Evaluation of Neuromuscular Diseases and Complaints by Quantitative Muscle MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Assessments
2.3. MRI Acquisition and Processing
2.4. Statistical Analysis
3. Results
3.1. Fat Fraction

3.2. Water T2 Relaxation Time
| LGMDR1 | IBM | LOPD | PCC | CON | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Thigh | FF [%] | 56.2 ± 23.7 | 26.6 ± 12.3 | 22.2 ± 7.3 | 5.8 ± 1.2 | 4.8 ± 0.4 |
| T2 [ms] | 27.6 ± 2.9 | 31.8 ± 1.4 | 30.3 ± 1.0 | 28.9 ± 0.9 | 29.4 ± 0.6 | |
| FA | 0.35 ± 0.07 | 0.26 ± 0.02 | 0.24 ± 0.05 | 0.22 ± 0.02 | 0.21 ± 0.01 | |
| MD [10−3 m/s2] | 1.08 ± 0.25 | 1.40 ± 0.11 | 1.50 ± 0.08 | 1.51 ± 0.04 | 1.54 ± 0.03 | |
| Leg | FF [%] | 50.0 ± 18.0 | 16.8 ± 15.0 | 5.7 ± 3.4 | 5.0 ± 1.0 | 4.4 ± 0.7 |
| T2 [ms] | 29.5 ± 1.8 | 31.5 ± 0.6 | 31.0 ± 0.8 | 29.9 ± 0.9 | 30.3 ± 0.7 | |
| FA | 0.29 ± 0.07 | 0.24 ± 0.03 | 0.23 ± 0.04 | 0.21 ± 0.01 | 0.21 ± 0.01 | |
| MD [10−3 m/s2] | 1.22 ± 0.20 | 1.48 ± 0.13 | 1.58 ± 0.09 | 1.50 ± 0.04 | 1.52 ± 0.04 |
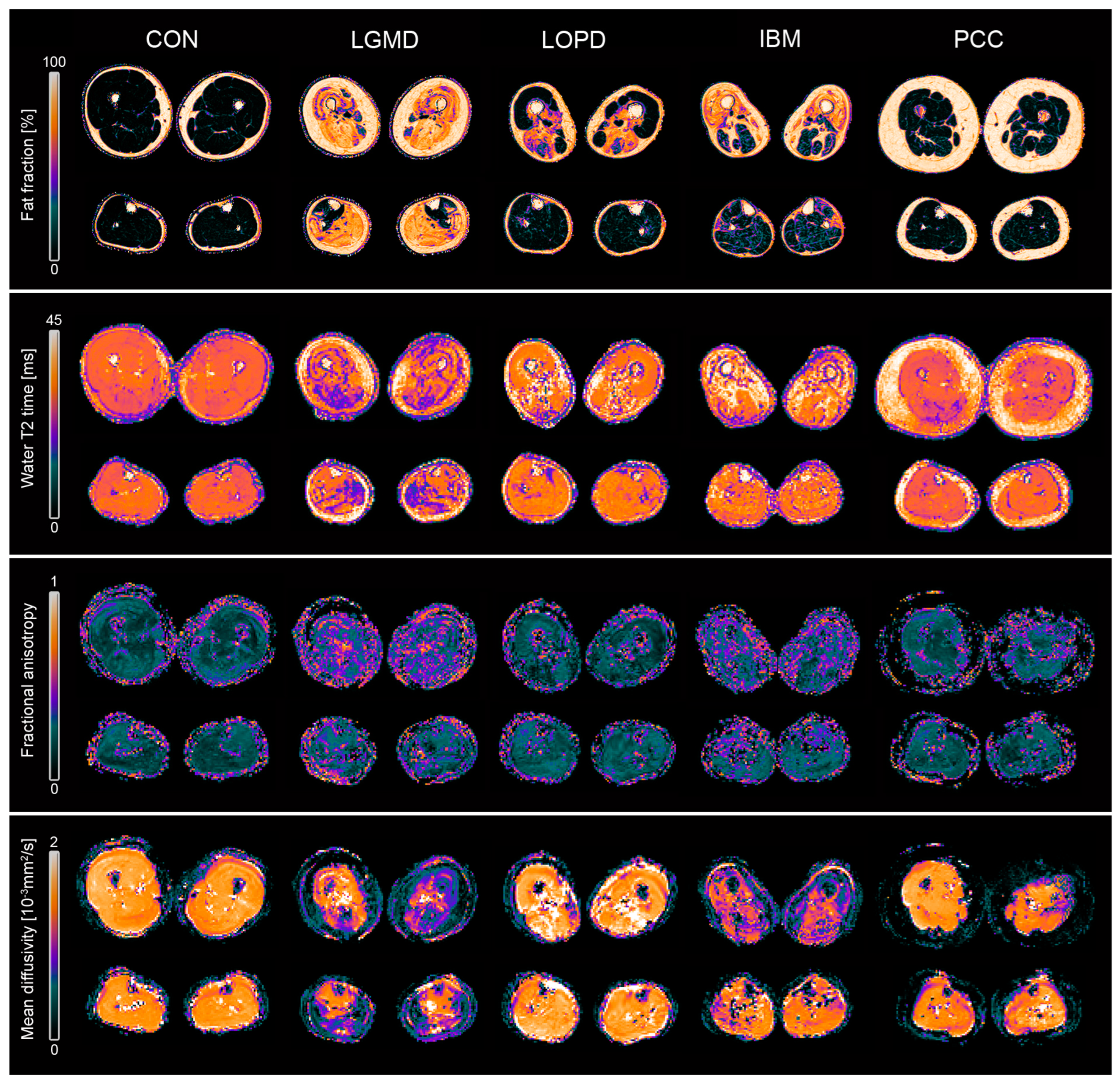
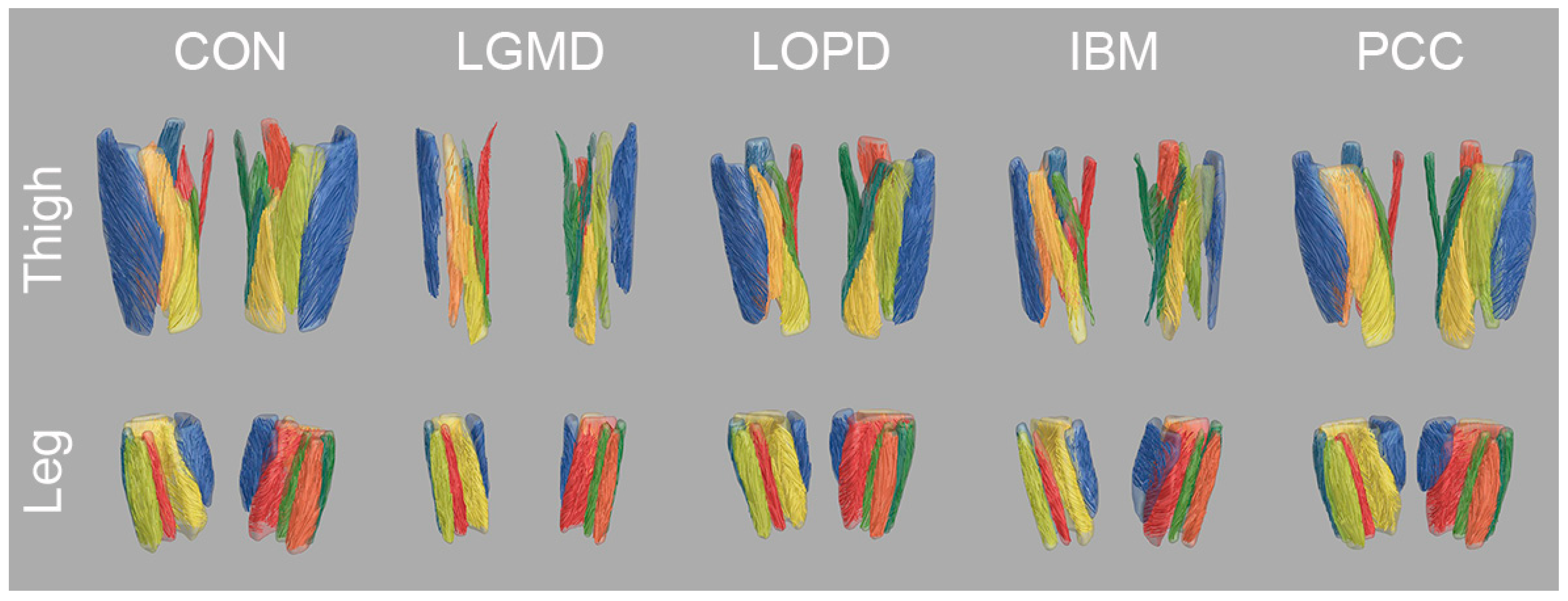
3.3. Diffusion Metrics
3.4. Correlation with Clinical Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenberg, S.A. Inclusion body myositis: Clinical features and pathogenesis. Nat. Rev. Rheumatol. 2019, 15, 257–272. [Google Scholar] [CrossRef]
- Hightower, R.M.; Alexander, M.S. Genetic modifiers of Duchenne and facioscapulohumeral muscular dystrophies. Muscle Nerve 2018, 57, 6–15. [Google Scholar] [CrossRef]
- Angelini, C.; Burlina, A.; Blau, N.; Ferreira, C.R. Clinical and biochemical footprints of inherited metabolic disorders: X. Metabolic myopathies. Mol. Genet. Metab. 2022, 137, 213–222. [Google Scholar] [CrossRef]
- Pasnoor, M.; Barohn, R.J.; Dimachkie, M.M. Toxic Myopathies. Neurol. Clin. 2014, 32, 647–670. [Google Scholar] [CrossRef]
- Jacob, S.; Kapadia, R.; Soule, T.; Luo, H.; Schellenberg, K.L.; Douville, R.N.; Pfeffer, G. Neuromuscular Complications of SARS-CoV-2 and Other Viral Infections. Front. Neurol. 2022, 13, 914411. [Google Scholar] [CrossRef] [PubMed]
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain Commun. 2022, 4, fcab297. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021, 3099, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Balcom, E.F.; Nath, A.; Power, C. Acute and chronic neurological disorders in COVID-19: Potential mechanisms of disease. Brain 2021, 144, 3576–3588. [Google Scholar] [CrossRef]
