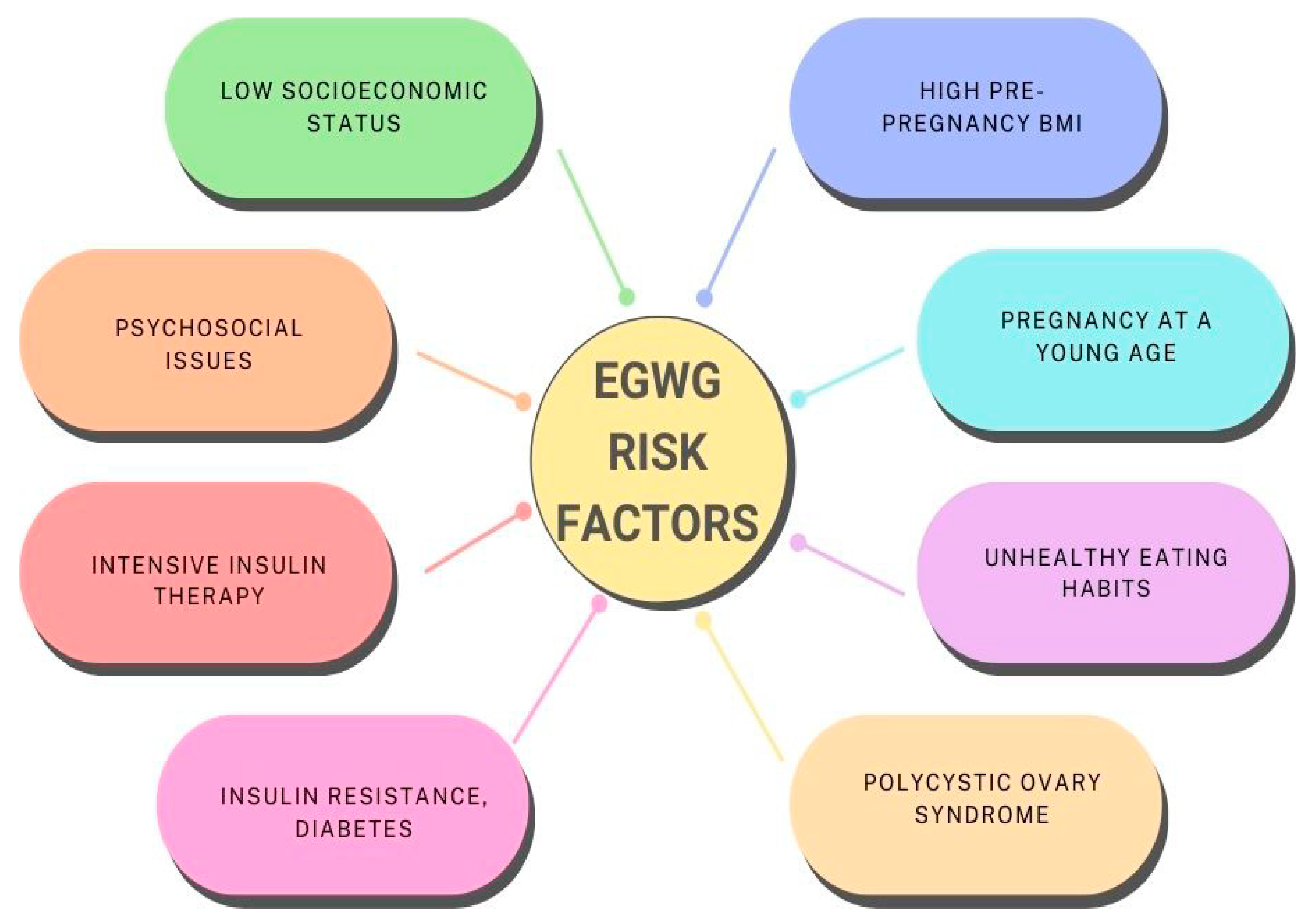
4. Pathophysiology and Risk Factors of EGWG
5. EGWG Prevention—Dietary and Training Recommendations
5.1. Physical Activity
5.2. Diet and Nutrition
5.3. Remote Methods of GWG Control
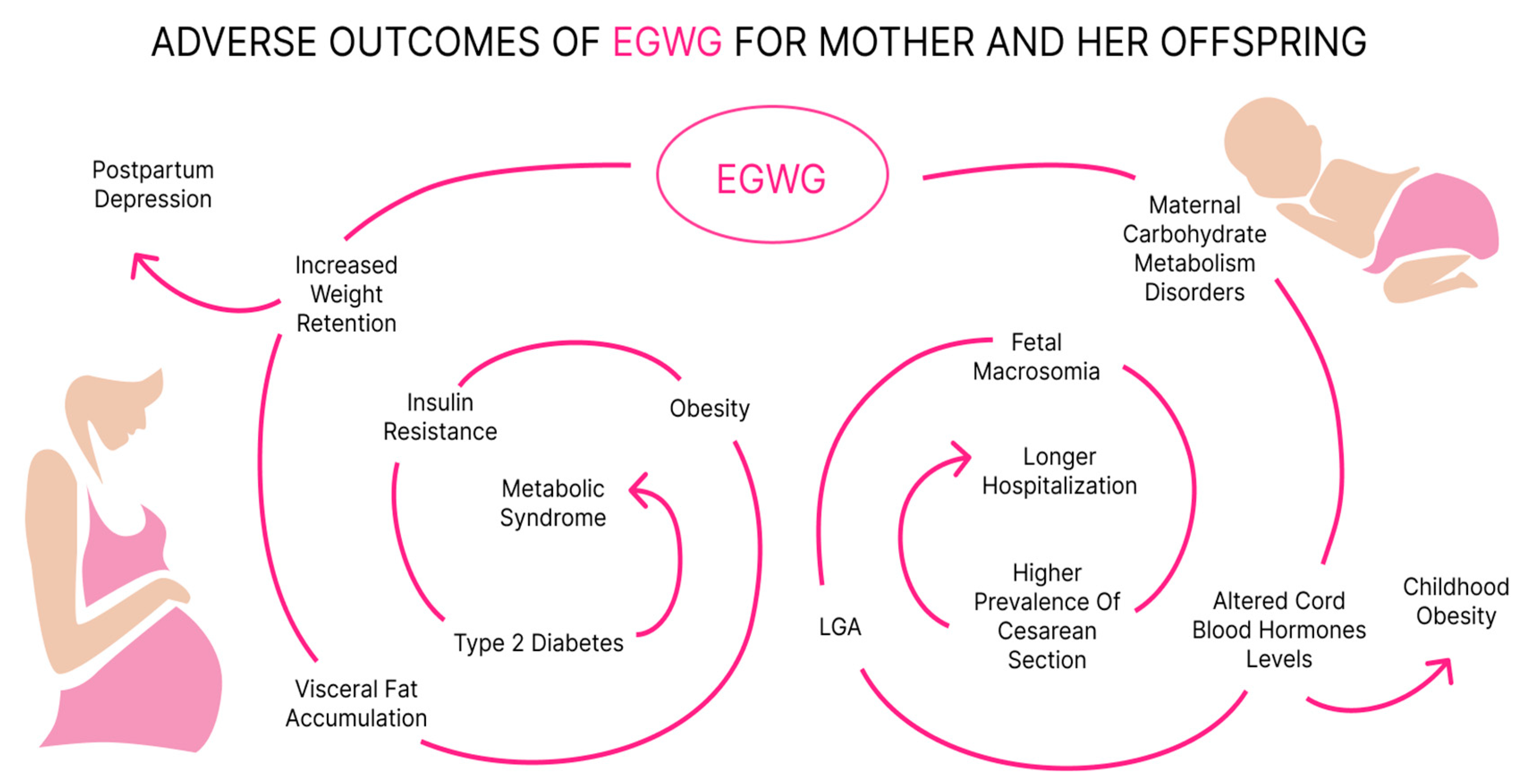
6. Long-Term Maternal Consequences
7. Fetal Programming for Metabolic Diseases in Children Born to Mothers with a History of EGWG
8. Comparison between the Effects of Pre-Pregnancy Overweight or Obesity and EGWG
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruager, M.R.; Hyde, M.J.; Modi, N. Maternal obesity and infant outcomes. Early Hum. Dev. 2010, 86, 715–722. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Siega-Riz, A.M.; Viswanathan, M.; Moos, M.K.; Deierlein, A.; Mumford, S.; Knaack, J.; Thieda, P.; Lux, L.J.; Lohr, K.N. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: Birthweight, fetal growth, and postpartum weight retention. Am. J. Obstet. Gynecol. 2009, 201, 339.e1–339.e14. [Google Scholar] [CrossRef]
- Nohr, E.A.; Vaeth, M.; Baker, J.L.; Sørensen, T.I.A.; Olsen, J.; Rasmussen, K.M. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am. J. Clin. Nutr. 2008, 87, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. (Eds.) Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Champion, M.L.; Harper, L.M. Gestational Weight Gain: Update on Outcomes and Interventions. Curr. Diab. Rep. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P. Childbearing and Obesity in Women: Weight Before, During, and After Pregnancy. Obstet. Gynecol. Clin. N. Am. 2009, 36, 317–332. [Google Scholar] [CrossRef]
- McDowell, M.; Cain, M.A.; Brumley, J. Excessive Gestational Weight Gain. J. Midwifery Womens Health 2019, 64, 46–54. [Google Scholar] [CrossRef]
- Restall, A.; Taylor, R.S.; Thompson, J.M.D.; Flower, D.; Dekker, G.A.; Kenny, L.C.; Poston, L.; McCowan, L.M.E. Risk Factors for Excessive Gestational Weight Gain in a Healthy, Nulliparous Cohort. J. Obes. 2014, 2014, 148391. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.; Dodson, W.C.; Kunselman, A.; Pauli, J.; Stone, A.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Alvero, R.; Casson, P.; et al. Gestational Weight Gain in Women with Polycystic Ovary Syndrome: A Controlled Study. J. Clin. Endocrinol. Metab. 2018, 103, 4315–4323. [Google Scholar] [CrossRef]
- Rastogi, S.; Rastogi, D. The Epidemiology and Mechanisms of Lifetime Cardiopulmonary Morbidities Associated with Pre-Pregnancy Obesity and Excessive Gestational Weight Gain. Front. Cardiovasc. Med. 2022, 9, 844905. [Google Scholar] [CrossRef]
