A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Sample
- Geographical region: studies were categorized by the continent where the research was conducted, including Asia, Europe, South America, and North America;
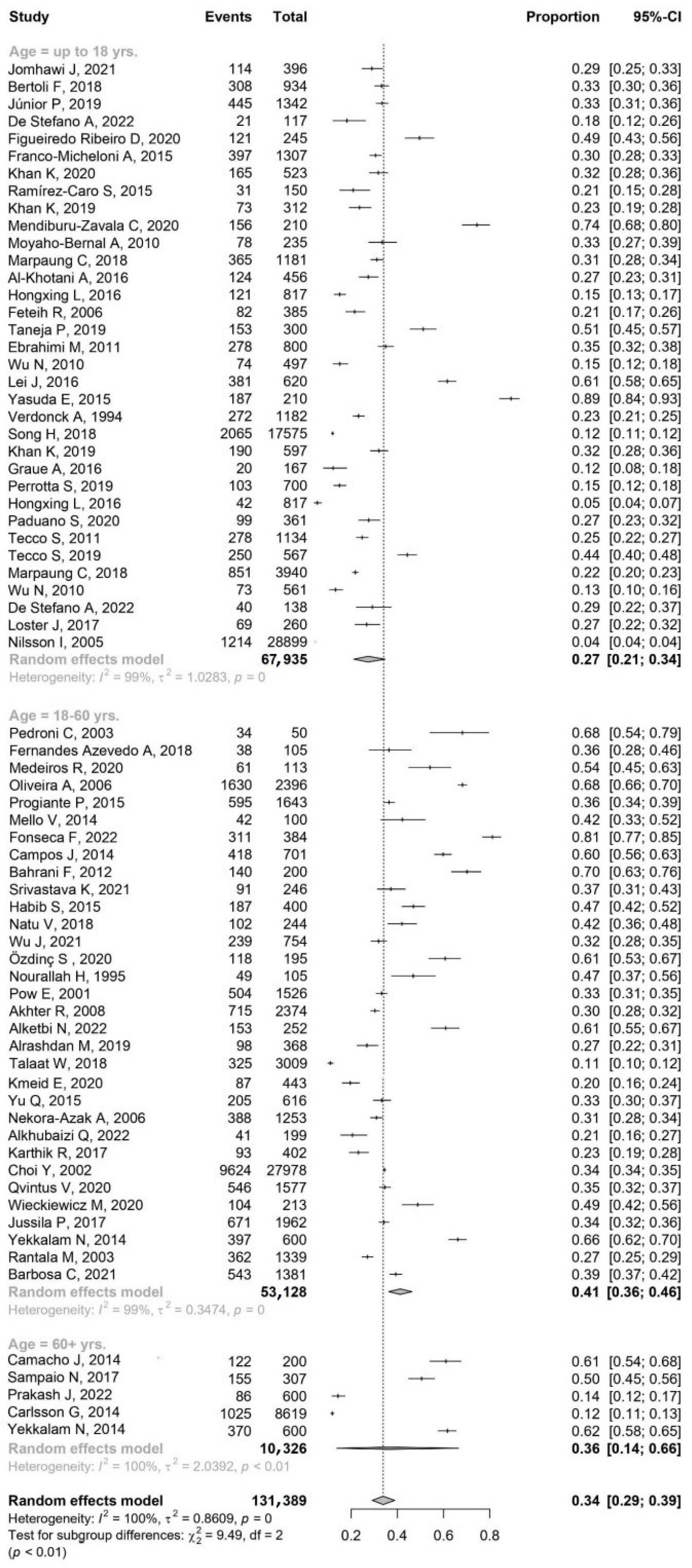
- Age group: patient age is categorized into three distinct groups—younger patients (up to 18 yrs.), young and middle-aged patients (18–60 yrs.), older patients (over 60 yrs.);
- Sample size: includes the overall size of the sample and the gender distribution within the sample (number of males and females).
