Celiac Disease-Related Enamel Defects: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Processing
2.2. Inclusion Criteria
2.3. Quality Assessment
3. Results
Quality Assessment and Risk of Bias
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Control patient |
| CC | Case control |
| CD | Celiac disease |
| CG | Control group |
| DC | Dental caries |
| DED | Dental enamel defects |
| DMFT | Decayed, missing, and filled teeth |
| DFS | Decayed, filled tooth surfaces |
| DO | Dental opacity |
| EAPD | European Academy of Pediatric Dentistry |
| ECOHIS | Early Childhood Oral Health Impact Scale |
| GFD | Gluten-free diet |
| MIH | Molar incisor hypomineralization |
| Ob S | Observational study |
| OE | Oral examination |
| OHRQoL | Oral health related to quality of life |
| PEB | Post-eruptive breakdown |
| PS | Preventive study |
| RAS | Recurrent aphthous stomatitis |
| SED | Specific enamel diseases |
| (TTG) IgA | Tissue transglutaminase antibodies |
References
- Ahmed, A.; Singh, A.; Kajal, S.; Chauhan, A.; Rajput, M.S.; Banyal, V.; Ahuja, V.; Makharia, G.K. Dental Enamel Defects and Oral Cavity Manifestations in Asian Patients with Celiac Disease. Indian J. Gastroenterol. 2021, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Beth, S.A.; Jansen, M.A.E.; Elfrink, M.E.C.; Kiefte-de Jong, J.C.; Wolvius, E.B.; Jaddoe, V.W.V.; van Zelm, M.C.; Moll, H.A. Generation R Birth Cohort Study Shows That Specific Enamel Defects Were Not Associated with Elevated Serum Transglutaminase Type 2 Antibodies. Acta Paediatr. 2016, 105, e485–e491. [Google Scholar] [CrossRef] [PubMed]
- Aine, L.; Mäki, M.; Collin, P.; Keyriläinen, O. Dental Enamel Defects in Celiac Disease. J. Oral. Pathol. Med. 1990, 19, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Villanacci, V. Coeliac Disease. J. Clin. Pathol. 2005, 58, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Emami, M.H.; Karimi, S.; Kouhestani, S.; Hashemi, M.; Taheri, H. Diagnostic Accuracy of IgA Anti-Tissue Transglutaminase in Patients Suspected of Having Coeliac Disease in Iran. J. Gastrointestin Liver Dis. 2008, 17, 141–146. [Google Scholar] [PubMed]
- Karlin, S.; Karlin, E.; Meiller, T.; Bashirelahi, N. Dental and Oral Considerations in Pediatric Celiac Disease. J. Dent. Child. 2016, 83, 67–70. [Google Scholar]
- Laurikka, P.; Nurminen, S.; Kivelä, L.; Kurppa, K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018, 10, 1015. [Google Scholar] [CrossRef]
- Lopes, C.M.I.; Cavalcanti, M.C.; Alves E Luna, A.C.; Marques, K.M.G.; Rodrigues, M.J.; DE Menezes, V.A. Enamel Defects and Tooth Eruption Disturbances in Children with Sickle Cell Anemia. Braz. Oral. Res. 2018, 32, e87. [Google Scholar] [CrossRef]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian. J. Pediatr. 2018, 85, 202–206. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Villabruna, B.; Inchingolo, A.M.; Dipalma, G. Severe Anisocoria after Oral Surgery under General Anesthesia. Int. J. Med. Sci. 2010, 7, 314–318. [Google Scholar] [CrossRef]
- Rapone, B.; Inchingolo, A.D.; Trasarti, S.; Ferrara, E.; Qorri, E.; Mancini, A.; Montemurro, N.; Scarano, A.; Inchingolo, A.M.; Dipalma, G.; et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. J. Clin. Med. 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Auricchio, R.; Discepolo, V.; Troncone, R. Extra-Intestinal Manifestations of Coeliac Disease in Children: Clinical Features and Mechanisms. Front. Pediatr. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Nieri, M.; Tofani, E.; Defraia, E.; Giuntini, V.; Franchi, L. Enamel Defects and Aphthous Stomatitis in Celiac and Healthy Subjects: Systematic Review and Meta-Analysis of Controlled Studies. J. Dent. 2017, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nota, A.; Abati, S.; Bosco, F.; Rota, I.; Polizzi, E.; Tecco, S. General Health, Systemic Diseases and Oral Status in Adult Patients with Coeliac Disease. Nutrients 2020, 12, 3836. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.P.; Kirkham, E.N.; John, R.; Staines, K.; Basude, D. Coeliac Disease in Children—An Update for General Dental Practitioners. Br. Dent. J. 2016, 220, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Popp, A.; Mäki, M. Gluten-Induced Extra-Intestinal Manifestations in Potential Celiac Disease-Celiac Trait. Nutrients 2019, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Tokuc, M.; Aydin, M.N.; Civan, H.A.; Polat, E.; Dogan, G.; Altuntas, C.; Bayrak, N.A.; Beser, O.F. Advancing Dentistry: Fractal Assessment of Bone Health in Pediatric Patients with Celiac Disease Using Dental Images. Quintessence Int. 2023, 54, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Wright-Hughes, A.; Alderson, S.L.; Ow, P.-L.; Ridd, M.J.; Foy, R.; Bianco, G.; Bishop, F.L.; Chaddock, M.; Cook, H.; et al. Amitriptyline at Low-Dose and Titrated for Irritable Bowel Syndrome as Second-Line Treatment in Primary Care (ATLANTIS): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 402, 1773–1785. [Google Scholar] [CrossRef]
- Naud, S.; Ibrahim, A.; Valles, C.; Maatouk, M.; Bittar, F.; Alou, M.T.; Raoult, D. Candidate Phyla Radiation, an Underappreciated Division of the Human Microbiome, and Its Impact on Health and Disease. Clin. Microbiol. Rev. 2022, 35, e00140-21. [Google Scholar] [CrossRef]
- Kondapalli, A.V.; Kamanda-Kosseh, M.; Williams, J.M.; Shiau, S.; Bucovsky, M.; Colon, I.; Shane, E.; Cohen, A. Clinical Characteristics of Pregnancy and Lactation Associated Osteoporosis: An Online Survey Study. Osteoporos. Int. 2023, 34, 1477–1489. [Google Scholar] [CrossRef]
- Al-Zahrani, M.S.; Alhassani, A.A.; Zawawi, K.H. Clinical Manifestations of Gastrointestinal Diseases in the Oral Cavity. Saudi Dent. J. 2021, 33, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Turska-Szybka, A.; Dąbrowska, E.; Głogowska, K.; Janczewska, A.; Olczak-Kowalczyk, D.; Piekoszewska-Ziętek, P. Coeliac Disease and Its Implications on the Oral Health of Children: A Systematic Review. J. Paediatr. Child. Health 2023, 59, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Fernandes, G.S.; Sarmanova, A.; Valdes, A.M.; Walsh, D.A.; Coupland, C.; Doherty, M.; Zhang, W. Comorbidities and Use of Analgesics in People with Knee Pain: A Study in the Nottingham Knee Pain and Health in the Community (KPIC) Cohort. Rheumatol. Adv. Pract. 2022, 6, rkac049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Lin, I.-C.; Chen, P.-C.; Lee, C.-C.; Meng, M. Crystal Structure of a Burkholderia Peptidase and Modification of the Substrate-Binding Site for Enhanced Hydrolytic Activity toward Gluten-Derived pro-Immunogenic Peptides. Int. J. Biol. Macromol. 2022, 222, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Marzban, H.; Goleij, P.; Sahebkar, A.; Morshedi, K.; Rezaei, S.; Mahjoubin-Tehran, M.; Tarrahimofrad, H.; Hamblin, M.R.; Mirzaei, H. Effects of Therapeutic Probiotics on Modulation of microRNAs. Cell Commun. Signal. 2021, 19, 4. [Google Scholar] [CrossRef]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the Effects of a Standardized Polyphenol Mixture Extracted from Poplar-Type Propolis on Healthy and Diseased Human Gut Microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef]
- Guarnieri, K.M.; Saba, N.K.; Schwartz, J.T.; Devonshire, A.L.; Bufford, J.; Casale, T.B.; Rothenberg, M.E.; Andorf, S. Food Allergy Characteristics Associated With Coexisting Eosinophilic Esophagitis in FARE Registry Participants. J. Allergy Clin. Immunol. Pract. 2023, 11, 1509–1521.e6. [Google Scholar] [CrossRef]
- Despot, R.; Zitko, V.; Kadic, A.J. Gastrointestinal Manifestation of Food Allergy. Lijec. Vjesn. 2022, 144, 99–103. [Google Scholar] [CrossRef]
- Bak, J.C.G.; Serné, E.H.; de Valk, H.W.; Valk, N.K.; Kramer, M.H.H.; Nieuwdorp, M.; Verheugt, C.L. Gender Gaps in Type 1 Diabetes Care. Acta Diabetol. 2023, 60, 425–434. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Lebwohl, B.; Song, M.; Sun, Q.; Green, P.H.R.; Giovannucci, E.L.; Willett, W.C.; Chan, A.T. Gluten Intake and Risk of Digestive System Cancers in 3 Large Prospective Cohort Studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1986–1996.e11. [Google Scholar] [CrossRef]