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal muscular atrophy. Nat. Rev. Dis. Prim. 2022, 8, 52. [Google Scholar] [CrossRef]
- Dahlqvist, J.R.; Widholm, P.; Leinhard, O.D.; Vissing, J. MRI in Neuromuscular Diseases: An Emerging Diagnostic Tool and Biomarker for Prognosis and Efficacy. Ann. Neurol. 2020, 88, 669–681. [Google Scholar] [CrossRef]
- Roy, B.; Lucchini, M.; Lilleker, J.B.; Goyal, N.A.; Naddaf, E.; Adler, B.; Alfano, L.N.; Malandraki, G.A.; Focht Garand, K.L.; Mochel, D.; et al. Current status of clinical outcome measures in inclusion body myositis: A systematised review. Clin. Exp. Rheumatol. 2023, 41, 370–378. [Google Scholar] [CrossRef]
- Barp, A.; Ferrero, A.; Casagrande, S.; Morini, R.; Zuccarino, R. Circulating Biomarkers in Neuromuscular Disorders: What Is Known, What Is New. Biomolecules 2021, 11, 1246. [Google Scholar] [CrossRef]
- Vincenten, S.C.C.; Mul, K.; van As, D.; Jansen, J.J.; Heskamp, L.; Heerschap, A.; van Engelen, B.G.M.; Voermans, N.C. Five-year follow-up study on quantitative muscle magnetic resonance imaging in facioscapulohumeral muscular dystrophy: The link to clinical outcome. J. Cachexia. Sarcopenia Muscle 2023, 14, 1695–1706. [Google Scholar] [CrossRef]
- Marty, B.; Baudin, P.-Y.; Caldas de Almeida Araujo, E.; Fromes, Y.; Wahbi, K.; Reyngoudt, H. Assessment of Extracellular Volume Fraction in Becker Muscular Dystrophy by Using MR Fingerprinting. Radiology 2023, 307, e221115. [Google Scholar] [CrossRef]
- Forsting, J.; Rohm, M.; Froeling, M.; Güttsches, A.K.; Südkamp, N.; Roos, A.; Vorgerd, M.; Schlaffke, L.; Rehmann, R. Quantitative muscle MRI captures early muscle degeneration in calpainopathy. Sci. Rep. 2022, 12, 19676. [Google Scholar] [CrossRef]
- De Wel, B.; Huysmans, L.; Peeters, R.; Goosens, V.; Ghysels, S.; Byloos, K.; Putzeys, G.; D’Hondt, A.; De Bleecker, J.L.; Dupont, P.; et al. Prospective Natural History Study in 24 Adult Patients with LGMDR12 over 2 Years of Follow-up: Quantitative MRI and Clinical Outcome Measures. Neurology 2022, 99, E638–E649. [Google Scholar] [CrossRef]