- Perichart-Perera, O.; Muñoz-Manrique, C.; Reyes-López, A.; Tolentino-Dolores, M.; Espino y Sosa, S.; Ramírez-González, M.C. Metabolic Markers during Pregnancy and Their Association with Maternal and Newborn Weight Status. PLoS ONE 2017, 12, e0180874. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Solis Paredes, J.M.; Perichart Perera, O.; Montoya Estrada, A.; Reyes Muñoz, E.; Espino Y Sosa, S.; Ortega Castillo, V.; Medina Bastidas, D.; Tolentino Dolores, M.; Sanchez Martinez, M.; Nava Salazar, S.; et al. Gestational Weight Gain Influences the Adipokine-Oxidative Stress Association during Pregnancy. Obes. Facts 2021, 14, 604–612. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of Leptin and Adiponectin in Insulin Resistance. Clin. Chim. Acta Int. J. Clin. Chem. 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Resistance. Adv. Exp. Med. Biol. 2018, 1090, 145–163. [Google Scholar]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Godínez-Martínez, E.; Sánchez-Jiménez, B.; Montiel-Ojeda, D.; Tolentino, M. Serum Concentration of Leptin in Pregnant Adolescents Correlated with Gestational Weight Gain, Postpartum Weight Retention and Newborn Weight/Length. Nutrients 2017, 9, 1067. [Google Scholar] [CrossRef]
- Tessier, D.R.; Ferraro, Z.M.; Gruslin, A. Role of Leptin in Pregnancy: Consequences of Maternal Obesity. Placenta 2013, 34, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Darling, A.M.; McDonald, C.R.; Perumal, N.; Liu, E.; Wang, M.; Aboud, S.; Urassa, W.; Conroy, A.L.; Hayford, K.T.; et al. Plasma Concentrations of Leptin at Mid-Pregnancy Are Associated with Gestational Weight Gain among Pregnant Women in Tanzania: A Prospective Cohort Study. BMC Pregnancy Childbirth 2021, 21, 675. [Google Scholar] [CrossRef] [PubMed]
- Molin, J.; Vanky, E.; Løvvik, T.S.; Dehlin, E.; Bixo, M. Gestational Weight Gain, Appetite Regulating Hormones, and Metformin Treatment in Polycystic Ovary Syndrome: A Longitudinal, Placebo-controlled Study. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Franco-Sena, A.; Rebelo, F.; Pinto, T.; Farias, D.; Silveira, G.; Mendes, R.; Henriques, V.; Kac, G. The Effect of Leptin Concentrations and Other Maternal Characteristics on Gestational Weight Gain Is Different According to Pre-Gestational BMI: Results from a Prospective Cohort. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1804–1813. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Obstetric and Metabolic Implications of Excessive Gestational Weight Gain in Pregnancy. Obesity 2014, 22, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Domali, E.; Messinis, I.E. Leptin in Pregnancy. J. Matern. Fetal Neonatal Med. 2002, 12, 222–230. [Google Scholar] [CrossRef]
- Cade, W.T.; Mittendorfer, B.; Patterson, B.W.; Haire-Joshu, D.; Cahill, A.G.; Stein, R.I.; Schechtman, K.B.; Tinius, R.A.; Brown, K.; Klein, S. Effect of Excessive Gestational Weight Gain on Insulin Sensitivity and Insulin Kinetics in Women with Overweight/Obesity. Obesity 2022, 30, 2014–2022. [Google Scholar] [CrossRef]
