Low Bone Mass in Ambulatory Spinal Muscular Atrophy: A Proactive Approach for an Often-Overlooked Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Six-Minute Walk Test (6MWT)
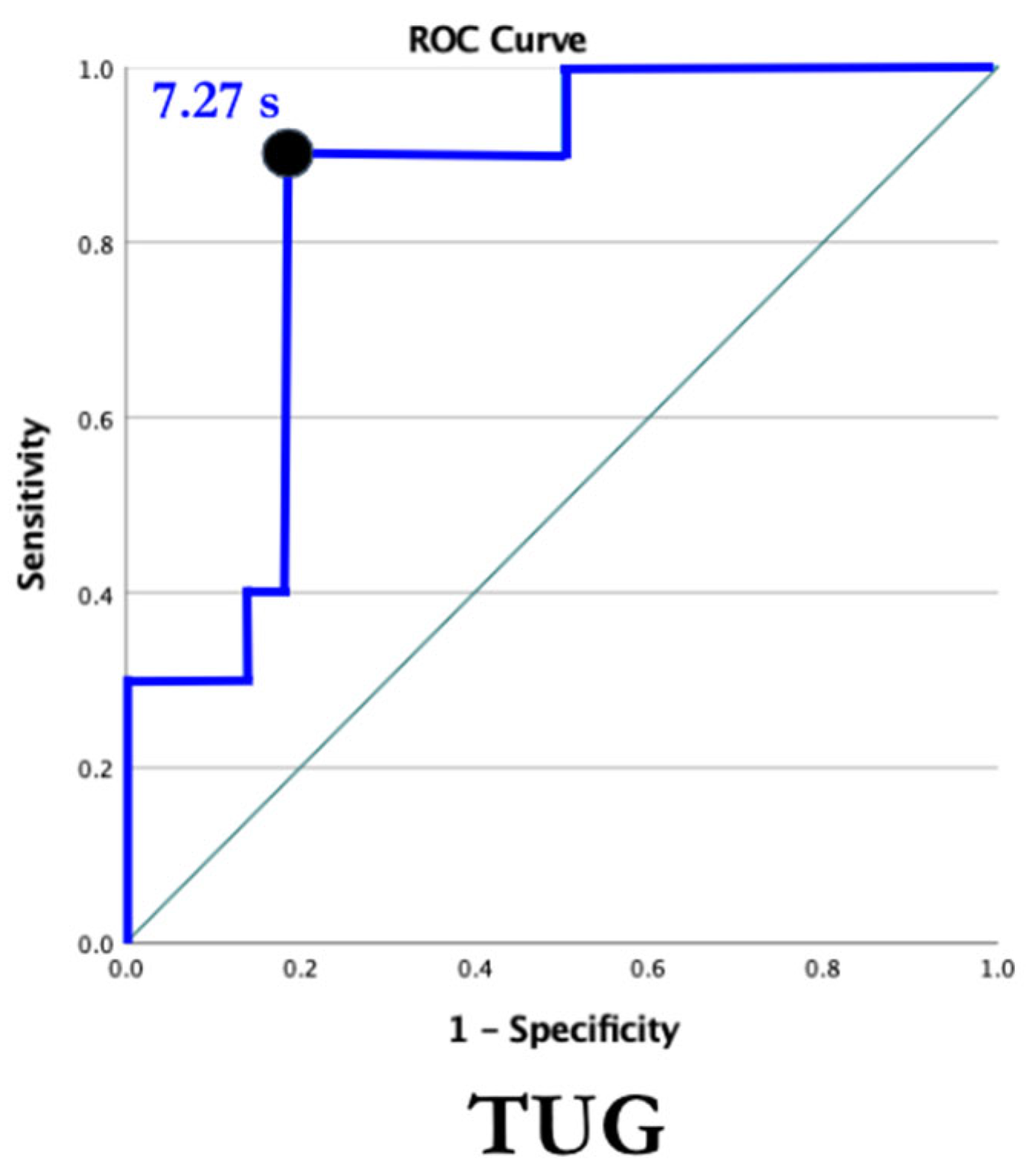
2.2. The Timed Up and Go (TUG)
2.3. The International Physical Activity Questionnaire—Short Form (IPAQ-SF)
2.4. Dual X-ray Absorptiometry (DEXA)
2.5. Statistical Methods
3. Results
4. Discussion
4.1. Six-Minute Walk Test
4.2. Timed Up and Go Test
4.3. Bone Density across the Lifespan
4.4. Extrinsic Mechanisms
4.5. Intrinsic Mechanisms
4.6. Future of BMD in SMA
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Munsat, T.L.; Davies, K.E. International SMA Consortium Meeting (26–28 June 1992, Bonn, Germany). Neuromuscul. Disord. 1992, 2, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.O.; Pardo, C.A. The Neurobiology of Childhood Spinal Muscular Atrophy. Neurobiol. Dis. 1996, 3, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Hjartarson, H.T.; Nathorst-Böös, K.; Sejersen, T. Disease Modifying Therapies for the Management of Children with Spinal Muscular Atrophy (5q SMA): An Update on the Emerging Evidence. Drug Des. Dev. Ther. 2022, 16, 1865–1883. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018, 28, 197–207. [Google Scholar] [CrossRef]