2.2. Statistical Analysis
2.2.1. Significance Level
2.2.2. Effect Size Estimation
2.2.3. Identifying Outliers and Influential Cases
2.2.4. Subgroup Analysis
2.2.5. Publication Bias
2.2.6. Statistical Environment
3. Results
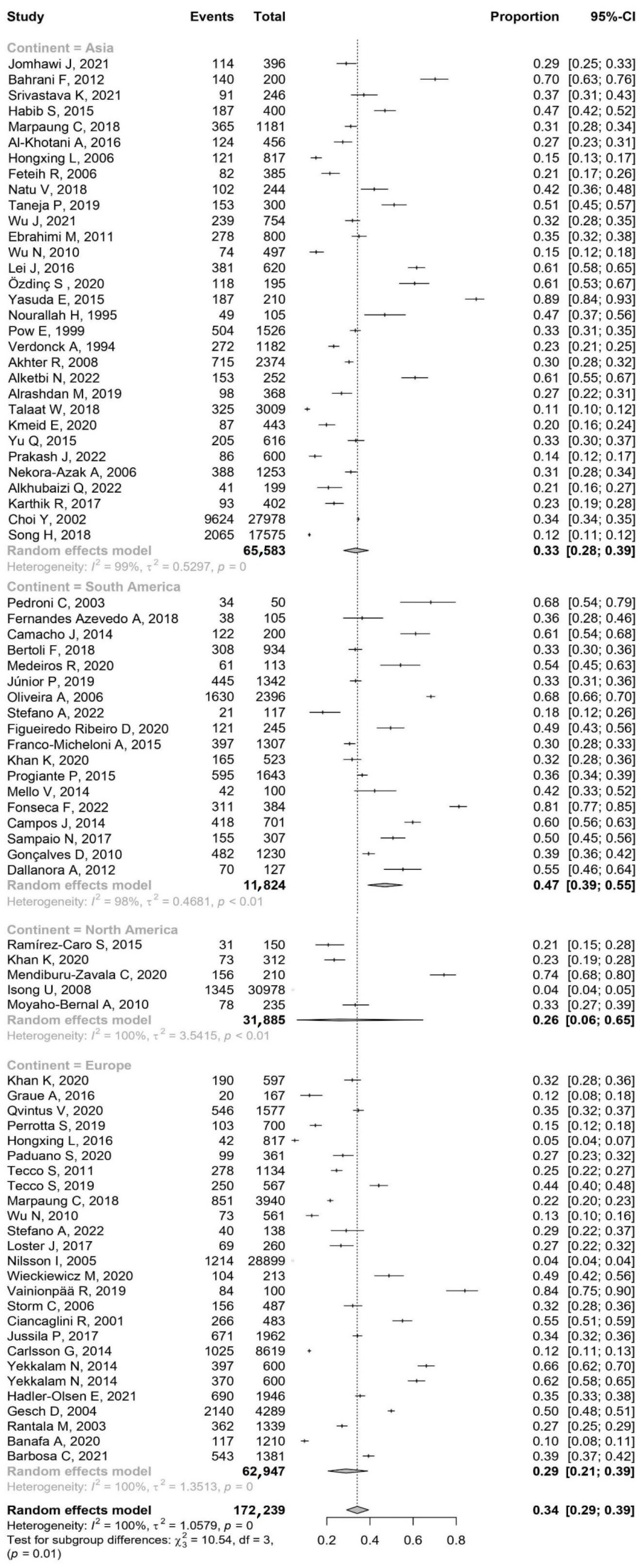
3.1. The Prevalence of TMDs by Continent and Age
3.1.1. Asia
3.1.2. South America
3.1.3. North America
3.1.4. Europe
3.1.5. Prevalence of TMDs Female-to-Male Ratio (F:M)
4. Discussion
5. Conclusions
- This study suggests that geographical location may play a role in the epidemiology of TMDs. The prevalence of TMDs was significantly higher in South America (47%) compared to Asia (33%) and Europe (29%).
- This study suggests a higher incidence of TMDs in females compared to males in these age groups compared to the 18–60 age group.
- From the data presented, the female group was, on average, 9% to 56% larger than the male group in each continent. The highest female-to-male ratio was reported in South America (1.56), whereas the lowest F:M was reported in Europe (1.09), suggesting an almost equal distribution of males and females.