- Kawakita, T.; Bowers, K.; McWhorter, K.; Rosen, B.; Adams, M.; Miodovnik, M.; Khoury, J.C. Characterizing Gestational Weight Gain According to Institute of Medicine Guidelines in Women with Type 1 Diabetes Mellitus: Association with Maternal and Perinatal Outcome. Am. J. Perinatol. 2016, 33, 1266–1272. [Google Scholar]
- Bashir, M.; Naem, E.; Taha, F.; Konje, J.C.; Abou-Samra, A.-B. Outcomes of Type 1 Diabetes Mellitus in Pregnancy; Effect of Excessive Gestational Weight Gain and Hyperglycaemia on Fetal Growth. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 84–88. [Google Scholar]
- Morrens, A.; Verhaeghe, J.; Vanhole, C.; Devlieger, R.; Mathieu, C.; Benhalima, K. Risk Factors for Large-for-Gestational Age Infants in Pregnant Women with Type 1 Diabetes. BMC Pregnancy Childbirth 2016, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, J.; García-Patterson, A.; Chico, A.; Mateu-Salat, M.; Amigó, J.; Adelantado, J.M.; Corcoy, R. Gestational Weight Gain in Women with Type 1 and Type 2 Diabetes Mellitus Is Related to Both General and Diabetes-Related Clinical Characteristics. Hormones 2023, 23, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hartley, E.; McPhie, S.; Skouteris, H.; Fuller-Tyszkiewicz, M.; Hill, B. Psychosocial Risk Factors for Excessive Gestational Weight Gain: A Systematic Review. Women Birth J. Aust. Coll. Midwives 2015, 28, e99–e109. [Google Scholar] [CrossRef]
- Samura, T.; Steer, J.; Michelis, L.D.; Carroll, L.; Holland, E.; Perkins, R. Factors Associated with Excessive Gestational Weight Gain: Review of Current Literature. Glob. Adv. Health Med. 2016, 5, 87–93. [Google Scholar] [CrossRef]
- Rugină, C.; Mărginean, C.O.; Meliţ, L.E.; Giga, D.V.; Modi, V.; Mărginean, C. Relationships between Excessive Gestational Weight Gain and Energy and Macronutrient Intake in Pregnant Women. J. Int. Med. Res. 2020, 48, 0300060520933808. [Google Scholar] [CrossRef]
- Chuang, C.H.; Stengel, M.R.; Hwang, S.W.; Velott, D.; Kjerulff, K.H.; Kraschnewski, J.L. Behaviours of Overweight and Obese Women during Pregnancy Who Achieve and Exceed Recommended Gestational Weight Gain. Obes. Res. Clin. Pract. 2014, 8, e577–e583. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S. Pre-Pregnancy Obesity, Excessive Gestational Weight Gain, and the Risk of Pregnancy-Induced Hypertension and Gestational Diabetes Mellitus. J. Clin. Med. 2020, 9, 1980. [Google Scholar] [CrossRef]
- Van Ha, A.V.; Zhao, Y.; Pham, N.M.; Nguyen, C.L.; Chu, T.K.; Binns, C.W.; Lee, A.H. Physical activity and sedentary behaviour during pregnancy are associated with gestational weight gain in Vietnamese women. Asia Pac. J. Clin. Nutr. 2020, 29, 136–143. [Google Scholar]