- Montes, J.; Young, S.D.; Mazzone, E.S.; Pasternak, A.; Glanzman, A.M.; Finkel, R.S.; Darras, B.T.; Muntoni, F.; Mercuri, E.; De Vivo, D.C.; et al. Nusinersen improves walking distance and reduces fatigue in later-onset spinal muscular atrophy. Muscle Nerve 2019, 60, 409–414. [Google Scholar] [CrossRef]
- Carter, M.I.; Hinton, P.S. Physical Activity and Bone Health. Mo. Med. 2014, 111, 59–64. [Google Scholar]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and Molecular Regulation of Bone Remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef]
- Robling, A.G.; Turner, C.H. Mechanical Signaling for Bone Modeling and Remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef]
- Baxter-Jones, A.D.; Kontulainen, S.A.; Faulkner, R.A.; Bailey, D.A. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone 2008, 43, 1101–1107. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Olofsson, H.; Jensevik, K.; Larsson, S.; Mallmin, H.; Berglund, L.; Vessby, B.; Melhus, H. Leisure Physical Activity and the Risk of Fracture in Men. PLoS Med. 2007, 4, e199. [Google Scholar] [CrossRef]
- Nilsson, M.; Ohlsson, C.; Mellström, D.; Lorentzon, M. Previous Sport Activity During Childhood and Adolescence Is Associated with Increased Cortical Bone Size in Young Adult Men. J. Bone Miner. Res. 2009, 24, 125–133. [Google Scholar] [CrossRef]
- Kroksmark, A.; Alberg, L.; Tulinius, M.; Magnusson, P.; Söderpalm, A. Low bone mineral density and reduced bone-specific alkaline phosphatase in 5q spinal muscular atrophy type 2 and type 3: A 2-year prospective study of bone health. Acta Paediatr. 2023, 112, 2589–2600. [Google Scholar] [CrossRef]