- Alonso-Jiménez, A.; Nuñez-Peralta, C.; Montesinos, P.; Alonso-Pérez, J.; García, C.; Montiel, E.; Belmonte, I.; Pedrosa, I.; Segovia, S.; Llauger, J.; et al. Different Approaches to Analyze Muscle Fat Replacement with Dixon MRI in Pompe Disease. Front. Neurol. 2021, 12, 675781. [Google Scholar] [CrossRef]
- Burakiewicz, J.; Sinclair, C.D.J.; Fischer, D.; Walter, G.A.; Kan, H.E.; Hollingsworth, K.G. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J. Neurol. 2017, 264, 2053–2067. [Google Scholar] [CrossRef]
- Veeger, T.T.J.; van de Velde, N.M.; Keene, K.R.; Niks, E.H.; Hooijmans, M.T.; Webb, A.G.; de Groot, J.H.; Kan, H.E. Baseline fat fraction is a strong predictor of disease progression in Becker muscular dystrophy. NMR Biomed. 2022, 35, e4691. [Google Scholar] [CrossRef]
- Wang, F.; Fang, S.; Li, J.; Yuan, L.; Hou, B.; Zhu, J.; Jiao, Y.; Liu, Z.; Qian, M.; Santini, F.; et al. Correlation analysis of quantitative MRI measurements of thigh muscles with histopathology in patients with idiopathic inflammatory myopathy. Eur. Radiol. Exp. 2023, 7, 51. [Google Scholar] [CrossRef]
- Schlaeger, S.; Weidlich, D.; Zoffl, A.; Becherucci, E.A.; Kottmaier, E.; Montagnese, F.; Deschauer, M.; Schoser, B.; Zimmer, C.; Baum, T.; et al. Beyond mean value analysis—A voxel-based analysis of the quantitative MR biomarker water T2 in the presence of fatty infiltration in skeletal muscle tissue of patients with neuromuscular diseases. NMR Biomed. 2022, 35, e4805. [Google Scholar] [CrossRef] [PubMed]
- Martín-Noguerol, T.; Barousse, R.; Wessell, D.E.; Rossi, I.; Luna, A. Clinical applications of skeletal muscle diffusion tensor imaging. Skeletal Radiol. 2023, 52, 1639–1649. [Google Scholar] [CrossRef]
- Cameron, D.; Reiter, D.A.; Adelnia, F.; Ubaida-Mohien, C.; Bergeron, C.M.; Choi, S.; Fishbein, K.W.; Spencer, R.G.; Ferrucci, L. Age-related changes in human skeletal muscle microstructure and architecture assessed by diffusion-tensor magnetic resonance imaging and their association with muscle strength. Aging Cell 2023, 22, e13851. [Google Scholar] [CrossRef]
- Cameron, D.; Abbassi-Daloii, T.; Heezen, L.G.M.; van de Velde, N.M.; Koeks, Z.; Veeger, T.T.J.; Hooijmans, M.T.; el Abdellaoui, S.; van Duinen, S.G.; Verschuuren, J.J.G.M.; et al. Diffusion-tensor magnetic resonance imaging captures increased skeletal muscle fibre diameters in Becker muscular dystrophy. J. Cachexia. Sarcopenia Muscle 2023, 14, 1546–1557. [Google Scholar] [CrossRef]
