Correspondence between Expected, Perceived, and Measured Effects of BoNT-A Treatment in Calf Muscles among Children and Adolescents with Cerebral Palsy: A Mixed Methods Study
Abstract
:1. Introduction
2. Materials and Methods
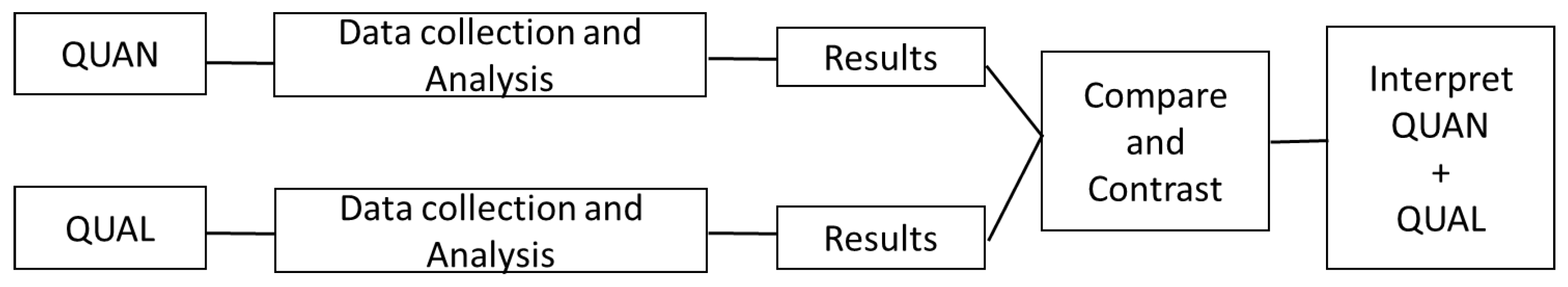
2.1. Design
2.2. Participants and Recruitment
2.3. Ethical Considerations
2.4. Data Collection
2.4.1. Qualitative Data—Interviews
2.4.2. Quantitative Data—Measured Outcomes
2.5. Data Analysis
2.5.1. Qualitative Analysis
2.5.2. Quantitative Analysis
3. Results
3.1. Participants Characteristics
3.2. Qualitative Results
3.2.1. Expectations to Treatment
Theme 1a: Parents’ Expectations
- Category 1: Approaching normality
“Perhaps she’ll avoid being bullied. It’s easy to target those who are perceived to have obvious weaknesses or visible functional impairment. Her mother is always afraid of this. We also hope she can keep up in sports for longer. It’s not enjoyable when everyone is much better than her at the ages of 12, 13, and 14, but this may not happen”. (parent 9)
“The physiotherapist is very concerned that he must learn to ride a bike for the social aspect, so he can be with friends. Therefore, the focus is on enabling him to participate and be part of the group, to be one of the others”. (parent 12)
- Category 2: Saving energy
“It’s typical that she can fall asleep when she gets home from school. And that she has to opt out in a way… She has good friends in the neighborhood, whom she has to exclude herself from for periods of time”. (parent 13)
Theme 1b: Childrens’ Expectations
- Category 1: Just doing it
- Category 2: Pros and cons
Interpretation of Parent’s and Children’s Expectations
3.2.2. Perceived Effect of Treatment
Theme 2a: Parents’ Perceptions
- Category 1: Less stiff and stumbling
- Category 2: More energy
“Before the treatment we somehow couldn’t make appointments because she was so tired in the afternoons. She had to relax….She has so much energy now…That means a lot, because we can take part in activities, we do not have to sit home with her. We also know that she’ll be fine in the kindergarten the day after”. (parent 3).