- Larger epidemiological studies of TMDs in African and Australian populations are recommended.
- In conclusion, both visual and statistical assessments suggest that the results of our meta-analysis are robust and unlikely to be significantly affected by publication bias.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- List, T.; Jensen, R.H. Temporomandibular Disorders: Old Ideas and New Concepts. Cephalalgia Int. J. Headache 2017, 37, 692–704. [Google Scholar] [CrossRef]
- Maini, K.; Dua, A. Temporomandibular Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Manfredini, D. Tinnitus in Temporomandibular Disorders Patients: Any Clinical Implications from Research Findings? Evid. Based Dent. 2019, 20, 30–31. [Google Scholar] [CrossRef]
- Al-Jewair, T.; Shibeika, D.; Ohrbach, R. Temporomandibular Disorders and Their Association with Sleep Disorders in Adults: A Systematic Review. J. Oral Facial Pain Headache 2021, 35, 41–53. [Google Scholar] [CrossRef]
- Liou, Y.-J.; Bai, Y.-M.; Tsai, S.-J.; Chen, T.-J.; Chen, M.-H.; Lo, W.-L. Bidirectional Associations of Temporomandibular Joint Disorders with Major Depressive and Anxiety Disorders. J. Evid.-Based Dent. Pract. 2023, 23, 101860. [Google Scholar] [CrossRef]
- Matheus, H.R.; Özdemir, Ş.D.; Guastaldi, F.P.S. Stem Cell-Based Therapies for Temporomandibular Joint Osteoarthritis and Regeneration of Cartilage/Osteochondral Defects: A Systematic Review of Preclinical Experiments. Osteoarthr. Cartil. 2022, 30, 1174–1185. [Google Scholar] [CrossRef]
- Garstka, A.A.; Kozowska, L.; Kijak, K.; Brzózka, M.; Gronwald, H.; Skomro, P.; Lietz-Kijak, D. Accurate Diagnosis and Treatment of Painful Temporomandibular Disorders: A Literature Review Supplemented by Own Clinical Experience. Pain Res. Manag. 2023, 2023, 1002235. [Google Scholar] [CrossRef] [PubMed]
- Chisnoiu, A.M.; Picos, A.M.; Popa, S.; Chisnoiu, P.D.; Lascu, L.; Picos, A.; Chisnoiu, R. Factors Involved in the Etiology of Temporomandibular Disorders—A Literature Review. Clujul Med. 2015, 88, 473–478. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.F.; LeResche, L. Research Diagnostic Criteria for Temporomandibular Disorders: Review, Criteria, Examinations and Specifications, Critique. J. Craniomandib. Disord. Facial Oral Pain 1992, 6, 301–355. [Google Scholar]
- Fonseca, D.M.d.; Bonfante, G.; do Valle, A.L.; Freitas, S.F.T. de Diagnóstico pela anamnese da disfunção craniomandibular. RGO Porto Alegre 1994, 42, 23–28. [Google Scholar]
- Pires, P.F.; de Castro, E.M.; Pelai, E.B.; de Arruda, A.B.C.; Rodrigues-Bigaton, D. Analysis of the Accuracy and Reliability of the Short-Form Fonseca Anamnestic Index in the Diagnosis of Myogenous Temporomandibular Disorder in Women. Braz. J. Phys. Ther. 2018, 22, 276–282. [Google Scholar] [CrossRef]
- Helkimo, M. Studies on Function and Dysfunction of the Masticatory System. II. Index for Anamnestic and Clinical Dysfunction and Occlusal State. Sven. Tandlak. Tidskr. Swed. Dent. J. 1974, 67, 101–121. [Google Scholar]
- Giannakopoulos, N.N.; Rammelsberg, P.; Eberhard, L.; Schmitter, M. A New Instrument for Assessing the Quality of Studies on Prevalence. Clin. Oral Investig. 2012, 16, 781–788. [Google Scholar] [CrossRef]
- Figueiredo Ribeiro, D.C.; Ferreira Gradella, C.M.; Franco Rocha Rodrigues, L.L.; Abanto, J.; Oliveira, L.B. The Impact of Temporomandibular Disorders on the Oral Health-Related Quality of Life of Brazilian Children: A Cross-Sectional Study. J. Dent. Child. Chic. Ill 2020, 87, 103–109. [Google Scholar]