- Sun, J.J.; Chien, L.Y. Decreased Physical Activity during Pregnancy Is Associated with Excessive Gestational Weight Gain. Int. J. Environ. Res. Public Health 2021, 18, 12597. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, D.; Liu, X.; Liu, Y. Impact of exercise on maternal gestational weight gain: An updated meta-analysis of randomized controlled trials. Medicine 2019, 98, e16199. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.B.; Knudsen, S.D.; Alomairah, S.A.; Jessen, A.D.; Jensen, I.K.B.; Brændstrup, N.; Molsted, S.; Jensen, A.K.; Stallknecht, B.; Bendix, J.M.; et al. Effects of prenatal exercise on gestational weight gain, obstetric and neonatal outcomes: FitMum randomized controlled trial. BMC Pregnancy Childbirth 2023, 23, 214. [Google Scholar] [CrossRef] [PubMed]
- Global Health Observatory Data Repository. Prevalence of Overweight Among Adults, BMI ≥ 25, Age-Standardized. Estimates by Country. Available online: https://apps.who.int/gho/data/view.main.CTRY2430A?lang=en (accessed on 5 February 2024).
- Syed, H.; Slayman, T.; DuChene Thoma, K. ACOG Committee Opinion No. 804: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2021, 137, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Terrones, M.; Nagpal, T.S. Impact of exercise during pregnancy on gestational weight gain and birth weight: An overview. Braz. J. Phys. Ther. 2019, 23, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Saccone, G. Exercise in pregnancy! Am. J. Obstet. Gynecol. 2017, 216, 335–337. [Google Scholar] [CrossRef]
- Green, J.; Larkey, L.; Leiferman, J.A.; Buman, M.; Oh, C.; Huberty, J. Prenatal yoga and excessive gestational weight gain: A review of evidence and potential mechanisms. Complement. Ther. Clin. Pract. 2022, 46, 101551. [Google Scholar] [CrossRef]
- Barakat, R.; Lucia, A.; Ruiz, J.R. Resistance exercise training during pregnancy and newborn’s birth size: A randomised controlled trial. Int. J. Obes. 2009, 33, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Murtezani, A.; Pacarada, M.; Ibraimi, Z.; Nevzati, A.; Abazi, N. The impact of exercise during pregnancy on neonatal outcomes: A randomized controlled trial. J. Sports Med. Phys. Fit. 2014, 54, 802–808. [Google Scholar]
- Clapp, J.F., 3rd. The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Haug, L.S.; Sakhi, A.K.; Andrusaityte, S.; Basagaña, X.; Brantsaeter, A.L.; Casas, M.; Fernández-Barrés, S.; Grazuleviciene, R.; Knutsen, H.K.; et al. Diet as a source of exposure to environmental contaminants for pregnant women and children from six European countries. Environ. Health Perspect. 2019, 127, 107005. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-I.; Cardenas, A.; Rifas-Shiman, S.L.; Hivert, M.-F.; James-Todd, T.; Amarasiriwardena, C.; Wright, R.O.; Rahman, M.L.; Oken, E. Diet and erythrocyte metal concentrations in early pregnancy—Cross-sectional analysis in Project Viva. Am. J. Clin. Nutr. 2021, 114, 540–549. [Google Scholar] [CrossRef]
- Australian Living Evidence Collaboration. (2023 Version 1). Australian Pregnancy Care Guidelines. Available online: https://leappguidelines.org/ (accessed on 27 February 2024).