- Hooijmans, M.T.; Damon, B.M.; Froeling, M.; Versluis, M.J.; Burakiewicz, J.; Verschuuren, J.J.G.M.; Niks, E.H.; Webb, A.G.; Kan, H.E. Evaluation of skeletal muscle DTI in patients with duchenne muscular dystrophy. NMR Biomed. 2015, 28, 1589–1597. [Google Scholar] [CrossRef]
- Aeles, J.; Bolsterlee, B.; Kelp, N.Y.; Dick, T.J.M.; Hug, F. Regional variation in lateral and medial gastrocnemius muscle fibre lengths obtained from diffusion tensor imaging. J. Anat. 2022, 240, 131–144. [Google Scholar] [CrossRef]
- Bolsterlee, B.; Souza, A.D.; Herbert, R.D. Reliability and robustness of muscle architecture measurements obtained using diffusion tensor imaging with anatomically constrained tractography. J. Biomech. 2019, 86, 71–78. [Google Scholar] [CrossRef]
- Chianca, V.; Vincenzo, B.; Cuocolo, R.; Zappia, M.; Guarino, S.; Di Pietto, F.; Del Grande, F. MRI Quantitative Evaluation of Muscle Fatty Infiltration. Magnetochemistry 2023, 9, 111. [Google Scholar] [CrossRef]
- Schlaffke, L.; Rehmann, R.; Rohm, M.; Otto, L.A.M.; De Luca, A.; Burakiewicz, J.; Baligand, C.; Monte, J.; den Harder, C.; Hooijmans, M.T.; et al. Multi-center evaluation of stability and reproducibility of quantitative MRI measures in healthy calf muscles. NMR Biomed. 2019, 32, e4119. [Google Scholar] [CrossRef] [PubMed]
- Van Capelle, C.I.; Van Der Beek, N.A.M.E.; De Vries, J.M.; Van Doorn, P.A.; Duivenvoorden, H.J.; Leshner, R.T.; Hagemans, M.L.C.; Van Der Ploeg, A.T. The quick motor function test: A new tool to rate clinical severity and motor function in Pompe patients. J. Inherit. Metab. Dis. 2012, 35, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Froeling, M. QMRTools: A Mathematica toolbox for quantitative MRI analysis. J. Open Source Softw. 2019, 4, 1204. [Google Scholar] [CrossRef]
- Reeder, S.B.; Pineda, A.R.; Wen, Z.; Shimakawa, A.; Yu, H.; Brittain, J.H.; Gold, G.E.; Beaulieu, C.H.; Pelc, N.J. Iterative Decomposition of Water and Fat With Echo Asymmetry and Least-Squares Estimation (IDEAL): Application with Fast Spin-Echo Imaging. Magn. Reson. Med. 2005, 644, 636–644. [Google Scholar] [CrossRef]