Theme 2b: Children’s Perceptions
- Category 1: They say
- Category 2: Can do more
Interpretation of Parent’s and Children’s Perceptions
3.3. Quantitative Results
3.4. Comparing and Contrasting Qualitative and Quantitative Results
4. Discussion
4.1. Measured and Perceived Treatment Effect
4.2. The Impact of Expectations to Perceived Treatment Effects
4.3. Diverse Perspectives of Parents and Children
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Koman, L.A.; Smith, B.P.; Shilt, J.S. Cerebral palsy. Lancet 2004, 363, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Horber, V.; Fares, A.; Platt, M.J.; Arnaud, C.; Krägeloh-Mann, I.; Sellier, E. Severity of Cerebral Palsy-The Impact of Associated Impairments. Neuropediatrics 2020, 51, 120–128. [Google Scholar] [CrossRef]
- Brændvik, S.M.; Goihl, T.; Braaten, R.S.; Vereijken, B. The Effect of Increased Gait Speed on Asymmetry and Variability in Children With Cerebral Palsy. Front. Neurol. 2019, 10, 1399. [Google Scholar] [CrossRef]
- Saether, R.; Helbostad, J.L.; Adde, L.; Braendvik, D.; Lydersen, S.; Vik, T. The relationship between trunk control in sitting and during gait in children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 2014, 20, 12628. [Google Scholar] [CrossRef] [PubMed]
- Nardon, M.; Ruzzante, F.; O’donnell, L.; Adami, A.; Dayanidhi, S.; Bertucco, M. Energetics of walking in individuals with cerebral palsy and typical development, across severity and age: A systematic review and meta-analysis. Gait Posture 2021, 90, 388–407. [Google Scholar] [CrossRef]
- Brunton, L.K.; Rice, C.L. Fatigue in cerebral palsy: A critical review. Dev. Neurorehabil. 2012, 15, 54–62. [Google Scholar] [CrossRef]
- Vila-Nova, F.; Sá, C.D.S.C.d.; Oliveira, R.; Cordovil, R. Differences in Leisure Physical Activity Participation in Children with Typical Development and Cerebral Palsy. Dev. Neurorehabil. 2021, 24, 180–186. [Google Scholar] [CrossRef]
- Maher, C.A.; Toohey, M.; Ferguson, M. Physical activity predicts quality of life and happiness in children and adolescents with cerebral palsy. Disabil. Rehabil. 2016, 38, 865–869. [Google Scholar] [CrossRef]
- Ohata, K.; Tsuboyama, T.; Haruta, T.; Ichihashi, N.; Kato, T.; Nakamura, T. Relation between muscle thickness, spasticity, and activity limitations in children and adolescents with cerebral palsy. Dev. Med. Child Neurol. 2008, 50, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Schranz, C.; Kruse, A.; Tilp, M.; Svehlik, M. Is there a relationship between muscle-tendon properties and a variety of functional tasks in children with spastic cerebral palsy? Gait Posture 2021, 85, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.L.; Quinlivan, J.; Owen, B.F.; Shaffrey, M.; Abel, M.F. Spasticity versus strength in cerebral palsy: Relationships among involuntary resistance, voluntary torque, and motor function. Eur. J. Neurol. 2001, 8 (Suppl. S5), 40–49. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Mc Namara, M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Ross Raftemo, A.E.; Mahendran, A.; Hollung, S.J.; Jahnsen, R.B.; Lydersen, S.; Vik, T. Use of botulinum toxin A in children with cerebral palsy. Tidsskr Nor Laegeforen 2019, 139. [Google Scholar]
- Hägglund, G.; Hollung, S.J.; Ahonen, M.; Andersen, G.L.; Eggertsdóttir, G.; Gaston, M.S.; Jahnsen, R.; Jeglinsky-Kankainen, I.; Nordbye-Nielsen, K.; Tresoldi, I.; et al. Treatment of spasticity in children and adolescents with cerebral palsy in Northern Europe: A CP-North registry study. BMC Neurol. 2021, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Sätilä, H. Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections? Toxins 2020, 12, 440. [Google Scholar] [CrossRef]