- Khan, K.; Muller-Bolla, M.; Anacleto Teixeira Junior, O.; Gornitsky, M.; Guimarães, A.S.; Velly, A.M. Comorbid Conditions Associated with Painful Temporomandibular Disorders in Adolescents from Brazil, Canada and France: A Cross-Sectional Study. J. Oral Rehabil. 2020, 47, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Marpaung, C.; van Selms, M.K.A.; Lobbezoo, F. Prevalence and Risk Indicators of Pain-Related Temporomandibular Disorders among Indonesian Children and Adolescents. Community Dent. Oral Epidemiol. 2018, 46, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, S.; Bucci, R.; Simeon, V.; Martina, S.; Michelotti, A.; Valletta, R. Prevalence of Malocclusion, Oral Parafunctions and Temporomandibular Disorder-Pain in Italian Schoolchildren: An Epidemiological Study. J. Oral Rehabil. 2019, 46, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Zhang, M.-J.; Lei, J.; Fu, K.-Y. Diagnostic Accuracy of the Short-Form Fonseca Anamnestic Index in Relation to the Diagnostic Criteria for Temporomandibular Disorders. J. Prosthet. Dent. 2022, 128, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Zhang, M.J.; Lei, J.; Fu, K.-Y. Accuracy of the Fonseca Anamnestic Index for Identifying Pain-Related and/or Intra-Articular Temporomandibular Disorders. Cranio J. Craniomandib. Pract. 2021, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, G.; Maracci, L.M.; de Oliveira Chami, V.; Pereira, D.D.; Tomazoni, F.; Bernardon Silva, T.; Ferrazzo, V.; Marquezan, M. TMD Diagnosis: Sensitivity and Specificity of the Fonseca Anamnestic Index. Cranio J. Craniomandib. Pract. 2023, 41, 199–203. [Google Scholar] [CrossRef]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of Temporomandibular Joint Disorders: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Prevalence of Temporomandibular Disorders in Children and Adolescents Evaluated with Diagnostic Criteria for Temporomandibular Disorders: A Systematic Review with Meta-Analysis. J. Oral Rehabil. 2023, 50, 522–530. [Google Scholar] [CrossRef]
- Melo, V.; Monteiro, L.; Orge, C.; Sales, M.; Melo, J.; Rodrigues, B.; Melo, A. Prevalence of Temporomandibular Disorders in the Brazilian Population: A Systematic Review and Meta-Analysis. Cranio J. Craniomandib. Pract. 2023, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Lin, M.; Yang, H.; Ren, A. Prevalence of Temporomandibular Disorders and Its Clinical Signs in Chinese Students, 1979–2017: A Systematic Review and Meta-Analysis. Oral Dis. 2019, 25, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, E.J.; Power, H.; Fawcett, J.M. Women Are at Greater Risk of OCD Than Men: A Meta-Analytic Review of OCD Prevalence Worldwide. J. Clin. Psychiatry 2020, 81, 19r13085. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Wagaw, Z.A.; Clements, A.C.A. Mapping Tuberculosis Prevalence in Ethiopia: Protocol for a Geospatial Meta-Analysis. BMJ OPEN 2020, 10, e034704. [Google Scholar] [CrossRef] [PubMed]
- Booth, A. Over 85% of Included Studies in Systematic Reviews Are on MEDLINE. J. Clin. Epidemiol. 2016, 79, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Halladay, C.W.; Trikalinos, T.A.; Schmid, I.T.; Schmid, C.H.; Dahabreh, I.J. Using Data Sources beyond PubMed Has a Modest Impact on the Results of Systematic Reviews of Therapeutic Interventions. J. Clin. Epidemiol. 2015, 68, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Rice, D.B.; Kloda, L.A.; Levis, B.; Qi, B.; Kingsland, E.; Thombs, B.D. Are MEDLINE Searches Sufficient for Systematic Reviews and Meta-Analyses of the Diagnostic Accuracy of Depression Screening Tools? A Review of Meta-Analyses. J. Psychosom. Res. 2016, 87, 7–13. [Google Scholar] [CrossRef]