- Rothburn, N.; Fairchild, R.M.; Morgan, M.Z. Gluten-Free Foods: A “health Halo” Too Far for Oral Health? Br. Dent. J. 2022. [Google Scholar] [CrossRef] [PubMed]
- Moisidis-Tesch, C.M.; Shulman, L.P. Iron Deficiency in Women’s Health: New Insights into Diagnosis and Treatment. Adv. Ther. 2022, 39, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Kanikowska, A.; Janisz, S.; Mańkowska-Wierzbicka, D.; Gabryel, M.; Dobrowolska, A.; Eder, P. Management of Adult Patients with Gastrointestinal Symptoms from Food Hypersensitivity—Narrative Review. J. Clin. Med. 2022, 11, 7326. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, L.; Xiao, B.; Zhang, B.; Zuo, Z.; Ji, P.; Zheng, J.; Li, X.; Zhao, F. Maternal and Neonatal Viromes Indicate the Risk of Offspring’s Gastrointestinal Tract Exposure to Pathogenic Viruses of Vaginal Origin during Delivery. mLife 2022, 1, 303–310. [Google Scholar] [CrossRef]
- Ariño-Pérez, I.; Martínez-Domínguez, S.J.; Alfaro Almajano, E.; Carrera-Lasfuentes, P.; Lanas, Á. Mistakes in the Diagnosis and Treatment of Helicobacter Pylori Infection in Daily Clinical Practice. Helicobacter 2023, 28, e12957. [Google Scholar] [CrossRef] [PubMed]
- Bugălă, N.M.; Carsote, M.; Stoica, L.E.; Albulescu, D.M.; Ţuculină, M.J.; Preda, S.A.; Boicea, A.-R.; Alexandru, D.O. New Approach to Addison Disease: Oral Manifestations Due to Endocrine Dysfunction and Comorbidity Burden. Diagnostics 2022, 12, 2080. [Google Scholar] [CrossRef] [PubMed]
- Kriström, B.; Ankarberg-Lindgren, C.; Barrenäs, M.-L.; Nilsson, K.O.; Albertsson-Wikland, K. Normalization of Puberty and Adult Height in Girls with Turner Syndrome: Results of the Swedish Growth Hormone Trials Initiating Transition into Adulthood. Front. Endocrinol. 2023, 14, 1197897. [Google Scholar] [CrossRef]
- Bulut, M.; Tokuc, M.; Aydin, M.; Ayyildiz Civan, H.; Polat, E.; Dogan, G.; Altuntas, C.; Bayrak, N.; Beser, O. Nutrition and Oral Health in Children with Recently and Previously Diagnosed Celiac Disease. Clin. Oral. Investig. 2023, 27, 3579–3588. [Google Scholar] [CrossRef]
- Banyai, D.; Vegh, D.; Vegh, A.; Ujpal, M.; Payer, M.; Biczo, Z.; Triebl, Z.; Mukaddam, K.; Herber, V.; Jakse, N.; et al. Oral Health Status of Children Living with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 545. [Google Scholar] [CrossRef]
- Coelho, M.; Bernardo, M.; Mendes, S. Oral Health-Related Quality of Life in Celiac Portuguese Children: A Cross-Sectional Study. Eur. Arch. Paediatr. Dent. 2023, 24, 759–767. [Google Scholar] [CrossRef]
- El-Housseiny, A.A.; Alsadat, F.A.; Alamoudi, N.M.; Felemban, O.M.; Mosli, R.H.; Saadah, O.I. Oral Health-Related Quality of Life in Children with Celiac Disease. Qual. Life Res. 2022, 31, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Nastro, F.; Di Vico, F.; Fabbrocini, G.; Annunziata, M.C.; Genco, L. Oral Isotretinoin for Acne: A Complete Overview. Expert. Opin. Drug Saf. 2022, 21, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, U.K. Oral Prevotella Species and Their Connection to Events of Clinical Relevance in Gastrointestinal and Respiratory Tracts. Front. Microbiol. 2022, 12, 798763. [Google Scholar] [CrossRef] [PubMed]
- Pukhalskaya, T.; Smoller, B.R.; Becker, M.; Maly, A.; Zadik, Y.; Elad, S. Oral White Lesion in Patients Post-Hematopoietic Stem Cell Transplantation: A Case Series Demonstrating the Diagnostic Dilemma. Support. Care Cancer 2021, 29, 7999–8007. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lundemann, A.-K.J.; Reibel, J.; Pedersen, A.M.L. Salivary Gland Involvement and Oral Health in Patients with Coeliac Disease. Eur. J. Oral. Sci. 2022, 130, e12861. [Google Scholar] [CrossRef] [PubMed]