- Shao, B.; Mo, M.; Xin, X.; Jiang, W.; Wu, J.; Huang, M.; Wang, S.; Muyiduli, X.; Si, S.; Shen, Y.; et al. The interaction between prepregnancy BMI and gestational vitamin D deficiency on the risk of gestational diabetes mellitus subtypes with elevated fasting blood glucose. Clin. Nutr. 2020, 39, 2265–2273. [Google Scholar] [CrossRef]
- Rodrigues, C.Z.; Cardoso, M.A.; Maruyama, J.M.; Neves, P.A.R.; Qi, L.; Lourenço, B.H. Vitamin D insufficiency, excessive weight gain, and insulin resistance during pregnancy. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2121–2128. [Google Scholar] [CrossRef]
- Niemiec, T.; Karowicz-Bilińska, A.; Kotarski, J.; Kurowska-Mroczek, E.; Poręba, R.; Spaczyński, M. Stanowisko Zespołu Ekspertów Polskiego Towarzystwa Ginekologicznego dotyczącego spożycia wody pitnej przez kobiety w okresie rozrodczym, ciężarne oraz karmiące piersią. [The statement of Polish Gynaecologic Society experts concerning drinking water consumption in women in reproductive age, pregnancy and breast feeding]. Ginekol. Pol. 2009, 80, 538–547. [Google Scholar]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131, 213–253. [Google Scholar] [CrossRef]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W., Jr.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef]
- Saldiva, S.R.D.M.; De Arruda Neta, A.D.C.P.; Teixeira, J.A.; Peres, S.V.; Marchioni, D.M.L.; Carvalho, M.A.; Vieira, S.E.; Francisco, R.P.V. Dietary Pattern Influences Gestational Weight Gain: Results from the ProcriAr Cohort Study-São Paulo, Brazil. Nutrients 2022, 14, 4428. [Google Scholar] [CrossRef] [PubMed]
- Radwan, H.; Hashim, M.; Hasan, H.; Abbas, N.; Obaid, R.R.S.; Al Ghazal, H.; Naja, F. Adherence to the Mediterranean diet during pregnancy is associated with lower odds of excessive gestational weight gain and postpartum weight retention: Results of the Mother-Infant Study Cohort. Br. J. Nutr. 2022, 128, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Sandborg, J.; Söderström, E.; Henriksson, P.; Bendtsen, M.; Henström, M.; Leppänen, M.H.; Maddison, R.; Migueles, J.H.; Blomberg, M.; Löf, M. Effectiveness of a Smartphone App to Promote Healthy Weight Gain, Diet, and Physical Activity during Pregnancy (HealthyMoms): Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e26091. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A.; Hedderson, M.M.; Brown, S.D.; Ehrlich, S.F.; Tsai, A.-L.; Feng, J.; Galarce, M.; Marcovina, S.; Catalano, P.; Quesenberry, C.P. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): A randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Noever, K.; Schubert, J.; Reuschel, E.; Timmesfeld, N.; Arabin, B. Changes in Maternal Body Mass Index, Weight Gain and Outcome of Singleton Pregnancies from 2000 to 2015: A Population-based Retrospective Cohort Study in Hesse/Germany. Geburtshilfe Frauenheilkd. 2020, 80, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Klempel-Donchenko, M.; Redman, L.M. Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Semin. Perinatol. 2015, 39, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Nehring, I.; Schmoll, S.; Beyerlein, A.; Hauner, H.; von Kries, R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1225–1231. [Google Scholar] [CrossRef]
- Davis, E.M.; Stange, K.C.; Horwitz, R.I. Childbearing, stress and obesity disparities in women: A public health perspective. Matern. Child Health J. 2012, 16, 109–118. [Google Scholar] [CrossRef]
- Hutchins, F.; Abrams, B.; Brooks, M.; Colvin, A.; Moore Simas, T.; Rosal, M.; Sternfeld, B.; Crawford, S. The Effect of Gestational Weight Gain across Reproductive History on Maternal Body Mass Index in Midlife: The Study of Women’s Health arcoss the Nation. J. Womens Health 2020, 29, 148–157. [Google Scholar] [CrossRef]
- McClure, C.K.; Catov, J.M.; Ness, R.; Bodnar, L.M. Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. Am. J. Clin. Nutr. 2013, 98, 1218–1225. [Google Scholar] [CrossRef]
- Gur, E.B.; Genc, M.; Eskicioglu, F.; Kurtulmus, S.; Guclu, S. Ultrasonographic visceral fat thickness measurement may be a good scan test for prediction of gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2015, 28, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.M.; Tita, A.; Biggio, J.R. The Institute of Medicine Guidelines for Gestational Weight Gain after a Diagnosis of Gestational Diabetes and Pregnancy Outcomes. Am. J. Perinatol. 2014, 32, 239–246. [Google Scholar] [PubMed]
- Hutchins, F.; El Khoudary, S.R.; Catov, J.; Krafty, R.; Colvin, A.; Barinas-Mitchell, E.; Brooks, M.M. Excessive Gestational Weight Gain and Long-Term Maternal Cardiovascular Risk Profile: The Study of Women’s Health arcoss the Nation. J. Womens Health 2022, 31, 808–818. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Scifres, C.M.; Catov, J.M.; Simhan, H.N. The impact of maternal obesity and gestational weight gain on early and mid-pregnancy lipid profiles. Obesity 2014, 22, 932–938. [Google Scholar] [CrossRef]