- Marty, B.; Baudin, P.; Reyngoudt, H.; Azzabou, N.; Araujo, E.C.A.; Carlier, P.G.; de Sousa, P.L. Simultaneous muscle water T2 and fat fraction mapping using transverse relaxometry with stimulated echo compensation. NMR Biomed. 2016, 29, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Leemans, A.; Jones, D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009, 61, 1336–1349. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-Aron, B.; Sijbers, J.; Fieremans, E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016, 142, 394–406. [Google Scholar] [CrossRef]
- Veraart, J.; Sijbers, J.; Sunaert, S.; Leemans, A.; Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 2013, 81, 335–346. [Google Scholar] [CrossRef]
- Rohm, M.; Markmann, M.; Forsting, J.; Rehmann, R.; Froeling, M.; Schlaffke, L. 3D Automated Segmentation of Lower Leg Muscles Using Machine Learning on a Heterogeneous Dataset. Diagnostics 2021, 11, 1747. [Google Scholar] [CrossRef]
- Klein, S.; Staring, M.; Murphy, K.; Viergever, M.A.; Pluim, J. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Trans. Med. Imaging 2010, 29, 196–205. [Google Scholar] [CrossRef]
- Froeling, M.; Tax, C.M.W.; Vos, S.B.; Luijten, P.R.; Leemans, A. “MASSIVE” brain dataset: Multiple acquisitions for standardization of structural imaging validation and evaluation. Magn. Reson. Med. 2017, 77, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Forsting, J.; Rehmann, R.; Froeling, M.; Vorgerd, M.; Tegenthoff, M.; Schlaffke, L. Diffusion tensor imaging of the human thigh: Consideration of DTI-based fiber tracking stop criteria. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Barp, A.; Laforet, P.; Bello, L.; Tasca, G.; Vissing, J.; Monforte, M.; Ricci, E.; Choumert, A.; Stojkovic, T.; Malfatti, E.; et al. European muscle MRI study in limb girdle muscular dystrophy type R1/2A (LGMDR1/LGMD2A). J. Neurol. 2020, 267, 45–56. [Google Scholar] [CrossRef]
- Morishima, R.; Schoser, B. A Straightforward Approach to Analyze Skeletal Muscle MRI in Limb-Girdle Muscular Dystrophy for Differential Diagnosis: A Systematic Review. Muscles 2023, 2, 374–388. [Google Scholar] [CrossRef]