- Fiot, E.; Alauze, B.; Donadille, B.; Samara-Boustani, D.; Houang, M.; De Filippo, G.; Bachelot, A.; Delcour, C.; Beyler, C.; Bois, E.; et al. Turner Syndrome: French National Diagnosis and Care Protocol (NDCP; National Diagnosis and Care Protocol). Orphanet J. Rare Dis. 2022, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Sóñora, C.; Arbildi, P.; Rodríguez-Camejo, C.; Beovide, V.; Marco, A.; Hernández, A. Enamel Organ Proteins as Targets for Antibodies in Celiac Disease: Implications for Oral Health. Eur. J. Oral. Sci. 2016, 124, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Trotta, L.; Biagi, F.; Bianchi, P.I.; Marchese, A.; Vattiato, C.; Balduzzi, D.; Collesano, V.; Corazza, G.R. Dental Enamel Defects in Adult Coeliac Disease: Prevalence and Correlation with Symptoms and Age at Diagnosis. Eur. J. Intern. Med. 2013, 24, 832–834. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; Mancini, A.; et al. COVID-19 Infection in Children and Infants: Current Status on Therapies and Vaccines. Children 2022, 9, 249. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral Infection by Staphylococcus Aureus in Patients Affected by White Sponge Nevus: A Description of Two Cases Occurred in the Same Family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Sammartino, G.; Charrier, J.-B. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Human Cell Cultures: Growth Factor Release and Contradictory Results. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endodontology 2010, 110, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-Syndromic Multiple Supernumerary Teeth in a Family Unit with a Normal Karyotype: Case Report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Di Blasio, M.; Ronsivalle, V.; Cicciù, M. Children Oral Health and Parents Education Status: A Cross Sectional Study. BMC Oral. Health 2023, 23, 787. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.K.V.; Madithati, P.; Narapureddy, B.R.; Ravula, S.R.; Vaddamanu, S.K.; Alhamoudi, F.H.; Minervini, G.; Chaturvedi, S. Perception about Health Applications (Apps) in Smartphones towards Telemedicine during COVID-19: A Cross-Sectional Study. J. Pers. Med. 2022, 12, 1920. [Google Scholar] [CrossRef]
- Rathi, S.; Chaturvedi, S.; Abdullah, S.; Rajput, G.; Alqahtani, N.M.; Chaturvedi, M.; Gurumurthy, V.; Saini, R.; Bavabeedu, S.S.; Minervini, G. Clinical Trial to Assess Physiology and Activity of Masticatory Muscles of Complete Denture Wearer Following Vitamin D Intervention. Medicina 2023, 59, 410. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Post-traumatic Stress, Prevalence of Temporomandibular Disorders in War Veterans: Systematic Review with Meta-analysis. J. Oral. Rehabil. 2023, 50, 1101–1109. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Almeida, L.E.; Ronsivalle, V.; Cicciù, M. Prevalence of Temporomandibular Disorders (TMD) in Obesity Patients: A Systematic Review and Meta-analysis. J. Oral. Rehabil. 2023, 50, 1544–1553. [Google Scholar] [CrossRef]
- van Gils, T.; Brand, H.S.; de Boer, N.K.H.; Mulder, C.J.J.; Bouma, G. Gastrointestinal Diseases and Their Oro-Dental Manifestations: Part 3: Coeliac Disease. Br. Dent. J. 2017, 222, 126–129. [Google Scholar] [CrossRef]
- Ceratti, C.; Maspero, C.; Consonni, D.; Caprioglio, A.; Connelly, S.T.; Inchingolo, F.; Tartaglia, G.M. Cone-Beam Computed Tomographic Assessment of the Mandibular Condylar Volume in Different Skeletal Patterns: A Retrospective Study in Adult Patients. Bioengineering 2022, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Pede, C.D.; et al. The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int. J. Environ. Res. Public Health 2022, 19, 988. [Google Scholar] [CrossRef] [PubMed]
- Coloccia, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Montenegro, V.; Patano, A.; Marinelli, G.; Laudadio, C.; Limongelli, L.; Di Venere, D.; et al. Effectiveness of Dental and Maxillary Transverse Changes in Tooth-Borne, Bone-Borne, and Hybrid Palatal Expansion through Cone-Beam Tomography: A Systematic Review of the Literature. Medicina 2021, 57, 288. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Amato, M.; Caggiano, M.; Ciacci, C. Dental Manifestations and Celiac Disease-An Overview. J. Clin. Med. 2023, 12, 2801. [Google Scholar] [CrossRef] [PubMed]