- Tomedi, L.E.; Simhan, H.N.; Chang, C.C.; McTigue, K.M.; Bodnar, L.M. Gestational weight gain, early pregnancy maternal adiposity distribution, and maternal hyperglycemia. Matern. Child. Health J. 2014, 18, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Mannan, M.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M.; Callaway, L.K. Association between gestational weight gain and postpartum diabetes: Evidence from a community based large cohort study. PLoS ONE 2013, 8, e75679. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, S.; Yan, J. Gestational weight gain and risk of postpartum depression: A meta-analysis of observational studies. Psychiatry Res. 2022, 310, 114448. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.L.; Ertel, K.A.; Dole, N.; Chasan-Taber, L. The role of body image in prenatal and postpartum depression: A critical review of the literature. Arch. Womens Ment. Health 2015, 18, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Minschart, C.; Myngheer, N.; Maes, T.; De Block, C.; Van Pottelbergh, I.; Abrams, P.; Vinck, W.; Leuridan, L.; Driessens, S.; Mathieu, C.; et al. Weight retention and glucose intolerance in early postpartum after gestational diabetes. Eur. J. Endocrinol. 2023, 19, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, V.; Giliberti, L.; Giliberti, E.; Grassi, A.; Perin, V.; Parotto, M.; Soldera, G.; Straface, G. The role of gestational weight gain disorders in symptoms of maternal postpartum depression. Int. J. Gynaecol. Obstet. 2021, 153, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [PubMed]
- Öztürk, H.N.O.; Türker, P.F. Fetal Programming: Could Intrauterine Life Affect Health Status in Adulthood? Obstet. Gynecol. Sci. 2021, 64, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Guiomar de Almeida Brasiel, P.; Cristina Potente Dutra Luquetti, S. Metabolic Programming and Nutrition. In New Insights Into Metabolic Syndrome; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic Programming and Fetal Metabolic Programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef]
- Page, L.; Younge, N.; Freemark, M. Hormonal Determinants of Growth and Weight Gain in the Human Fetus and Preterm Infant. Nutrients 2023, 15, 4041. [Google Scholar] [CrossRef]
- Karakosta, P.; Georgiou, V.; Fthenou, E.; Papadopoulou, E.; Roumeliotaki, T.; Margioris, A.; Castanas, E.; Kampa, M.; Kogevinas, M.; Chatzi, L. Maternal Weight Status, Cord Blood Leptin, and Fetal Growth: A Prospective Mother-Child Cohort Study (Rhea Study). Paediatr. Perinat. Epidemiol. 2013, 27, 461–471. [Google Scholar] [CrossRef]
- Lemas, D.J.; Brinton, J.T.; Shapiro, A.L.; Glueck, D.H.; Friedman, J.E.; Dabelea, D. Associations of Maternal Weight Status Prior and during Pregnancy with Neonatal Cardiometabolic Markers at Birth: The Healthy Start Study. Int. J. Obes. 2015, 39, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.A.; Bornemann, R.; Koenig, W.; Reister, F.; Walter, V.; Fantuzzi, G.; Weyermann, M.; Brenner, H.; Genuneit, J.; Rothenbacher, D. Gestational Weight Gain and Fetal-Maternal Adiponectin, Leptin, and CRP: Results of Two Birth Cohort Studies. Sci. Rep. 2017, 7, 41847. [Google Scholar] [CrossRef] [PubMed]
- Patro-Małysza, J.; Trojnar, M.; Skórzyńska-Dziduszko, K.E.; Kimber-Trojnar, Ż.; Darmochwał-Kolarz, D.; Czuba, M.; Leszczyńska-Gorzelak, B. Leptin and Ghrelin in Excessive Gestational Weight Gain: Association Between Mothers and Offspring. Int. J. Mol. Sci. 2019, 20, 2398. [Google Scholar] [CrossRef] [PubMed]
- Rifas-Shiman, S.L.; Fleisch, A.; Hivert, M.F.; Mantzoros, C.; Gillman, M.W.; Oken, E. First and Second Trimester Gestational Weight Gains Are Most Strongly Associated with Cord Blood Levels of Hormones at Delivery Important for Glycemic Control and Somatic Growth. Metabolism 2017, 69, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, S.; Waffarn, F.; Ohashi, M.; Sumiyoshi, K.; Ikenoue, C.; Buss, C.; Gillen, D.L.; Simhan, H.N.; Entringer, S.; Wadhwa, P.D. Prospective Association of Fetal Liver Blood Flow at 30 Weeks Gestation with Newborn Adiposity. Am. J. Obstet. Gynecol. 2017, 217, 204.e1–204.e8. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Ebbing, C.; Rasmussen, S.; Qvigstad, E.; Kiserud, T.; Kessler, J. Pre-gestational Diabetes: Maternal Body Mass Index and Gestational Weight Gain Are Associated with Augmented Umbilical Venous Flow, Fetal Liver Perfusion, and Thus Birthweight. PLoS ONE 2021, 16, e0256171. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of Maternal Body Mass Index and Gestational Weight Gain on Pregnancy Complications: An Individual Participant Data Meta-analysis of European, North American and Australian Cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, S.; Gu, S.; Mou, Z.; Dong, L.; Luo, Z.; Zhang, J.; Hua, X. Gestational Weight Gain and Adverse Pregnancy Outcomes: A Prospective Cohort Study. BMJ Open 2020, 10, e038187. [Google Scholar] [CrossRef]