- Cox, F.M.; Reijnierse, M.; van Rijswijk, C.S.P.; Wintzen, A.R.; Verschuuren, J.J.; Badrising, U.A. Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology 2011, 50, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Ansari, B.; Salort-Campana, E.; Ogier, A.; Le Troter PhD, A.; De Sainte Marie, B.; Guye, M.; Delmont, E.; Grapperon, A.M.; Verschueren, A.; Bendahan, D.; et al. Quantitative muscle MRI study of patients with sporadic inclusion body myositis. Muscle Nerve 2020, 61, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.M.; Sinclair, C.D.J.; Fischmann, A.; Machado, P.M.; Reilly, M.M.; Yousry, T.A.; Thornton, J.S.; Hanna, M.G. MRI biomarker assessment of neuromuscular disease progression: A prospective observational cohort study. Lancet Neurol. 2016, 15, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Bonaparte, S.; Llauger, J.; Segovia, S.; Belmonte, I.; Pedrosa, I.; Montiel, E.; Montesinos, P.; Sánchez-González, J.; Alonso-Jiménez, A.; Gallardo, E.; et al. Quantitative muscle MRI to follow up late onset Pompe patients: A prospective study. Sci. Rep. 2018, 8, 10898. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, R.; Froeling, M.; Rohm, M.; Forsting, J.; Kley, R.A.; Schmidt-Wilcke, T.; Karabul, N.; Tegenthoff, M.; Vorgerd, M.; Schlaffke, L. Muscle Diffusion tensor imaging reveals changes in non-fat infiltrated muscles in late-onset Pompe disease. Muscle Nerve 2020, 62, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Reyngoudt, H.; Smith, F.E.; Caldas de Almeida Araújo, E.; Wilson, I.; Fernández-Torrón, R.; James, M.K.; Moore, U.R.; Díaz-Manera, J.; Marty, B.; Azzabou, N.; et al. Three-year quantitative magnetic resonance imaging and phosphorus magnetic resonance spectroscopy study in lower limb muscle in dysferlinopathy. J. Cachexia. Sarcopenia Muscle 2022, 13, 1850–1863. [Google Scholar] [CrossRef]
- Schlaeger, S.; Weidlich, D.; Klupp, E.; Montagnese, F.; Deschauer, M.; Schoser, B.; Bublitz, S.; Ruschke, S.; Zimmer, C.; Rummeny, E.J.; et al. Decreased water T2 in fatty infiltrated skeletal muscles of patients with neuromuscular diseases. NMR Biomed. 2019, 32, e4111. [Google Scholar] [CrossRef]
- Carlier, P.G.; Marty, B.; Scheidegger, O.; De Sousa, P.L.; Baudin, P.Y.; Snezhko, E.; Vlodavets, D. Skeletal Muscle Quantitative Nuclear Magnetic Resonance Imaging and Spectroscopy as an Outcome Measure for Clinical Trials. J. Neuromuscul. Dis. 2016, 3, 1–28. [Google Scholar] [CrossRef]