- Cantekin, K.; Arslan, D.; Delikan, E. Presence and Distribution of Dental Enamel Defects, Recurrent Aphthous Lesions and Dental Caries in Children with Celiac Disease. Pak. J. Med. Sci. 2015, 31, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Böhme Kristensen, C.; Asimakopoulou, K.; Scambler, S. Enhancing Patient-Centred Care in Dentistry: A Narrative Review. Br. Med. Bull. 2023, 148, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pecci-Lloret, M.P.; Ramirez-Santisteban, E.; Hergueta-Castillo, A.; Guerrero-Gironés, J.; Oñate-Sánchez, R.E. Oral Manifestations of Crohn’s Disease: A Systematic Review. J. Clin. Med. 2023, 12, 6450. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Ganeshkumar, P. Systematic Reviews and Meta-Analysis: Understanding the Best Evidence in Primary Healthcare. J. Fam. Med. Prim. Care 2013, 2, 9–14. [Google Scholar] [CrossRef]
- Maia, L.C.; Antonio, A.G. Systematic Reviews in Dental Research. A Guideline. J. Clin. Pediatr. Dent. 2012, 37, 117–124. [Google Scholar] [CrossRef]
- Krausch-Hofmann, S.; Tran, T.D.; Declerck, D.; de Almeida Mello, J.; Declercq, A.; Lesaffre, E.; De Lepeleire, J.; Duyck, J. Assessment of Oral Health Conditions Presented in Photographs—Is There a Difference between Dentists and Non-Dental Professional Caregivers? BMC Oral. Health 2020, 20, 188. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.M.; Pincock, D.; Veldhuyzen van Zanten, S. The ‘Natural History’ of Declined Outpatient Gastroenterology Referrals. Can. J. Gastroenterol. 2012, 26, 785–790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crincoli, V.; Anelli, M.G.; Quercia, E.; Piancino, M.G.; Di Comite, M. Temporomandibular Disorders and Oral Features in Early Rheumatoid Arthritis Patients: An Observational Study. Int. J. Med. Sci. 2019, 16, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, V.; Di Comite, M.; Guerrieri, M.; Rotolo, R.P.; Limongelli, L.; Tempesta, A.; Iannone, F.; Rinaldi, A.; Lapadula, G.; Favia, G. Orofacial Manifestations and Temporomandibular Disorders of Sjögren Syndrome: An Observational Study. Int. J. Med. Sci. 2018, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Crincoli, V.; Ballini, A.; Fatone, L.; Di Bisceglie, M.B.; Nardi, G.M.; Grassi, F.R. Cytokine Genotype Distribution in Patients with Periodontal Disease and Rheumatoid Arthritis or Diabetes Mellitus. J. Biol. Regul. Homeost. Agents 2016, 30, 863–866. [Google Scholar] [PubMed]
- Kishore, M.; Panat, S.R.; Aggarwal, A.; Agarwal, N.; Upadhyay, N.; Alok, A. Evidence Based Dental Care: Integrating Clinical Expertise with Systematic Research. J. Clin. Diagn. Res. 2014, 8, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Ekhator, C.; Abdelaziz, A.M.; Naveed, H.; Karski, A.; Cook, D.E.; Reddy, S.M.; Affaf, M.; Khan, S.J.; Bellegarde, S.B.; et al. Revolutionizing Inflammatory Bowel Disease Management: A Comprehensive Narrative Review of Innovative Dietary Strategies and Future Directions. Cureus 2023, 15, e44304. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
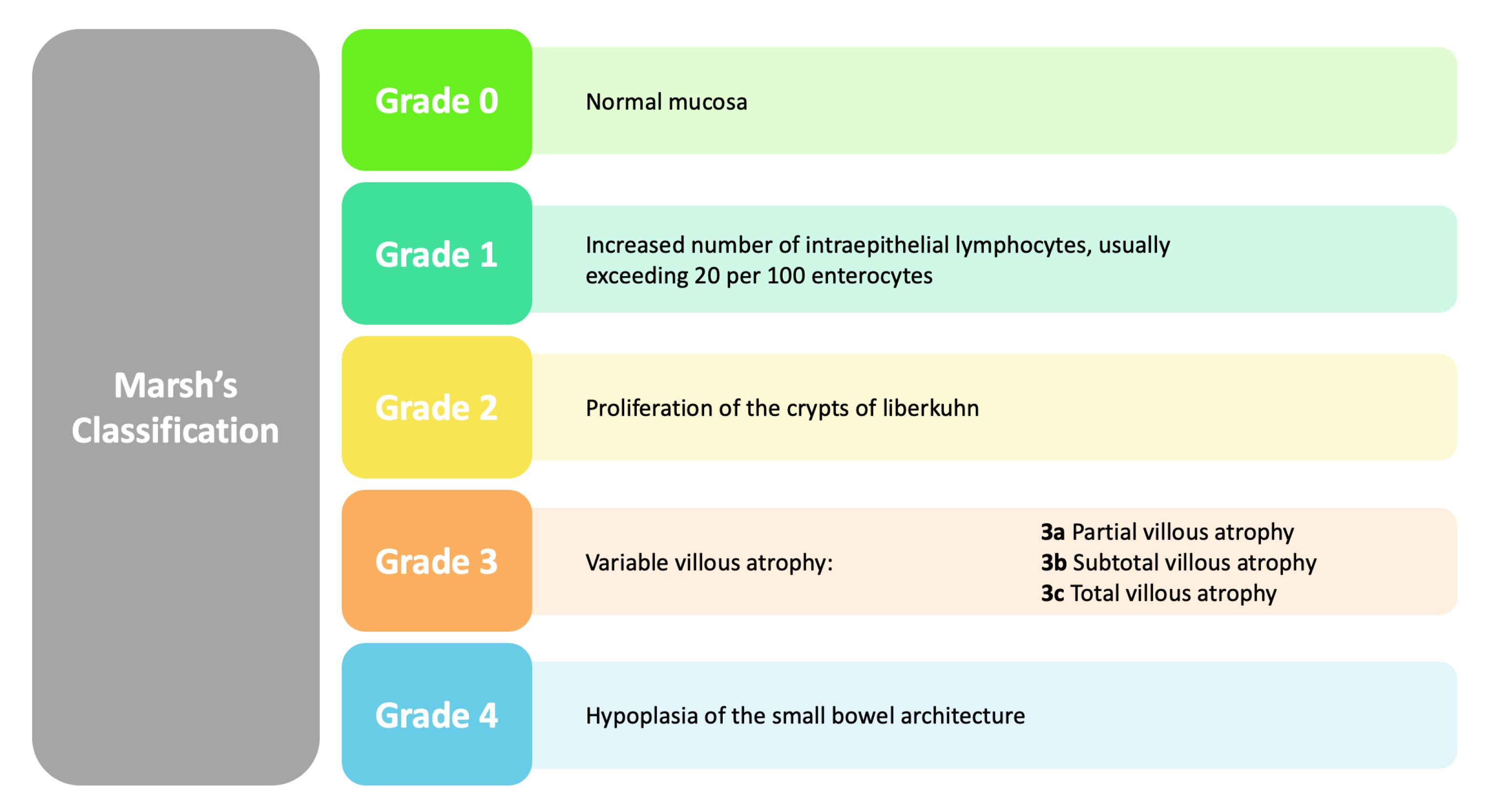
- Elbek-Cubukcu, C.; Arsoy, H.-A.; Ozkaya, G. Assessment of Oral Manifestations in Pediatric Patients with Celiac Disease in Relation to Marsh Types. Med. Oral. Patol. Oral. Cir. Bucal 2023, 28, e9–e15. [Google Scholar] [CrossRef]
- Ludovichetti, F.S.; Signoriello, A.G.; Girotto, L.; Del Dot, L.; Piovan, S.; Mazzoleni, S. Oro-Dental Lesions in Pediatric Patients with Celiac Disease: An Observational Retrospective Clinical Study. Rev. Esp. Enferm. Dig. 2022, 114, 654–659. [Google Scholar] [CrossRef]