- Blumetti, F.C.; Belloti, J.C.; Tamaoki, M.J.; A Pinto, J. Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy. Cochrane Database Syst. Rev. 2019, 10, Cd001408. [Google Scholar] [CrossRef]
- Sutherland, D.H.; Cooper, L.; Daniel, D. The role of the ankle plantar flexors in normal walking. J. Bone Joint Surg. Am. 1980, 62, 354–363. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F.; Tan, A.K.; Matthews, D.J. Botulinum toxin a treatment in children with cerebral palsy: Its effects on walking and energy expenditure. Am. J. Phys. Med. Rehabil. 2012, 91, 53–64. [Google Scholar] [CrossRef]
- Bjornson, K.; Hays, R.; Graubert, C.; Price, R.; Won, F.; McLaughlin, J.F.; Cohen, M. Botulinum toxin for spasticity in children with cerebral palsy: A comprehensive evaluation. Pediatrics 2007, 120, 49–58. [Google Scholar] [CrossRef]
- Ubhi, T.; Bhakta, B.B.; Ives, H.L.; Allgar, V.; Roussounis, S.H. Randomised double blind placebo controlled trial of the effect of botulinum toxin on walking in cerebral palsy. Arch. Dis. Child. 2000, 83, 481–487. [Google Scholar] [CrossRef]
- Hafliðadóttir, S.H.; Juhl, C.B.; Nielsen, S.M.; Henriksen, M.; Harris, I.A.; Bliddal, H.; Christensen, R. Placebo response and effect in randomized clinical trials: Meta-research with focus on contextual effects. Trials 2021, 22, 493. [Google Scholar] [CrossRef] [PubMed]
- Lorin, K.; Forsberg, A. Treatment with botulinum toxin in children with cerebral palsy: A qualitative study of parents’ experiences. Child. Care Health Dev. 2016, 42, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Karadag-Saygi, E.; Kenis-Coskun, Ö.; Unalan, P.C.; Evkaya-Acar, A.; Giray, E.; Akgulle, A.H. Pros and cons of botulinum toxin injection therapy in cerebral palsy: A qualitative study exploring caregivers’ perspective. Child. Care Health Dev. 2022, 48, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Di Rezze, B.; Mesterman, R.; Rosenbaum, P.; Gorter, J.W. Effects of Botulinum Toxin Treatment in Nonambulatory Children and Adolescents With Cerebral Palsy: Understanding Parents’ Perspectives. J. Child. Neurol. 2018, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, K.; Gaffney, M.; Taylor, N.; Gibson, B.E. The civil rights of disabled children in physiotherapy practices. Physiother. Theory Pract. 2023, 39, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, M.V.; Cole, D.C.; Frank, J.W. Does how you do depend on how you think you’ll do? A systematic review of the evidence for a relation between patients’ recovery expectations and health outcomes. Cmaj 2001, 165, 174–179. [Google Scholar]
- El-Haddad, C.; Hegazi, I.; Hu, W. Understanding Patient Expectations of Health Care: A Qualitative Study. J. Patient Exp. 2020, 7, 1724–1731. [Google Scholar] [CrossRef]
- Brændvik, S.M.; Roeleveld, K.; Andersen, G.L.; Raftemo, A.E.R.; Ramstad, K.; Majkic-Tajsic, J.; Lamvik, T.; Lund, B.; Follestad, T.; Vik, T. The WE-Study: Does botulinum toxin A make walking easier in children with cerebral palsy?: Study protocol for a randomized controlled trial. Trials 2017, 18, 58. [Google Scholar] [CrossRef]
- Størvold, G.V.; Jahnsen, R.B.; Evensen, K.A.I.; Bratberg, G.H. Is more frequent physical therapy associated with increased gross motor improvement in children with cerebral palsy? A national prospective cohort study. Disabil. Rehabil. 2020, 42, 1430–1438. [Google Scholar] [CrossRef]
- Edmonds, W.A.; Kennedy, D.T. An Applied Refereance Guide to Research Designs; SAGE Publications: Thousand Oaks, CA, USA, 2013; p. 206. [Google Scholar]