- Khatri, I.A.; Chaudhry, U.S.; Seikaly, M.G.; Browne, R.H.; Iannaccone, S.T. Low Bone Mineral Density in Spinal Muscular Atrophy. J. Clin. Neuromuscul. Dis. 2008, 10, 11–17. [Google Scholar] [CrossRef]
- Peng, X.; Qu, Y.; Li, X.; Liu, J.; Shan, X.; Wang, J.; Song, F. Bone mineral density and its influencing factors in Chinese children with spinal muscular atrophy types 2 and 3. BMC Musculoskelet. Disord. 2021, 22, 729. [Google Scholar] [CrossRef]
- Kinali, M.; Banks, L.M.; Mercuri, E.; Manzur, A.Y.; Muntoni, F. Bone Mineral Density in a Paediatric Spinal Muscular Atrophy Population. Neuropediatrics 2004, 35, 325–328. [Google Scholar] [CrossRef]
- Montes, J.; Goodwin, A.M.; McDermott, M.P.; Uher, D.; Hernandez, F.M.; Coutts, K.; Cocchi, J.; Hauschildt, M.; Cornett, K.M.; Rao, A.K.; et al. Diminished muscle oxygen uptake and fatigue in spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2021, 8, 1086–1095. [Google Scholar] [CrossRef]
- Young, S.D.; Montes, J.; Kramer, S.S.; Marra, J.; Salazar, R.; Cruz, R.; Chiriboga, C.A.; Garber, C.E.; De Vivo, D.C. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve 2016, 54, 836–842. [Google Scholar] [CrossRef]