- Villemur Moreau, L.; Dicky, O.; Mas, E.; Noirrit, E.; Marty, M.; Vaysse, F.; Olives, J.-P. Oral Manifestations of Celiac Disease in French Children. Arch. Pediatr. 2021, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.E.; Akbar, A.; Alkhubaizi, Q.; Qudeimat, M. Caries among Adult Patients with Controlled Celiac Disease: A Cross-Sectional Study. Spec. Care Dent. 2020, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kuklik, H.H.; Cruz, I.T.S.A.; Celli, A.; Fraiz, F.C.; da AssunÇÃo, L.R.S. Molar incisor hypomineralization and celiac disease. Arq. Gastroenterol. 2020, 57, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Macho, V.M.P.; de Barros Menéres Manso, M.C.A.; e Silva, D.M.V.; de Andrade, D.J.C. The Difference in Symmetry of the Enamel Defects in Celiac Disease versus Non-Celiac Pediatric Population. J. Dent. Sci. 2020, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, T.; Mehr, S.O.; Hill, I.D.; Shahraki, M. A Comparison of the Prevalence of Dental Enamel Defects and Other Oral Findings in Children with and without Celiac Disease. Iran. J. Pediatr. 2019, 29, e64353. [Google Scholar] [CrossRef]
- Zoumpoulakis, M.; Fotoulaki, M.; Topitsoglou, V.; Lazidou, P.; Zouloumis, L.; Kotsanos, N. Prevalence of Dental Enamel Defects, Aphthous-Like Ulcers and Other Oral Manifestations in Celiac Children and Adolescents: A Comparative Study. J. Clin. Pediatr. Dent. 2019, 43, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Zingone, F.; Caggiano, M.; Iovino, P.; Bucci, C.; Ciacci, C. Tooth Wear Is Frequent in Adult Patients with Celiac Disease. Nutrients 2017, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, A.M.; Arid, J.; de Carvalho, F.K.; da Silva, R.A.B.; Küchler, E.C.; Sawamura, R.; da Silva, L.A.B.; Nelson-Filho, P. Assessing the Proposed Association between DED and Gluten-Free Diet Introduction in Celiac Children. Spec. Care Dent. 2017, 37, 194–198. [Google Scholar] [CrossRef]
- de Carvalho, F.K.; de Queiroz, A.M.; Bezerra da Silva, R.A.; Sawamura, R.; Bachmann, L.; Bezerra da Silva, L.A.; Nelson-Filho, P. Oral Aspects in Celiac Disease Children: Clinical and Dental Enamel Chemical Evaluation. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2015, 119, 636–643. [Google Scholar] [CrossRef]
- Bramanti, E.; Cicciù, M.; Matacena, G.; Costa, S.; Magazzù, G. Clinical Evaluation of Specific Oral Manifestations in Pediatric Patients with Ascertained versus Potential Coeliac Disease: A Cross-Sectional Study. Gastroenterol. Res. Pr. 2014, 2014, 934159. [Google Scholar] [CrossRef]
- Shteyer, E.; Berson, T.; Lachmanovitz, O.; Hidas, A.; Wilschanski, M.; Menachem, M.; Shachar, E.; Shapira, J.; Steinberg, D.; Moskovitz, M. Oral Health Status and Salivary Properties in Relation to Gluten-Free Diet in Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 49–52. [Google Scholar] [CrossRef] [PubMed]

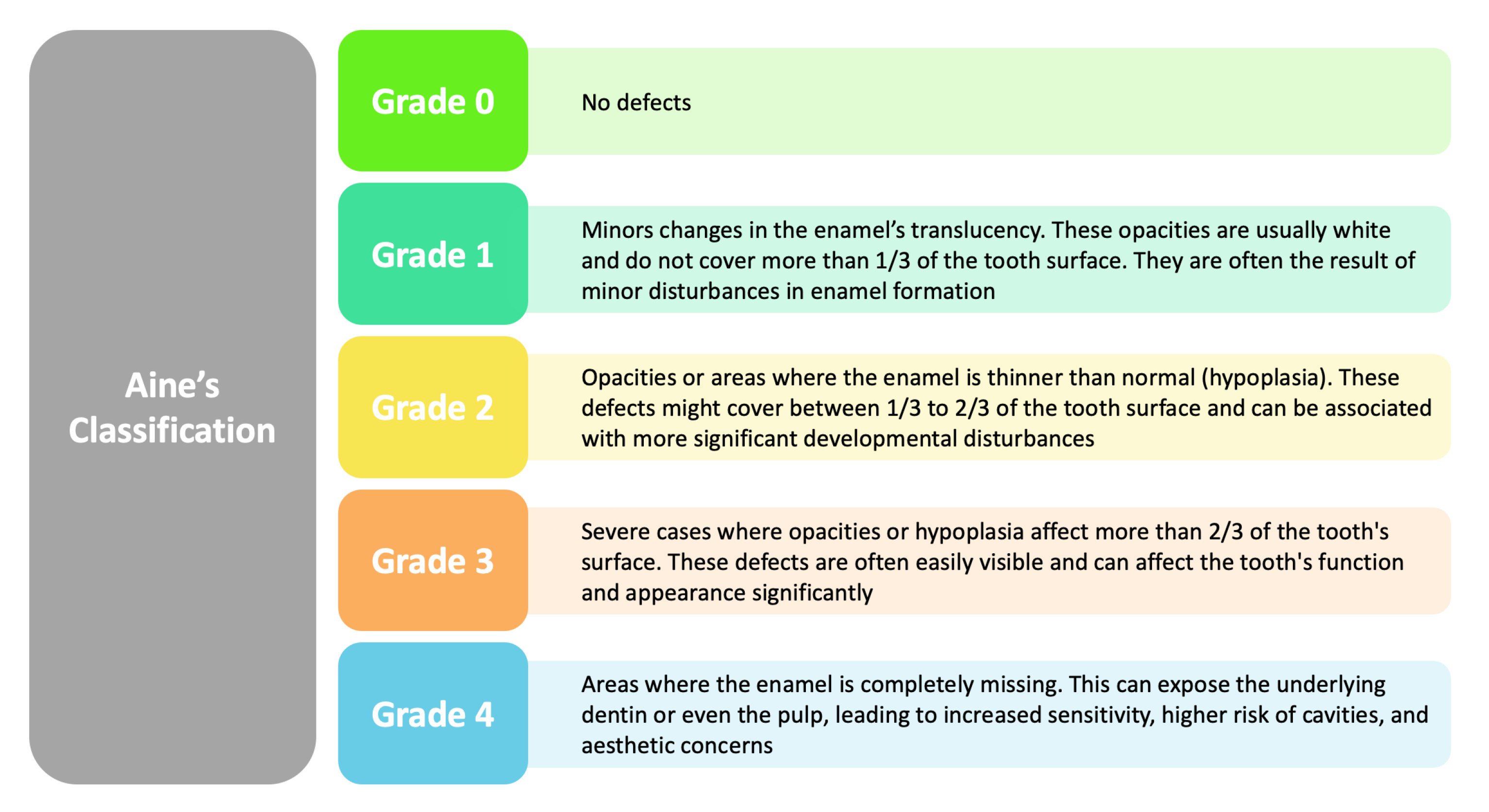

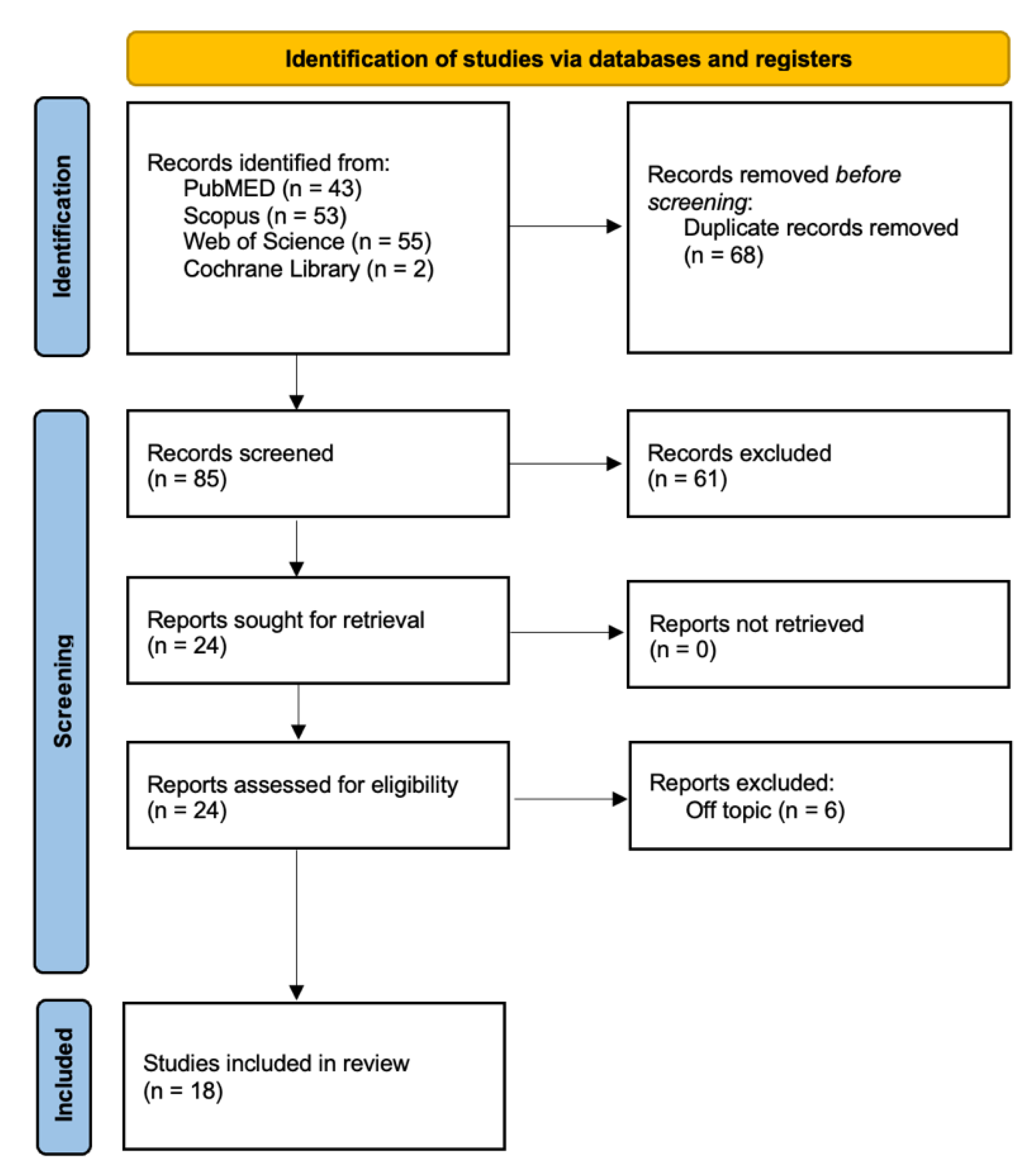

| Author (Year) | Type of Study | Sample Size | Age | Type and Gravity of the Defects | Type of Analysis Performed | Outcomes |
|---|---|---|---|---|---|---|
| Bulut et al. (2023) [38] | Clinical study | 78 P | 4 to 15 y.o. | Enamel defects as per Aine’s classification observed in 34.5% of patients (Grade 1 in most cases, Grade 2 in 3.8%). |
| One group had higher dental decay scores with no differences in salivary metrics, 34.5% had mild enamel defects, and aphthous stomatitis was more common in recently diagnosed individuals. |
| Coelho et al. (2023) [40] | Cross-sectional study | 146 CD P | 10.5 years | RAS, DC, and DO |
| RAS (46.6%), DC (45.2%), and DO (39%) was the most reported oral manifestation among CD P. |
| Elbek-Cubukcu et al. (2022) [79] | Prospective cohort study | 62 CD P, 64 CP | n.r. | MIH, RAS, poor oral hygiene, and dental status |
| Prevalence of DED: in the group with CD: 61% (MIH); in the control group 65.6% (no MIH). |
| Ludovichetti et al. (2022) [80] | Ob S | 114 P | 6–14 y.o. | DEDs, specifically hypoplasia; Severity classified by Aine’s classification (Grade 0, Grade I, Grade II/III) |
| DEDs were present in 31.6% Grade 0, 34.2% Grade I, 23.7% Grade II/III in the celiac disease (CD) group.