- Locher, N.; Wagner, B.; Balsiger, F.; Scheidegger, O. Quantitative water T2 relaxometry in the early detection of neuromuscular diseases: A retrospective biopsy-controlled analysis. Eur. Radiol. 2022, 32, 7910–7917. [Google Scholar] [CrossRef]
- Enax-Krumova, E.; Forsting, J.; Rohm, M.; Schwenkreis, P.; Tegenthoff, M.; Meyer-Frießem, C.H.; Schlaffke, L. Quantitative muscle magnetic resonance imaging depicts microstructural abnormalities but no signs of inflammation or dystrophy in post-COVID-19 condition. Eur. J. Neurol. 2023, 30, 970–981. [Google Scholar] [CrossRef]
- Carlier, P.G.; Azzabou, N.; De Sousa, P.L.; Hicks, A.; Boisserie, J.-M.; Amadon, A.; Carlier, R.-Y.; Wary, C.; Orlikowski, D.; Laforêt, P. Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. J. Inherit. Metab. Dis. 2015, 38, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, O.; Stenzel, W.; Hilton-Jones, D.; Sandri, M.; Boyer, O.; van Engelen, B.G.M. Amyloid deposits and inflammatory infiltrates in sporadic inclusion body myositis: The inflammatory egg comes before the degenerative chicken. Acta Neuropathol. 2015, 129, 611–624. [Google Scholar] [CrossRef]
- Otto, L.A.M.; van der Pol, W.L.; Schlaffke, L.; Wijngaarde, C.A.; Stam, M.; Wadman, R.I.; Cuppen, I.; van Eijk, R.P.A.; Asselman, F.-L.; Bartels, B.; et al. Quantitative MRI of skeletal muscle in a cross-sectional cohort of patients with spinal muscular atrophy types 2 and 3. NMR Biomed. 2020, 33, e4357. [Google Scholar] [CrossRef]
- Williams, S.E.; Heemskerk, A.M.; Welch, E.B.; Li, K.; Damon, B.M.; Park, J.H. Quantitative effects of inclusion of fat on muscle diffusion tensor MRI measurements. J. Magn. Reson. Imaging 2013, 38, 1292–1297. [Google Scholar] [CrossRef]
- Berry, D.B.; Regner, B.; Galinsky, V.; Ward, S.R.; Frank, L.R. Relationships Between Tissue Microstructure and the Diffusion Tensor in Simulated Skeletal Muscle. Magn. Reson. Med. 2018, 80, 317–329. [Google Scholar] [CrossRef]
- Rohm, M.; Russo, G.; Helluy, X.; Froeling, M.; Umathum, V.; Südkamp, N.; Manahan-Vaughan, D.; Rehmann, R.; Forsting, J.; Jacobsen, F.; et al. Muscle diffusion MRI correlates with autophagic buildup in a Pompe disease mouse model. Sci. Rep. 2023, 13, 22822. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, S.P.; Palmer, J.; Wagner, K.R.; Abdel-Hamid, H.Z.; Bertini, E.; Tian, C.; Mah, J.K.; Kostera-Pruszczyk, A.; Muntoni, F.; Guglieri, M.; et al. Quantitative magnetic resonance imaging measures as biomarkers of disease progression in boys with Duchenne muscular dystrophy: A phase 2 trial of domagrozumab. J. Neurol. 2022, 269, 4421–4435. [Google Scholar] [CrossRef]