- Dunaway, S.; Montes, J.; Garber, C.E.; Carr, B.; Kramer, S.S.; Kamil-Rosenberg, S.; Strauss, N.; Sproule, D.; De Vivo, D.C. Performance of the timed “up & go” test in spinal muscular atrophy. Muscle Nerve 2014, 50, 273–277. [Google Scholar] [CrossRef]
- Alomari, M.A.; Keewan, E.F.; Qhatan, R.; Amer, A.; Khabour, O.F.; Maayah, M.F.; Hurtig-Wennlöf, A. Blood Pressure and Circulatory Relationships with Physical Activity Level in Young Normotensive Individuals: IPAQ Validity and Reliability Considerations. Clin. Exp. Hypertens. 2011, 33, 345–353. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Andries, A.; van Walsem, M.R.; Frich, J.C. Self-reported physical activity in people with limb-girdle muscular dystrophy and Charcot-Marie-Tooth disease in Norway. BMC Musculoskelet. Disord. 2020, 21, 235. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.-L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Montes, J.; McDermott, M.P.; Martens, W.B.; Dunaway, S.; Glanzman, A.M.; Riley, S.; Quigley, J.; Montgomery, M.J.; Sproule, D.; Tawil, R.; et al. Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology 2010, 74, 833–838. [Google Scholar] [CrossRef]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J.; Norms Project Consortium. Reference values for developing responsive functional outcome measures across the lifespan. Neurology 2017, 88, 1512–1519. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef]