- Lin, L.H.; Lin, J.; Yan, J.Y. Interactive Affection of Pre-Pregnancy Overweight or Obesity, Excessive Gestational Weight Gain and Glucose Tolerance Test Characteristics on Adverse Pregnancy Outcomes Among Women with Gestational Diabetes Mellitus. Front. Endocrinol. 2022, 13, 942271. [Google Scholar] [CrossRef]
- Badon, S.E.; Dyer, A.R.; Josefson, J.L. HAPO Study Cooperative Research Group. Gestational Weight Gain and Neonatal Adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Study-North American Region. Obesity 2014, 22, 1731–1738. [Google Scholar] [CrossRef]
- Henriksson, P.; Eriksson, B.; Forsum, E.; Löf, M. Gestational Weight Gain According to Institute of Medicine Recommendations in Relation to Infant Size and Body Composition. Pediatr. Obes. 2015, 10, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.M.; Renault, K.M.; Nørgaard, K.; Nilas, L.; Jensen, J.E.; Hyldstrup, L.; Michaelsen, K.F.; Cortes, D.; Pryds, O. Newborn Regional Body Composition Is Influenced by Maternal Obesity, Gestational Weight Gain, and the Birthweight Standard Score. Acta Paediatr. 2014, 103, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lee, J.E. Large for Gestational Age and Obesity-Related Comorbidities. J. Obes. Metab. Syndr. 2021, 30, 124–131. [Google Scholar] [CrossRef]
- Ehrenthal, D.B.; Maiden, K.; Rao, A.; West, D.W.; Gidding, S.S.; Bartoshesky, L.; Carterette, B.; Ross, J.; Strobino, D. Independent Relation of Maternal Prenatal Factors to Early Childhood Obesity in the Offspring. Obstet. Gynecol. 2013, 121, 115–121. [Google Scholar] [CrossRef]
- Sridhar, S.B.; Darbinian, J.; Ehrlich, S.F.; Markman, M.A.; Gunderson, E.P.; Ferrara, A.; Hedderson, M.M. Maternal Gestational Weight Gain and Offspring Risk for Childhood Overweight or Obesity. Am. J. Obstet. Gynecol. 2014, 211, 259.e1–259.e8. [Google Scholar] [CrossRef]
- Tie, H.T.; Xia, Y.Y.; Zeng, Y.S.; Zhang, Y.; Dai, C.L.; Guo, J.J.; Zhao, Y. Risk of Childhood Overweight or Obesity Associated with Excessive Weight Gain during Pregnancy: A Meta-analysis. Arch. Gynecol. Obstet. 2014, 289, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bider-Canfield, Z.; Martinez, M.P.; Wang, X.; Yu, W.; Bautista, M.P.; Brookey, J.; Page, K.A.; Buchanan, T.A.; Xiang, A.H. Maternal Obesity, Gestational Diabetes, Breastfeeding and Childhood Overweight at Age 2 Years. Pediatr. Obes. 2017, 12, 171–178. [Google Scholar] [CrossRef]
- Mamun, A.A.; Mannan, M.; Doi, S.A. Gestational Weight Gain in Relation to Offspring Obesity Over the Life Course: A Systematic Review and Bias-Adjusted Meta-analysis. Obes. Rev. 2014, 15, 338–347. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal Body Mass Index, Gestational Weight Gain, and the Risk of Overweight and Obesity across Childhood: An Individual Participant Data Meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. Pre-Pregnancy Obesity vs. Other Risk Factors in Probability Models of Preeclampsia and Gestational Hypertension. Nutrients 2020, 12, 2681. [Google Scholar] [CrossRef]
- Shao, Y.; Qiu, J.; Huang, H.; Mao, B.; Dai, W.; He, X.; Cui, H.; Lin, X.; Lv, L.; Wang, D.; et al. Pre-pregnancy BMI, gestational weight gain and risk of preeclampsia: A birth cohort study in Lanzhou, China. BMC Pregnancy Childbirth 2017, 17, 400. [Google Scholar] [CrossRef]
- Swank, M.L.; Caughey, A.B.; Farinelli, C.K.; Main, E.K.; Melsop, K.A.; Gilbert, W.M.; Chung, J.H. The impact of change in pregnancy body mass index on the development of gestational hypertensive disorders. J. Perinatol. 2014, 34, 181–185. [Google Scholar] [CrossRef]
- Blondon, M.; Harrington, L.B.; Boehlen, F.; Robert-Ebadi, H.; Righini, M.; Smith, N.L. Pre-pregnancy BMI, delivery BMI, gestational weight gain and the risk of postpartum venous thrombosis. Thromb. Res. 2016, 145, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.Y.; Huang, K.; Yan, S.Q.; Zuo, A.Z.; Tao, R.W.; Cao, H.; Gu, C.L.; Tao, F.B. Pre-pregnancy BMI, gestational weight gain and breast-feeding: A cohort study in China. Public Health Nutr. 2017, 20, 1001–1008. [Google Scholar] [CrossRef]
- Castillo, H.; Santos, I.S.; Matijasevich, A. Maternal pre-pregnancy BMI, gestational weight gain and breastfeeding. Eur. J. Clin. Nutr. 2016, 70, 431–436. [Google Scholar] [CrossRef]
- Swank, M.L.; Caughey, A.B.; Farinelli, C.K.; Main, E.K.; Melsop, K.A.; Gilbert, W.M.; Chung, J.H. The impact of change in pregnancy body mass index on macrosomia. Obesity 2014, 22, 1997–2002. [Google Scholar] [CrossRef]