|
| Ahmed et al. (2021) [1] | Prospective CC study | 118 P | 20–37.23 | DED observed in 66.9% of patients with CD, specific/bilaterally symmetrical more common in treatment-naïve and GFD-treated patients compared to controls (20%) |
| Significantly higher percentage of CD patients (68.6%) reported xerostomia/dry mouth sensation compared to controls (7.5%), DED 8.1 (95% CI 3.4–19.2), xerostomia 27 (95% CI 7.8–93.2). |
| Villemur Moreau et al. (2021) [81] | Ob S | 28 CD and 59 CP | 3–12 y.o. | DEDs graded according to Aine’s classification (grades I to IV) |
| CD children had significantly more enamel defects and recurrent aphthous stomatitis than the control group (67.9% vs. 33.9% for ED, and 50.0% vs. 21.8% for RAS, respectively). No significant delay in dental eruption was observed in CD children. EDs in CD children were more severe than in the control group (p = 0.04). |
| Khalaf et al. (2020) [82] | CC | 23 CD P, 23 CP | 39.1 ± 14.4 years | Dental enamel hypoplasia, aphthous ulcers, dental caries (DMFT) |
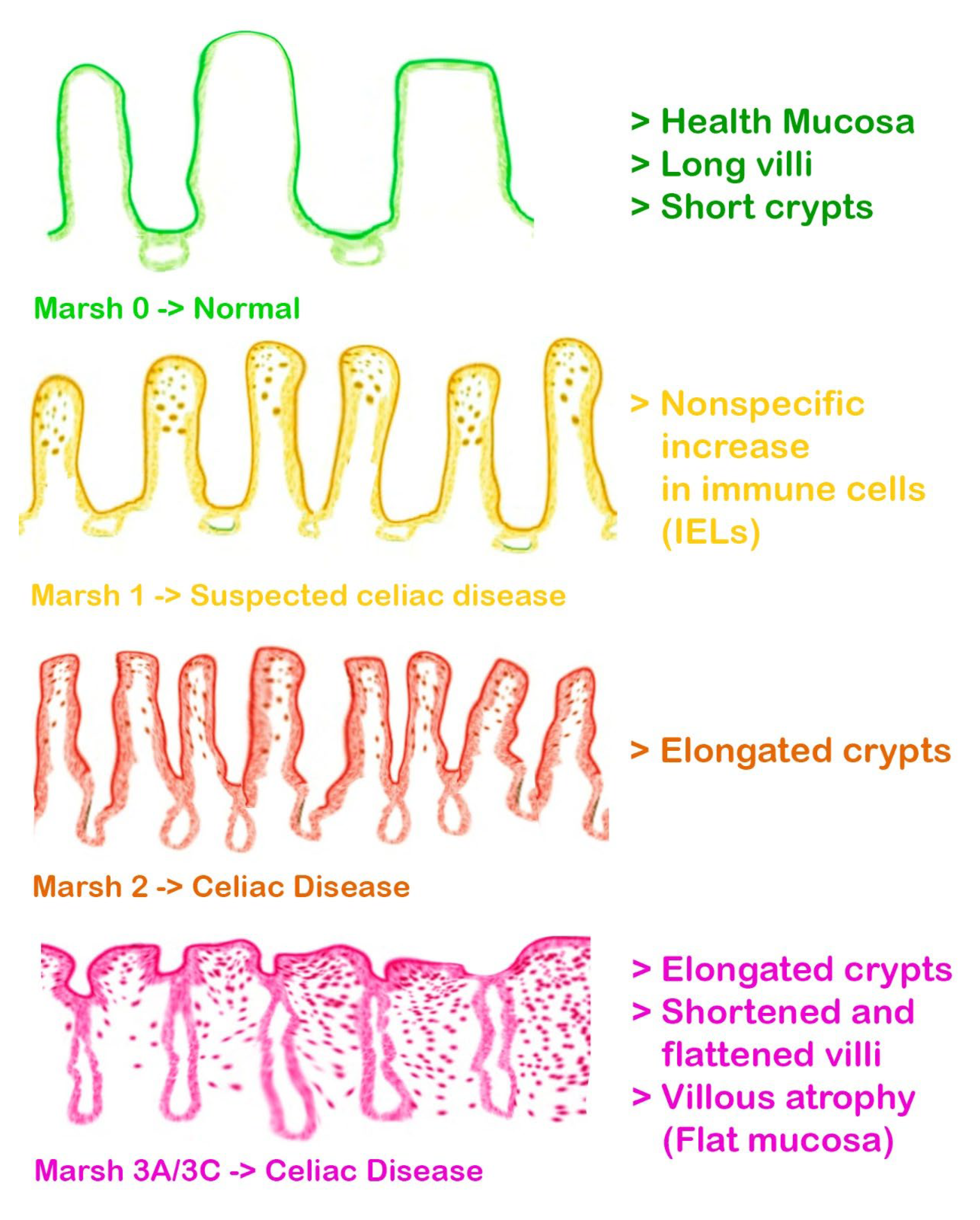
| Significant inverse relationship between MIH and age at diagnosis of celiac disease: Marsh 2 damage type in celiac disease is associated with an increased risk of dental caries. Reduced buffering capacity of saliva in children with celiac disease. |
| Kuklik et al. (2020) [83] | Prevalence study | 40 CD P 40 CP | 16.5 y.o. | MIH with demarcated opacities, post-eruptive breakdown (PEB), atypical restoration |
| Out of 80 participants, 10 had MIH (12.5%). Among the 40 celiac patients, 8 had MIH (20%). Among the 40 individuals without CD, 2 had MIH (5%). Celiac disease increased the likelihood of MIH occurrence by 4.75 times compared to the CG. |
| Pereira Macho et al. (2020) [84] | CC | 160 P | 6–18 y.o. | Mainly symmetrical enamel defects characterized by pitting, grooving, and loss of enamel |
| Grade I and Grade II defects were observed in both groups, but significantly higher in celiac group (p = 0.002, p = 0.003). Symmetric enamel defects were more prevalent in celiac group, particularly in first upper molars, first lower molars, lateral upper incisors, and central upper incisors (p < 0.05). |
| Shahraki et al. (2019) [85] | Prospective Ob S | 65 CD P 60 CP | Ages 3–16 y.o. | DED symmetric and non-symmetric; Grades I–IV based on Aine’s criteria |
| Half of the patients with celiac disease exhibited enamel defects, predominantly mild but including some severe cases, with a higher incidence of tooth decay in baby teeth, more frequent dry mouth symptoms, but not in adult teeth, despite reduced sugar intake compared to the control group. |
| Zoumpoulakis et al. (2019) [86] | Comparative, Cross-sectional | 45 CD P, 45 CP | 10.3 ± 4.1 y.o. | Systemic and non-systemic DED |
| Prevalence of systemic DED was significantly higher in CD patients (51.1%) compared to controls (11.1%). |
| Amato et al. (2017) [87] | CC | 49 CD P 51 CP | CD P: 31.8 ± 11.58 y.o., CP: 30.5 ± 8.7 y.o. | Enamel Hypoplasia: Aine Grade 1 (4 patients), Aine Grade 2 (3 patients); Non-specific Tooth Wear: Smith and Knight Index Grade 1 (4 patients), Grade 2 (3 patients), Grade 3 (2 patients) |
| RAS: CD Patients 53.0%, Controls 25.5%; Aphthosis during visit: CD Patients 0%, Controls 0%; Atrophic Glossitis: CD Patients 0%, Controls 0%; Enamel Hypoplasia: CD Patients 14.3%, Controls 0%; Non-specific Tooth Wear: CD Patients 18.3%, Controls 5.9%. |