- Sezer, A.; Altan, L.; Özdemir, Ö. Multiple Comparison of Age Groups in Bone Mineral Density under Heteroscedasticity. BioMed. Res. Int. 2015, 2015, 426847. [Google Scholar] [CrossRef]
- Baranello, G.; Vai, S.; Broggi, F.; Masson, R.; Arnoldi, M.T.; Zanin, R.; Mastella, C.; Bianchi, M.L. Evolution of bone mineral density, bone metabolism and fragility fractures in Spinal Muscular Atrophy (SMA) types 2 and 3. Neuromuscul. Disord. 2019, 29, 525–532. [Google Scholar] [CrossRef]
- Ribstein, C.; Courteix, D.; Rabiau, N.; Bommelaer, C.; Bourdeau, Y.; Pereira, B.; Sarret, C. Secondary Bone Defect in Neuromuscular Diseases in Childhood: A Longitudinal “Muscle-Bone Unit” Analysis. Neuropediatrics 2018, 49, 397–400. [Google Scholar] [CrossRef]
- Wasserman, H.M.; Hornung, L.N.; Stenger, P.J.; Rutter, M.M.; Wong, B.L.; Rybalsky, I.; Khoury, J.C.; Kalkwarf, H.J. Low bone mineral density and fractures are highly prevalent in pediatric patients with spinal muscular atrophy regardless of disease severity. Neuromuscul. Disord. 2017, 27, 331–337. [Google Scholar] [CrossRef]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef]
- Kurihara, N.; Menaa, C.; Maeda, H.; Haile, D.J.; Reddy, S.V. Osteoclast-stimulating Factor Interacts with the Spinal Muscular Atrophy Gene Product to Stimulate Osteoclast Formation. J. Biol. Chem. 2001, 276, 41035–41039. [Google Scholar] [CrossRef]