- Gujski, M.; Szukiewicz, D.; Chołuj, M.; Sawicki, W.; Bojar, I. Fetal and Placental Weight in Pre-Gestational Maternal Obesity (PGMO) vs. Excessive Gestational Weight Gain (EGWG): A Preliminary Approach to the Perinatal Outcomes in Diet-Controlled Gestational Diabetes Mellitus. J. Clin. Med. 2020, 9, 3530. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.J.; Ferguson, P.L.; Neelon, B.; Commodore, S.; Bloom, M.S.; Sciscione, A.C.; Grobman, W.A.; Kominiarek, M.A.; Newman, R.B.; Tita, A.T.; et al. The association between maternal pre-pregnancy BMI, gestational weight gain and child adiposity: A racial-ethnically diverse cohort of children. Pediatr. Obes. 2022, 17, e12911. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Lu, J.; Yan, S.; Tao, F.; Huang, K. Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study. Nutrients 2022, 14, 4613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, W.; Liu, C.; Liang, X.; Zhang, L.; Tian, Z.; Li, G. Associations between Pre-Pregnancy Body Mass Index and Gestational Weight Gain with Pregnancy Outcomes in Women with Polycystic Ovary Syndrome. Diabetol. Metab. Syndr. 2020, 12, 88. [Google Scholar] [CrossRef] [PubMed]
| Recommended Weight Gain during Pregnancy | ||
|---|---|---|
| Pre-pregnancy BMI | Recommended total weight gain during pregnancy (from the beginning of pregnancy to delivery) | Recommended average weekly weight gain in the II and III trimesters of pregnancy |
| Normal body weight (BMI 18.5–24.9 kg/m2) | 11.5–16.0 kg | 0.42 (0.35–0.50) kg/week |
| Overweight (BMI 25.0–29.9 kg/m2) | 7.0–11.5 kg | 0.28 (0.23–0.33) kg/week |
| Obesity (BMI > 30.0 kg/m2) | 5.0–9.0 kg | 0.22 (0.17–0.27) kg/week |
| Authors, Publication Years, References | Recommendations |
|---|---|
| Ha et al. (2020) Sun et al. (2021) Wang et al. (2019) ACOG Committee Opinion No. 650 (2015) [35,36,37,40] | Physically active pregnant women gain less weight than non-active ones, which can prevent EGWG. It is recommended to exercise at least 20–30 min a day on most days of the week. |
| Roland et al. (2023) [38] | Supervised exercise training 3 times a week and motivational counseling on physical activity are not superior to standard obstetric care. |
| Vargas-Terrones et al. (2019) Berghella et al. (2017) Green et al. (2022) [41,42,43] | Activities that are safe and beneficial include aerobic, strength, resistance, and stretching exercises. Walking, cycling, swimming, dancing, and hydrotherapy are recommended. Yoga exercise turned out to be ineffective in EGWG prevention. |
| Department of Health Clinical Practice Guidelines: Pregnancy Care (2020) [50] | Supplementation of 400–800 μg of folic acid, vitamin B1, and iodine is recommended. There is no need to supplement iron or calcium during healthy pregnancy. |
| Shao et al. (2020) Rodrigues et al. (2022) [51,52] | Vitamin D deficiency in pre-pregnancy overweight or obese women may significantly affect the risk of developing GDM. Its deficiency in third trimester might cause higher insulin concentration and development of insulin resistance. |
| Hanson et al. (2015) Marshall et al. (2022) Saldiva et al. (2022) Radwan et al. (2022) [54,55,56,57] | In the second and third trimester water consumption should be about 3 L a day. There is no need to limit salt intake; however, salt should be iodized. It is recommended to include fruits, vegetables, whole grain products, nuts, legumes, and fish in diet during pregnancy. The traditional Brazilian diet consisting of rice and beans, as well as consumption of vegetables and olive oil, are proven to be effective in preventing EGWG. |
| Sandborg et al. (2021) [58] | HealthyMom app usage for 6 months had no significant effect on EGWG; however, women with pre-pregnancy overweight or obesity gained less weight compared to controls. |
| Ferrara et al. (2020) [59] | Study group using telehealth lifestyle intervention had reduced total caloric intake and sedentary lifestyle. They also obtained better leptin and blood C-peptide levels compared to controls. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebrzydowska-Tatus, M.; Pełech, A.; Rekowska, A.K.; Satora, M.; Masiarz, A.; Kabała, Z.; Kimber-Trojnar, Ż.; Trojnar, M. Recent Insights and Recommendations for Preventing Excessive Gestational Weight Gain. J. Clin. Med. 2024, 13, 1461. https://doi.org/10.3390/jcm13051461
Niebrzydowska-Tatus M, Pełech A, Rekowska AK, Satora M, Masiarz A, Kabała Z, Kimber-Trojnar Ż, Trojnar M. Recent Insights and Recommendations for Preventing Excessive Gestational Weight Gain. Journal of Clinical Medicine. 2024; 13(5):1461. https://doi.org/10.3390/jcm13051461
Chicago/Turabian StyleNiebrzydowska-Tatus, Magdalena, Aleksandra Pełech, Anna K. Rekowska, Małgorzata Satora, Angelika Masiarz, Zuzanna Kabała, Żaneta Kimber-Trojnar, and Marcin Trojnar. 2024. "Recent Insights and Recommendations for Preventing Excessive Gestational Weight Gain" Journal of Clinical Medicine 13, no. 5: 1461. https://doi.org/10.3390/jcm13051461
APA StyleNiebrzydowska-Tatus, M., Pełech, A., Rekowska, A. K., Satora, M., Masiarz, A., Kabała, Z., Kimber-Trojnar, Ż., & Trojnar, M. (2024). Recent Insights and Recommendations for Preventing Excessive Gestational Weight Gain. Journal of Clinical Medicine, 13(5), 1461. https://doi.org/10.3390/jcm13051461