| de Queiroz et al. (2017) [88] | Retrospective Ob S | 45 CD p | Age range: 2–15 y.o. | DED Grades I-IV based on Aine’s criteria 55.6% |
| DED prevalence 55.6%. |
| Cantekin et al. (2015) [65] | RS | 25 CD P, 25 CP P | 8.94 ± 2.08 (CD) and 9.66 ± 4.26 (CP) y.o.; | DED prevalence was higher in CD children (48%) than healthy children (16%). Enamel defects were generally symmetrical and mostly observed in anterior teeth. |
| DMFT scores were significantly higher in CD children (3.75 ± 2.62) compared to the control group (1.83 ± 1.78). RAS prevalence was higher in CD children (44%) compared to the control group (0%). Significant differences were found between CD and control groups for both enamel defects (p = 0.01) and DMFT scores (p < 0.01). |
| de Carvalho et al. (2015) [89] | CC | 52 CD P and 52 CP; additional 50 DEDs | 2 to 15 y.o. | DEDs graded according to Aine’s classification (grades I to IV) |
| Children with celiac disease had more dental enamel defects and mouth ulcers but fewer cavities, showed signs of altered enamel chemistry with lower salivary flow and altered calcium-to-phosphorus ratios, although their carbonate-to-phosphate ratios were comparable to those of a control group. |
| Bramanti et al. (2014) [90] | Prospective Cohort | 116 PP | 2–16 years old | Specific Enamel Defects (SED)—Grades I-IV (severity) Unspecific Enamel Defects (UnSED) Dental Caries (DMFT/dmft indices) Dental Delayed Eruption (DDE) | Cross-Sectional Study | Anomalies found in oral hard tissues:
|
| Shteyer et al. (2013) [91] | PS | 90 P, 30 in each group (newly diagnosed CD, CD treated with Gluten Free Diet, and control). | 1.4 to 18 years; | DEDs graded according to Aine’s classification (grades I to IV). |
| Higher prevalence of enamel hypoplasia in CD children (66%). |
| Trotta et al. (2013) [48] | Prospective Ob S | 54 P | 37 ± 13 years | DED observed in 85.2% of CD P, predominantly Aine grade 1 type lesion (33.3%) |
| Severe DED (Aine grade 3 and 4) more common in classical CD (10/32) than non-classical CD (2/20), not statistically significant. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Dipalma, G.; Viapiano, F.; Netti, A.; Ferrara, I.; Ciocia, A.M.; Mancini, A.; Di Venere, D.; Palermo, A.; Inchingolo, A.M.; et al. Celiac Disease-Related Enamel Defects: A Systematic Review. J. Clin. Med. 2024, 13, 1382. https://doi.org/10.3390/jcm13051382
Inchingolo AD, Dipalma G, Viapiano F, Netti A, Ferrara I, Ciocia AM, Mancini A, Di Venere D, Palermo A, Inchingolo AM, et al. Celiac Disease-Related Enamel Defects: A Systematic Review. Journal of Clinical Medicine. 2024; 13(5):1382. https://doi.org/10.3390/jcm13051382
Chicago/Turabian StyleInchingolo, Alessio Danilo, Gianna Dipalma, Fabio Viapiano, Anna Netti, Irene Ferrara, Anna Maria Ciocia, Antonio Mancini, Daniela Di Venere, Andrea Palermo, Angelo Michele Inchingolo, and et al. 2024. "Celiac Disease-Related Enamel Defects: A Systematic Review" Journal of Clinical Medicine 13, no. 5: 1382. https://doi.org/10.3390/jcm13051382
APA StyleInchingolo, A. D., Dipalma, G., Viapiano, F., Netti, A., Ferrara, I., Ciocia, A. M., Mancini, A., Di Venere, D., Palermo, A., Inchingolo, A. M., & Inchingolo, F. (2024). Celiac Disease-Related Enamel Defects: A Systematic Review. Journal of Clinical Medicine, 13(5), 1382. https://doi.org/10.3390/jcm13051382