- Hensel, N.; Brickwedde, H.; Tsaknakis, K.; Grages, A.; Braunschweig, L.; Lüders, K.A.; Lorenz, H.M.; Lippross, S.; Walter, L.M.; Tavassol, F.; et al. Altered bone development with impaired cartilage formation precedes neuromuscular symptoms in spinal muscular atrophy. Hum. Mol. Genet. 2020, 29, 2662–2673. [Google Scholar] [CrossRef]
- Shanmugarajan, S.; Tsuruga, E.; Swoboda, K.J.; Maria, B.L.; Ries, W.L.; Reddy, S.V. Bone loss in survival motor neuron (Smn−/−SMN2) genetic mouse model of spinal muscular atrophy. J. Pathol. 2009, 219, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Vai, S.; Bianchi, M.L.; Moroni, I.; Mastella, C.; Broggi, F.; Morandi, L.; Arnoldi, M.T.; Bussolino, C.; Baranello, G. Bone and Spinal Muscular Atrophy. Bone 2015, 79, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.W.; Roach, K.E.; Nithman, R.W.; Betz, S.R.; Lindsey, C.; Fuchs, R.K.; Avin, K.G. Physical Therapist Management of Patients with Suspected or Confirmed Osteoporosis: A Clinical Practice Guideline From the Academy of Geriatric Physical Therapy. J. Geriatr. Phys. Ther. 2022, 44, E106–E119. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Vestergaard, P.; Glerup, H.; Steffensen, B.F.; Rejnmark, L.; Rahbek, J.; Mosekilde, L. Fracture risk in patients with muscular dystrophy and spinal muscular atrophy. J. Rehabilitation Med. 2001, 33, 150–155. [Google Scholar] [CrossRef]
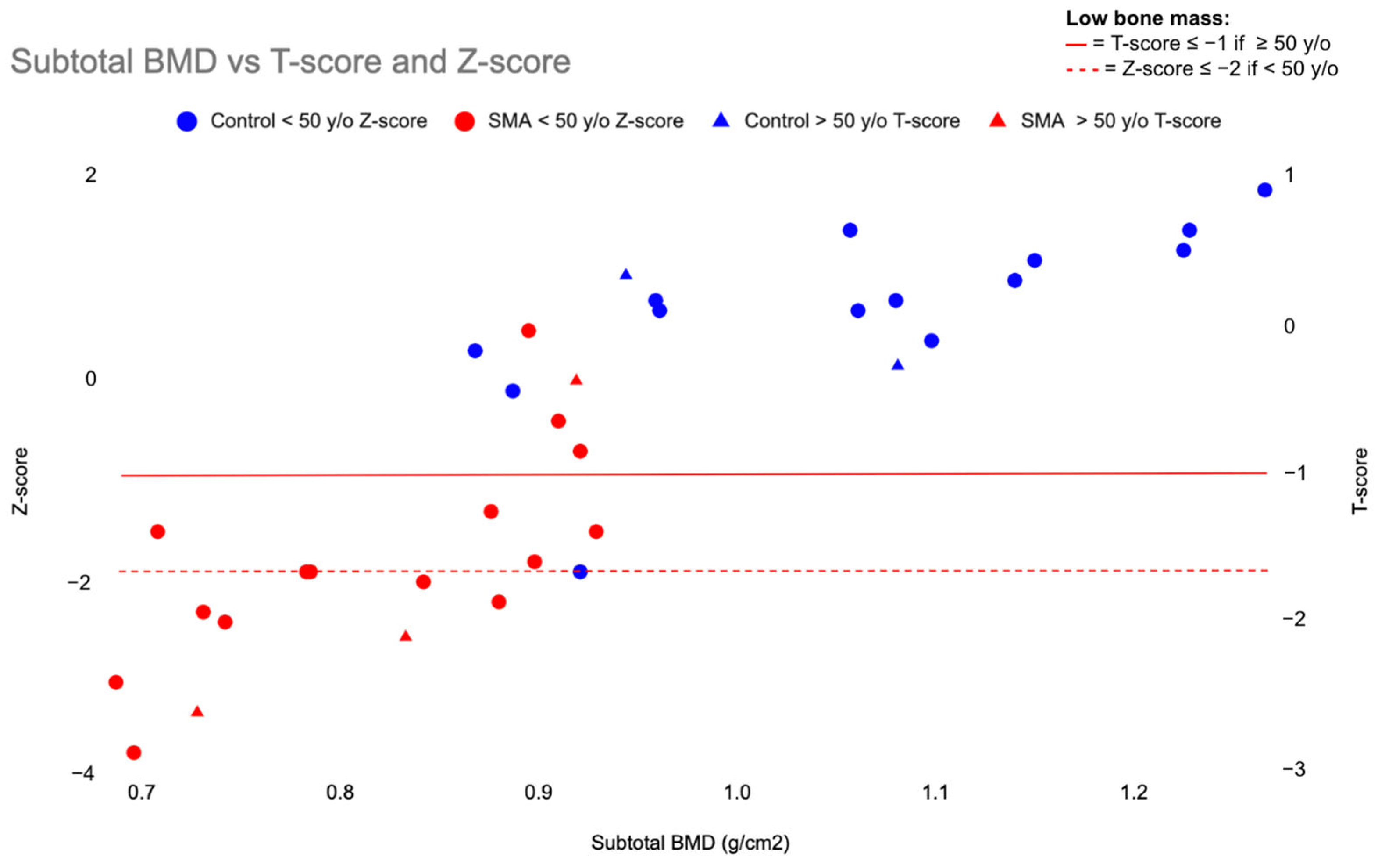
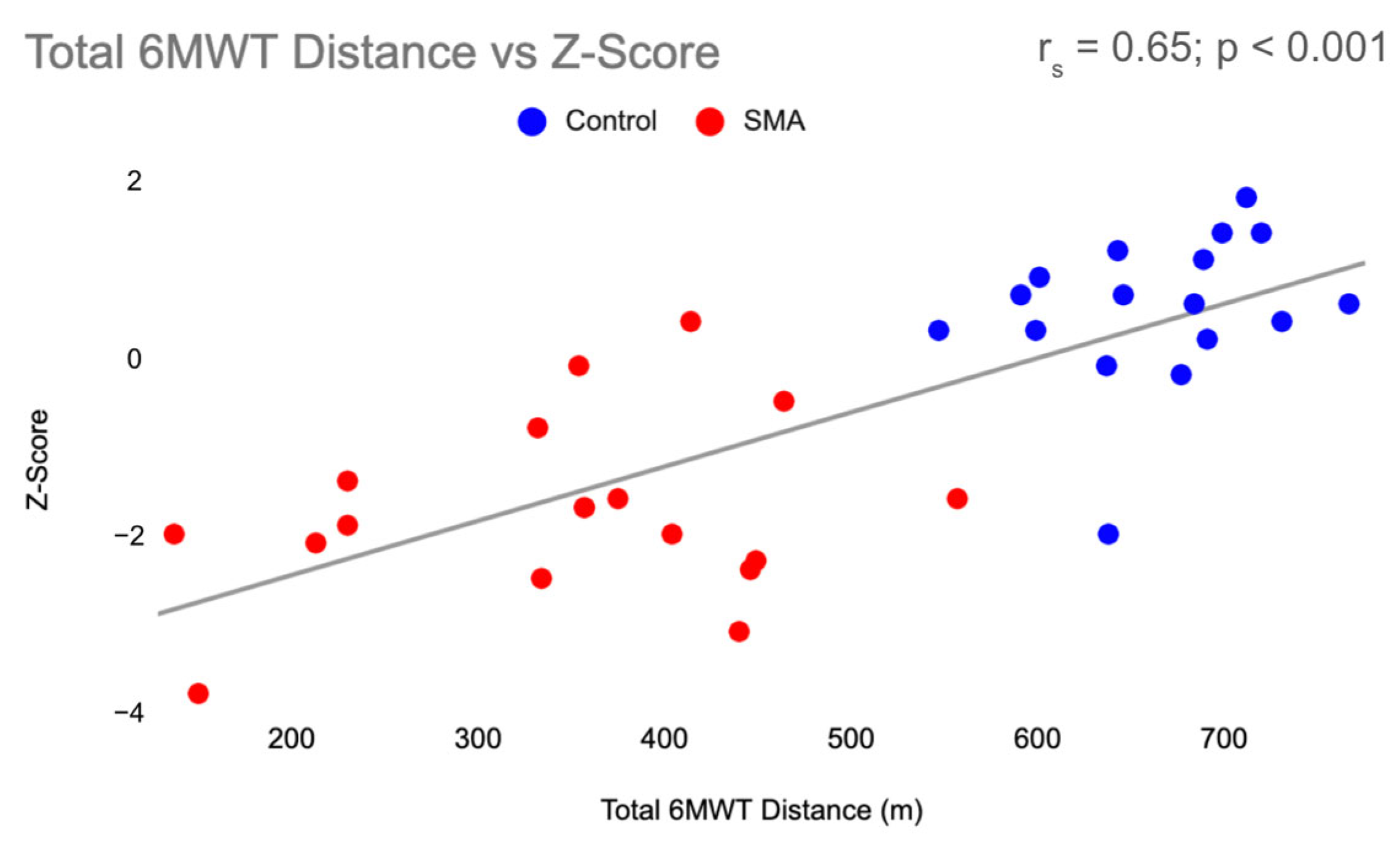