- Brown, D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot. Pract. 2020, 21, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W. Popping the (PICO) Question in Research and Evidence-Based Practice. Appl. Nurs. Res. 2002, 15, 197–198. [Google Scholar] [CrossRef]
- Adegbiji, W.A.; Olajide, G.T.; Agbesanwa, A.T.; Banjo, O.O. Otological Manifestation of Temporomandibular Joint Disorder in Ekiti, a Sub-Saharan African Country. J. Int. Med. Res. 2021, 49, 300060521996517. [Google Scholar] [CrossRef]
- Friedman Rubin, P.; Erez, A.; Peretz, B.; Birenboim-Wilensky, R.; Winocur, E. Prevalence of Bruxism and Temporomandibular Disorders among Orphans in Southeast Uganda: A Gender and Age Comparison. CRANIO® 2018, 36, 243–249. [Google Scholar] [CrossRef]
- Lung, J.; Bell, L.; Heslop, M.; Cuming, S.; Ariyawardana, A. Prevalence of Temporomandibular Disorders among a Cohort of University Undergraduates in Australia. J. Investig. Clin. Dent. 2018, 9, e12341. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and Influence Diagnostics for Meta-Analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Zwiri, A.M.A.; Al-Omiri, M.K. Prevalence of Temporomandibular Joint Disorder among North Saudi University Students. CRANIO® 2016, 34, 176–181. [Google Scholar] [CrossRef]
- Marpaung, C.; Lobbezoo, F.; van Selms, M.K.A. Temporomandibular Disorders among Dutch Adolescents: Prevalence and Biological, Psychological, and Social Risk Indicators. Pain Res. Manag. 2018, 2018, e5053709. [Google Scholar] [CrossRef]
- Hongxing, L.; Astrøm, A.N.; List, T.; Nilsson, I.-M.; Johansson, A. Prevalence of Temporomandibular Disorder Pain in Chinese Adolescents Compared to an Age-Matched Swedish Population. J. Oral Rehabil. 2016, 43, 241–248. [Google Scholar] [CrossRef]
- De Stefano, A.A.; Guercio-Mónaco, E.; Uzcátegui, A.; Boboc, A.M.; Barbato, E.; Galluccio, G. Temporomandibular Disorders in Venezuelan and Italian Adolescents. CRANIO® 2022, 40, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Guarda-Nardini, L.; Piccotti, F.; Mogno, G.; Favero, L.; Manfredini, D. Age-Related Differences in Temporomandibular Disorder Diagnoses. Cranio J. Craniomandib. Pract. 2012, 30, 103–109. [Google Scholar] [CrossRef]
- Yekkalam, N.; Wänman, A. Prevalence of Signs and Symptoms Indicative of Temporomandibular Disorders and Headaches in 35-, 50-, 65- and 75-Year-Olds Living in Västerbotten, Sweden. Acta Odontol. Scand. 2014, 72, 458–465. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Raudenbush, S.W. Analyzing Effect Sizes: Random-Effects Models. In The handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009; pp. 295–315. ISBN 978-0-87154-163-5. [Google Scholar]
- Jackson, D. Confidence Intervals for the between-Study Variance in Random Effects Meta-Analysis Using Generalised Cochran Heterogeneity Statistics. Res. Synth. Methods 2013, 4, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot–Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Coburn, K.; Vevea, J. Weightr: Estimating Weight-Function Models for Publication Bias. R Package Version 2.0.2. Available online: https://CRAN.R-project.org/package=weightr (accessed on 28 August 2023).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.; Wiernik, B. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. Available online: https://easystats.github.io/report/ (accessed on 16 November 2023).