- Brehm, M.A.; Becher, J.; Harlaar, J. Reproducibility evaluation of gross and net walking efficiency in children with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Himuro, N.; Abe, H.; Nishibu, H.; Seino, T.; Mori, M. Easy-to-use clinical measures of walking ability in children and adolescents with cerebral palsy: A systematic review. Disabil. Rehabil. 2017, 39, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Selvaag, A.M.; Ruperto, N.; Asplin, L.; Rygg, M.; Landgraf, J.M.; Forre, Ø.; Flatø, B.; Paediatric Rheumatology International Trials Organisation. The Norwegian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin. Exp. Rheumatol. 2001, 19 (Suppl. S23), S116–S120. [Google Scholar]
- Sakzewski, L.; Boyd, R.; Ziviani, J. Clinimetric properties of participation measures for 5- to 13-year-old children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2007, 49, 232–240. [Google Scholar] [CrossRef]
- Law, M.; Baptiste, S.; Carswell, A.; McColl, M.A.; Polatajko, H.J.; Pollock, N. Canadian Occupational Performance Measure, 5th ed.; CAOT Publications: Ottawa, ON, Canada, 2014. [Google Scholar]
- Malterud, K. Systematic text condensation: A strategy for qualitative analysis. Scand J. Public Health 2012, 40, 795–805. [Google Scholar] [CrossRef]
- Thoresen, L.; Rugseth, G.; Bonde, H. Fenomenologi i Helsefaglig Forskning; Universitetsforlaget: Oslo, Norway, 2020. [Google Scholar]
- Merleau-Ponty, M. Phenomenology of Perception, 1st ed.; Taylor & Francis Group: Oxfordshire, UK, 2012. [Google Scholar]
- Leder, D. The Abcent Body; University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- Wågby Gräfe, A.; Englander, M. Listening to the Social World of the Child: A Phenomenological Proposal. Barn Forsk. Om Barn Og Barndom I Nord. 2022, 40, 40–54. [Google Scholar] [CrossRef]
- Kaya Keles, C.S.; Ates, F. Botulinum Toxin Intervention in Cerebral Palsy-Induced Spasticity Management: Projected and Contradictory Effects on Skeletal Muscles. Toxins 2022, 14, 772. [Google Scholar] [CrossRef]
- Kahraman, A.; Seyhan, K.; Değer, Ü.; Kutlutürk, S.; Mutlu, A. Should botulinum toxin A injections be repeated in children with cerebral palsy? A systematic review. Dev. Med. Child Neurol. 2016, 58, 910–917. [Google Scholar] [CrossRef]
- Matsuda, M.; Tomita, K.; Yozu, A.; Nakayama, T.; Nakayama, J.; Ohguro, H.; Iwasaki, N. Effect of botulinum toxin type A treatment in children with cerebral palsy: Sequential physical changes for 3 months after the injection. Brain Dev. 2018, 40, 452–457. [Google Scholar] [CrossRef]
- Giuliani, C.A. Dorsal rhizotomy for children with cerebral palsy: Support for concepts of motor control. Phys. Ther. 1991, 71, 248–259. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; Valente, E.M.; Crivellini, M.; Ialongo, T.; Albertini, G. Computerized gait analysis of botulinum toxin treatment in children with cerebral palsy. Disabil.Rehabil. 2007, 29, 659–664. [Google Scholar] [CrossRef]
- Narayanan, U. Walking outcomes in cerebral palsy: What is the GOAL? Dev. Med. Child Neurol. 2024, 66, 9–10. [Google Scholar] [CrossRef]
- Thomason, P.; Tan, A.; Donnan, A.; Rodda, J.; Graham, H.K.; Narayanan, U. The Gait Outcomes Assessment List (GOAL): Validation of a new assessment of gait function for children with cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Coronado, R.A.; Seitz, A.L.; Pelote, E.; Archer, K.R.; Jain, N.B. Are Psychosocial Factors Associated With Patient-reported Outcome Measures in Patients With Rotator Cuff Tears? A Systematic Review. Clin. Orthop. Relat. Res. 2018, 476, 810–829. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Herren, D.B.; Vlieland, T.P.V.; Simmen, B.R.; Angst, F.; Goldhahn, J. Determinants of patient satisfaction after orthopedic interventions to the hand: A review of the literature. J. Hand Ther. 2011, 24, 303–312.e10, quiz 312. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.J.M.; Joseph, L.; Canby, G.; Paungmali, A.; Sitilertpisan, P.; Pirunsan, U. Are patient expectations associated with treatment outcomes in individuals with chronic low back pain? A systematic review of randomised controlled trials. Int. J. Clin. Pract. 2020, 74, e13680. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, B.; Clark, C.; Killingback, C.; Newell, D. Impact of contextual factors on patient outcomes following conservative low back pain treatment: Systematic review. Chiropr. Man. Ther. 2022, 30, 20. [Google Scholar] [CrossRef] [PubMed]
- Kwame, A.; Petrucka, P.M. A literature-based study of patient-centered care and communication in nurse-patient interactions: Barriers, facilitators, and the way forward. BMC Nurs. 2021, 20, 158. [Google Scholar] [CrossRef]
- Desloovere, K.; Molenaers, G.; De Cat, J.; Pauwels, P.; Van Campenhout, A.; Ortibus, E.; Fabry, G.; De Cock, P. Motor function following multilevel botulinum toxin type A treatment in children with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 56–61. [Google Scholar] [CrossRef]
- Tedroff, K.; Löwing, K.; Haglund-Akerlind, Y.; Gutierrez-Farewik, E.; Forssberg, H. Botulinum toxin A treatment in toddlers with cerebral palsy. Acta Paediatr. 2010, 99, 1156–1162. [Google Scholar] [CrossRef]
- Tedroff, K.; Granath, F.; Forssberg, H.; Haglund-Akerlind, Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dunn, N.; Shields, N.; Taylor, N.F.; Dodd, K.J. Comparing the self concept of children with cerebral palsy to the perceptions of their parents. Disabil. Rehabil. 2009, 31, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Shikako-Thomas, K.; Lach, L.; Majnemer, A.; Nimigon, J.; Cameron, K.; Shevell, M. Quality of life from the perspective of adolescents with cerebral palsy: “I just think I’m a normal kid, I just happen to have a disability”. Qual. Life Res. 2009, 18, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, V.; Sauve, K.; Klassen, A.F.; O’Donnell, M.; Cieza, A.; Mâsse, L.C. ‘He does not see himself as being different’: The perspectives of children and caregivers on relevant areas of functioning in cerebral palsy. Dev. Med. Child Neurol. 2014, 56, 853–861. [Google Scholar] [CrossRef]
| Id | Age | Gender | GMFCS | UL/BL | Parent | BoNT-A Earlier |
|---|---|---|---|---|---|---|
| 1 | 6 | m | I | BL | mo | no |
| 2 | 9 | m | I | UL | fa | yes |
| 3 | 4 | f | I | UL | mo | no |
| 4 | 4 | f | I | UL | fa | no |
| 5 | 11 | f | I | UL | mo | no |
| 6 | 8 | f | I | UL | mo | yes |
| 7 | 9 | f | I | UL | fa | no |
| 8 | 10 | m | I | UL | mo | yes |
| 9 | 7 | f | I | UL | fa | no |
| 10 | 11 | m | I | UL | fa | yes |
| 11 | 15 | m | II | UL | fa | no |
| 12 | 8 | m | I | UL | mo | yes |
| 13 | 12 | f | II | UL | mo | yes |
| 14 | 10 | m | I | UL | mo | yes |
| 15 | 7 | f | I | UL | mo | yes |
| 16 | 14 | m | I | UL | fa | yes |
| 17 | 8 | m | I | UL | mo | yes |
| 18 | 10 | m | I | UL | fa | yes |
| 19 | 9 | f | I | UL | mo | yes |
| 20 | 10 | f | II | UL | mo | yes |
| Theme | Category |
|---|---|
| 1a. Expectations to treatment parents | 1. Approaching normality |
| 2. Saving energy | |
| 1b. Expectations to treatment children | 1. Just doing it |
| 2. Pros and cons | |
| 2a. Perceived effect parents | 1. Less stiff and stumbling |
| 2. More energy | |
| 2b. Perceived effect children | 1. They say |
| 2. Can do more |
| ID | Group | Age | Gender | GMFCS | UL/ BL | EC Baseline | EC Diff 1 | WC Baseline | WC Diff 1 | COPM per Baseline | COPM per Diff 1 | Pain int Baseline | Pain int Diff 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P | 6 | m | I | BL | 5.85 | −1.14 | 97 | −2 | 3.0 | 1.0 | 4 | 0 |