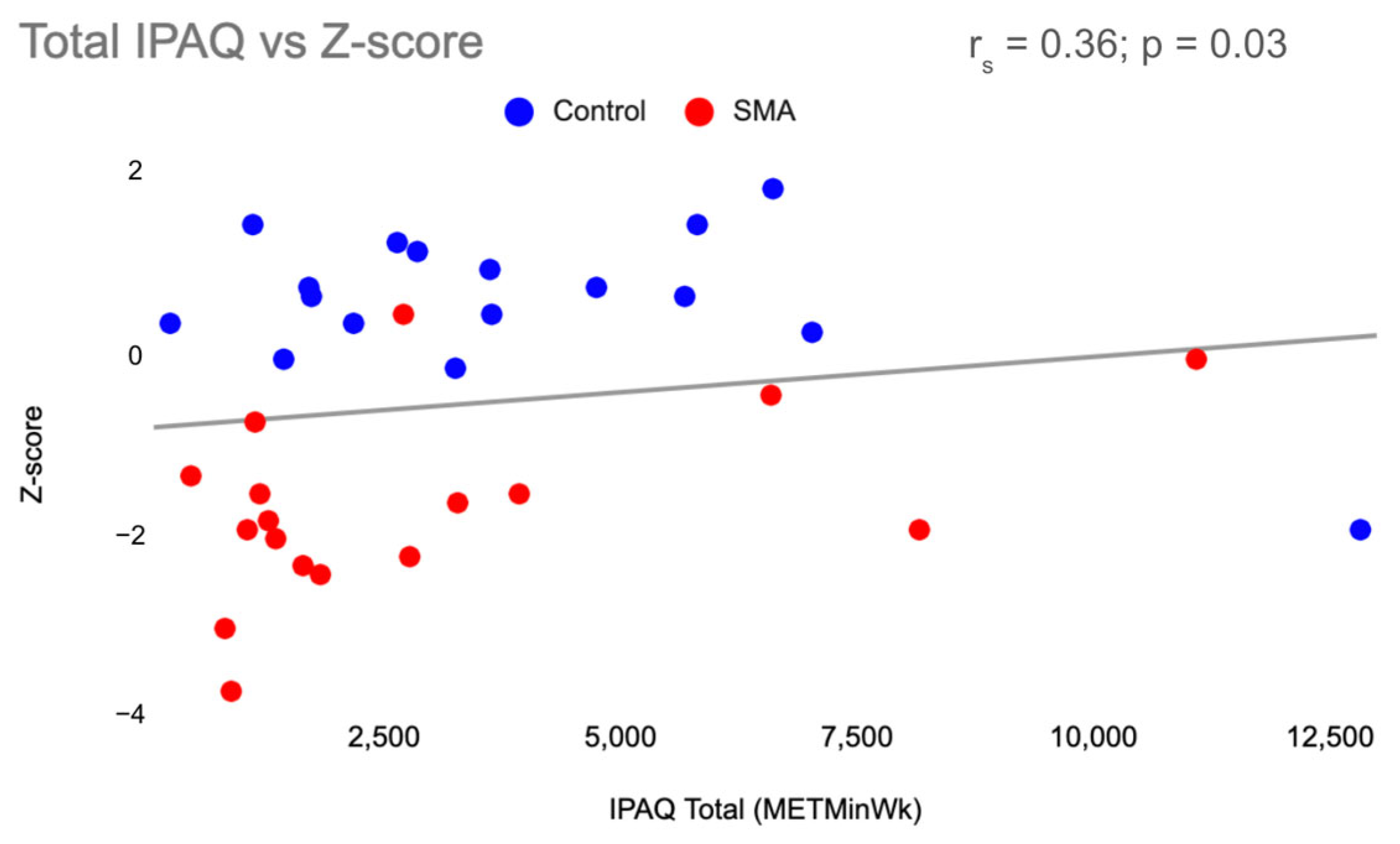
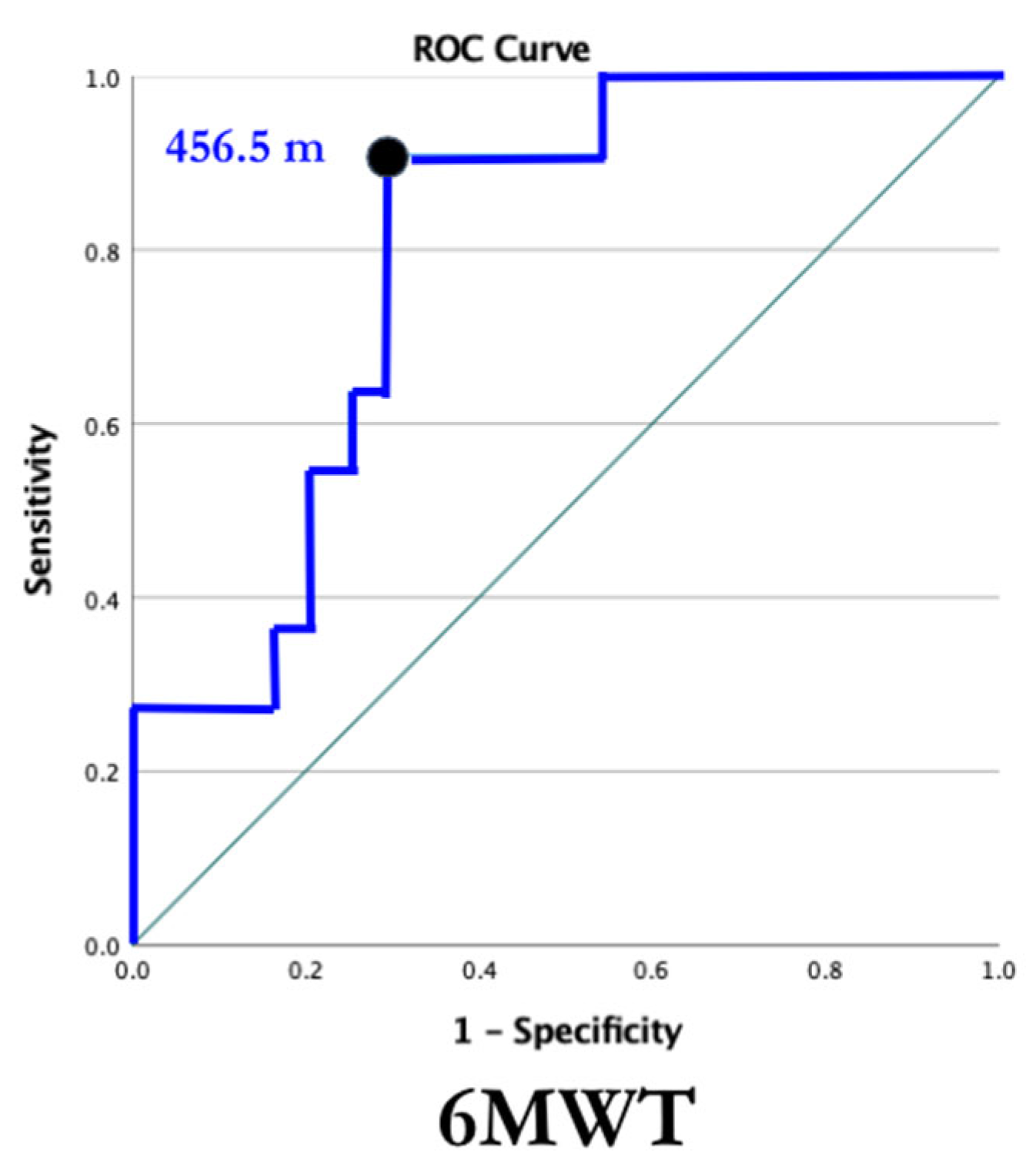
| Participant Characteristics | SMA (n = 19) Mean + SD | Control (n = 16) Mean + SD |
|---|---|---|
| Age (years) | 32.9 ± 15.4 | 28.1 ± 14.4 |
| Sex (% male) | 73.7% | 81.3% |
| Nusinersen Duration (years) | 1.0 ± 0.3 | - |
| Vitamin D Supplement (n) | 2 | 0 |
| Medication for Osteoporosis (n) | 1 | 0 |
| 6MWT Distance (meters) | 342.1 ± 113.5 | 670.3 ± 50.8 |
| TUG Time (seconds) | 21.3 ± 19.9 | 4.3 ± 0.7 |
| Physical Activity: IPAQ-SF Total 1 (METmin/week) | 1644.0 (2875.0) | 3437.3 (3934.4) |
| Physical Activity: IPAQ-SF Sitting Time 1 (METmin/week) | 480.0 (360.0) | 420.0 (120.0) |
| DXA Variable | SMA Mean + SD | Control Mean + SD | p-Value |
|---|---|---|---|
| Subtotal BMD (g/cm2) | 0.8194 ± 0.0900 | 1.0579 ± 0.1249 | <0.001 |
| T-score | −2.159 ± 1.2289 | −0.025 ± 1.3128 | <0.001 |
| Z-score | −1.729 ± 1.0475 | 0.563 ± 0.8793 | <0.001 |
| Left Arm BMD (g/cm2) | 0.7016 ± 0.0890 | 0.8303 ± 0.1125 | <0.001 |
| Right Arm BMD (g/cm2) | 0.7070 ± 0.0859 | 0.8260 ± 0.0926 | <0.001 |
| Thoracic Spine BMD (g/cm2) | 0.8269 ± 0.1287 | 0.8976 ± 0.1542 | 0.18 |
| Lumbar Spine BMD (g/cm2) | 1.0255 ± 0.1392 | 1.0916 ± 0.1780 | 0.37 |
| Left Leg BMD (g/cm2) | 0.9292 ± 0.1068 | 1.2818 ± 0.1689 | <0.001 |