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Metasens: Statistical Methods for Sensitivity Analysis in Meta-Analysis. 2023. Available online: https://CRAN.R-project.org/package=metasens (accessed on 28 August 2023).
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; Bryan, J. Posit, PBC (Copyright holder of all R. code and all C. code without explicit copyright; code), M.K. (Author of included R.; code), K.V. (Author of included libxls; code), C.L. (Author of included libxls; code), B.C. (Author of included libxls; code), D.H. (Author of included libxls; code), E.M. (Author of included libxls Readxl: Read Excel Files 2023. Available online: https://CRAN.R-project.org/package=readxl (accessed on 5 September 2023).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D.; Software, P. PBC Dplyr: A Grammar of Data Manipulation 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 5 September 2023).
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D. Dmetar: Companion r Package for the Guide ‘Doing Meta-Analysis in r’ (R Package Version 0.0.9000). Available online: https://dmetar.protectlab.org/ (accessed on 16 November 2023).
- Wickham, H.; Seidel, D. RStudio Scales: Scale Functions for Visualization 2022. Available online: https://CRAN.R-project.org/package=scales (accessed on 5 September 2023).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy; Committee on Temporomandibular Disorders (TMDs): From Research Discoveries to Clinical Treatment; Yost, O.; Liverman, C.T.; English, R.; Mackey, S.; Bond, E.C. Definitions and Scope: What Are TMDs? In Temporomandibular Disorders: Priorities for Research and Care; National Academies Press (US): Washington, DC, USA, 2020. [Google Scholar]
- Carapinha, I.H.A.; Canales, G.D.l.T.; Poluha, R.L.; Câmara-Souza, M.B.; Christidis, N.; Ernberg, M.; de Almeida, A.M.; Manso, A.C.G.d.M. Sociodemographic Profile: A Forgotten Factor in Temporomandibular Disorders? A Scoping Review. J. Pain Res. 2024, 17, 393–414. [Google Scholar] [CrossRef]