| 2 | P | 9 | m | I | UL | 3.38 | 0.96 | 100 | 0 | 5.3 | 1.0 | 2 | missing |
| 4 | P | 4 | f | I | UL | missing | missing | 61 | −6 | 4.7 | 2.0 | 4 | −2 |
| 5 | P | 11 | f | I | UL | 3.89 | −0.15 | 115 | 5 | 3.7 | 1.6 | 1 | 0 |
| 6 | P | 8 | f | I | UL | 6.43 | −0.53 | 125 | −42 | 6.3 | 3.0 | 1 | missing |
| 8 | P | 10 | m | I | UL | 5.64 | −0.14 | 111 | −17 | 2.0 | 2.5 | 1 | 3 |
| 9 | P | 7 | f | I | UL | 5.90 | −0.08 | 95 | 0 | 5.3 | 0.0 | 3 | −2 |
| 10 | P | 11 | m | I | UL | 4.42 | −0.85 | 93 | missing | 5.3 | 0.6 | 3 | −2 |
| 17 | P | 8 | m | I | UL | 4.67 | −0.66 | 114 | −2 | 5.0 | 2.0 | 1 | 0 |
| 18 | P | 10 | m | I | UL | 4.76 | −0.18 | 99 | −2 | 7.0 | −1.0 | 1 | 2 |
| 19 | P | 9 | f | I | UL | 4.94 | 1.54 | 113 | 0 | 3.5 | 1.5 | 3 | 0 |
| 3 | B | 4 | f | I | UL | missing | missing | 89 | 5 | 5.0 | 0.0 | 2 | 2 |
| 7 | B | 9 | f | I | UL | 5.84 | 0.25 | 82 | −12 | 2.0 | 0.7 | 1 | 0 |
| 11 | B | 16 | m | II | UL | 4.56 | 0.29 | 84 | 7 | 5.0 | 3.0 | 2 | 1 |
| 12 | B | 8 | m | I | UL | 5.05 | missing | 105 | missing | 5.7 | missing | 5 | missing |
| 13 | B | 12 | f | II | UL | 4.36 | 0.14 | 113 | −13 | 5.5 | −0.5 | 1 | 0 |
| 14 | B | 10 | m | I | UL | 4.62 | 0.72 | 119 | 0 | 6.3 | −5.0 | 1 | missing |
| 15 | B | 7 | f | I | UL | 6.41 | −1.58 | 77 | 18 | 3.0 | 1.0 | 5 | missing |
| 16 | B | 14 | m | I | UL | 3.06 | 0.10 | 114 | −7 | 3.5 | −1.0 | 2 | 1 |
| 20 | B | 10 | f | II | UL | 5.93 | 0.08 | 97 | −3 | 7.0 | −1.0 | 2 | 0 |
| ID | Measured Positive Effect | Measured Negative Effect | Perceived Effect Parents | Perceived Effect Children | Expectations Met Parents | Expectations Met Children | |
|---|---|---|---|---|---|---|---|
| Placebo group (P) | 1 | EC (−1.14) | yes | unsure | energy+ | ||
| 2 | EC (0.96) | yes | yes | normal * | energy+ | ||
| 4 | COPM (2), Pain int (−2) | WC (−6) | no | too young | |||
| 5 | no | no | |||||
| 6 | EC (−0.53), COPM (3) | WC (−42) | no | no | |||
| 8 | COPM (2.5) | WC (−17), Pain int (3) | no | no | |||
| 9 | Pain int (−2) | no | unsure | ||||
| 10 | EC (−0.85). Pain int (−2) | no | no | ||||
| 17 | EC (−0.66), COPM (2) | no | no | ||||
| 18 | Pain int (2) | no | no | ||||
| 19 | EC (1.54) | yes | no | ||||
| BoNT-A group (B) | 3 | Pain int (2) | yes | too young | energy+ | ||
| 7 | WC (−12) | yes | unsure | energy+ | |||
| 11 | WC/(7), COPM (3) | no P1 | no P1 | ||||
| 12 * | no P1 | no P1 | |||||
| 13 | WC (−13), COPM (−5) | yes | no | energy+ | |||
| 14 | EC (0.72) | no | unsure | ||||
| 15 | EC (−1.58), WC (18) | yes | no P1 | pain- | |||
| 16 | WC (−7) | no | no | ||||
| 20 | yes | yes | stifness- | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sæther, R.; Elvrum, A.-K.G.; Brændvik, S.M. Correspondence between Expected, Perceived, and Measured Effects of BoNT-A Treatment in Calf Muscles among Children and Adolescents with Cerebral Palsy: A Mixed Methods Study. J. Clin. Med. 2024, 13, 1453. https://doi.org/10.3390/jcm13051453
Sæther R, Elvrum A-KG, Brændvik SM. Correspondence between Expected, Perceived, and Measured Effects of BoNT-A Treatment in Calf Muscles among Children and Adolescents with Cerebral Palsy: A Mixed Methods Study. Journal of Clinical Medicine. 2024; 13(5):1453. https://doi.org/10.3390/jcm13051453
Chicago/Turabian StyleSæther, Rannei, Ann-Kristin Gunnes Elvrum, and Siri Merete Brændvik. 2024. "Correspondence between Expected, Perceived, and Measured Effects of BoNT-A Treatment in Calf Muscles among Children and Adolescents with Cerebral Palsy: A Mixed Methods Study" Journal of Clinical Medicine 13, no. 5: 1453. https://doi.org/10.3390/jcm13051453