| Right Leg BMD (g/cm2) | 0.9214 ± 0.0979 | 1.280 ± 0.1753 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trancho, C.; Stickney, B.; Kinirons, S.; Uher, D.; Kanner, C.H.; Rao, A.K.; McDermott, M.P.; Garber, C.E.; De Vivo, D.C.; Montes, J. Low Bone Mass in Ambulatory Spinal Muscular Atrophy: A Proactive Approach for an Often-Overlooked Impairment. J. Clin. Med. 2024, 13, 1336. https://doi.org/10.3390/jcm13051336
Trancho C, Stickney B, Kinirons S, Uher D, Kanner CH, Rao AK, McDermott MP, Garber CE, De Vivo DC, Montes J. Low Bone Mass in Ambulatory Spinal Muscular Atrophy: A Proactive Approach for an Often-Overlooked Impairment. Journal of Clinical Medicine. 2024; 13(5):1336. https://doi.org/10.3390/jcm13051336
Chicago/Turabian StyleTrancho, Caitlin, Bailey Stickney, Stacy Kinirons, David Uher, Cara H. Kanner, Ashwini K. Rao, Michael P. McDermott, Carol Ewing Garber, Darryl C. De Vivo, and Jacqueline Montes. 2024. "Low Bone Mass in Ambulatory Spinal Muscular Atrophy: A Proactive Approach for an Often-Overlooked Impairment" Journal of Clinical Medicine 13, no. 5: 1336. https://doi.org/10.3390/jcm13051336
APA StyleTrancho, C., Stickney, B., Kinirons, S., Uher, D., Kanner, C. H., Rao, A. K., McDermott, M. P., Garber, C. E., De Vivo, D. C., & Montes, J. (2024). Low Bone Mass in Ambulatory Spinal Muscular Atrophy: A Proactive Approach for an Often-Overlooked Impairment. Journal of Clinical Medicine, 13(5), 1336. https://doi.org/10.3390/jcm13051336