- Durham, J.; Steele, J.; Moufti, M.A.; Wassell, R.; Robinson, P.; Exley, C. Temporomandibular Disorder Patients’ Journey through Care. Community Dent. Oral Epidemiol. 2011, 39, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Di Giosia, M. The Role of Genetic Factors in the Etiology of Temporomandibular Disorders: A Review. CRANIO® 2016, 34, 43–51. [Google Scholar] [CrossRef]
- Warren, M.P.; Fried, J.L. Temporomandibular Disorders and Hormones in Women. Cells Tissues Organs 2001, 169, 187–192. [Google Scholar] [CrossRef]
- Jedynak, B.; Jaworska-Zaremba, M.; Grzechocińska, B.; Chmurska, M.; Janicka, J.; Kostrzewa-Janicka, J. TMD in Females with Menstrual Disorders. Int. J. Environ. Res. Public Health 2021, 18, 7263. [Google Scholar] [CrossRef]
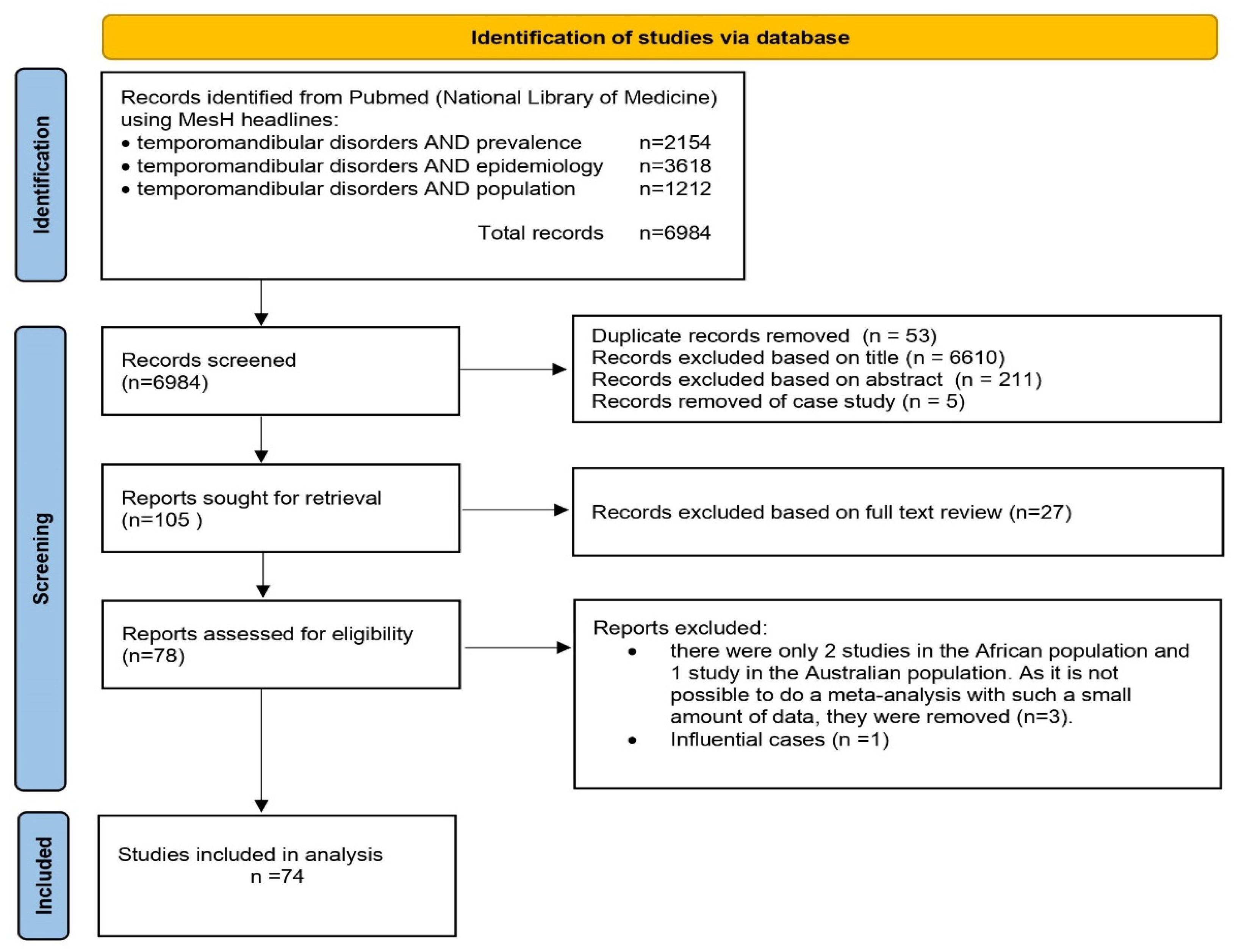

| Inclusion | Exclusion | |
|---|---|---|
| Patient | ||
| Adult and pediatric population | ||
| Intervention | ||
| Screening for TMDs | ||
| Outcome | ||
| Information in the study on: epidemiology, prevalence, TMD population. Research rated as good and outstanding by TQS. | Research rated as poor and fair by TQS. | |
| Comparison | ||
| TMDs vs. Subject health | ||
| Study Design | ||
| Studies to assess occurrence of TMDs in populations. The criterion for continents is at least 2 studies with a total of more than 500 participants. | Narrative Review Systematic Articles Meta-analysis Opinions Case reports or series patients Animal or biomechanical studies Publications in a language other than English Post-conference abstracts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. https://doi.org/10.3390/jcm13051365
Zieliński G, Pająk-Zielińska B, Ginszt M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. Journal of Clinical Medicine. 2024; 13(5):1365. https://doi.org/10.3390/jcm13051365
Chicago/Turabian StyleZieliński, Grzegorz, Beata Pająk-Zielińska, and Michał Ginszt. 2024. "A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders" Journal of Clinical Medicine 13, no. 5: 1365. https://doi.org/10.3390/jcm13051365
APA StyleZieliński, G., Pająk-Zielińska, B., & Ginszt, M. (2024). A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. Journal of Clinical Medicine, 13(5), 1365. https://doi.org/10.3390/jcm13051365