- Suslov, V.M.; Lieberman, L.N.; Carlier, P.G.; Ponomarenko, G.N.; Ivanov, D.O.; Rudenko, D.I.; Suslova, G.A.; Adulas, E.I. Efficacy and safety of hydrokinesitherapy in patients with dystrophinopathy. Front. Neurol. 2023, 14, 1230770. [Google Scholar] [CrossRef]
| Group | Sex (m/f) | Age (Years) | BMI | Disease Duration (Years) | QMFM | NSS | ACTIVLIM | 6-MWD (Meter) |
|---|---|---|---|---|---|---|---|---|
| LGMDR1 | 5/5 | 39.6 ± 12.1 | 23.0 ± 5.0 | 22.2 ± 7.3 | 28 ± 16 | 30 ± 7 | 31 ± 4 | 316 ± 78 |
| IBM | 5/5 | 69.3 ± 6.1 | 27.1 ± 4.2 | 8.8 ± 3.9 | 39 ± 11 | 30 ± 8 | 28 ± 10 | 387 ± 142 |
| LOPD | 5/5 | 47.2 ± 18.9 | 22.8 ± 3.3 | 12.8 ± 6.4 | 49 ± 14 | 22 ± 2 | 35 ± 7 | 461 ± 71 |
| PCC | 5/5 | 50.3 ± 11.0 | 28.7 ± 3.7 | 1.2 ± 0.4 | 64 ± 0 | - | - | 458 ± 84 |
| CON | 5/5 | 46.1 ± 12.8 | 23.4 ± 2.1 | - | 64 ± 0 | - | - | 569 ± 45 |
| LGMDR1 | IBM | LOPD | PCC | CON | |||
|---|---|---|---|---|---|---|---|
| Thigh | 6-MWD | FF | −0.932 ** | −0.353 | −0.798 * | −0.491 | 0.264 |
| T2 | 0.328 | −0.795 * | −0.701 | 0.682 | −0.099 | ||
| FA | −0.808 * | −0.523 | −0.527 | 0.095 | 0.079 | ||
| MD | 0.889 ** | 0.481 | −0.177 | −0.475 | 0.403 | ||
| QMFM | FF | −0.875 ** | −0.511 | −0.847 * | - | - | |
| T2 | 0.012 | −0.717 * | −0.991 ** | - | - | ||
| FA | −0.766 ** | −0.553 | 0.324 | - | - | ||
| MD | 0.918 ** | 0.553 | −0.775 * | - | - | ||
| Leg | 6-MWD | FF | −0.200 | −0.371 | −0.330 | −0.578 | 0.236 |
| T2 | 0.713 | 0.035 | 0.328 | 0.652 | −0.238 | ||
| FA | −0.443 | −0.722 * | −0.248 | 0.219 | −0.010 | ||
| MD | −0.159 | 0.690 | −0.319 | −0.884 ** | 0.182 | ||
| QMFM | FF | −0.322 | −0.225 | −0.814 * | - | - | |
| T2 | −0.322 | −0.498 | −0.407 | - | - | ||
| FA | −0.310 | −0.213 | −0.299 | - | - | ||
| MD | 0.286 | 0.097 | −0.695 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlaffke, L.; Rehmann, R.; Güttsches, A.-K.; Vorgerd, M.; Meyer-Frießem, C.H.; Dinse, H.R.; Enax-Krumova, E.; Froeling, M.; Forsting, J. Evaluation of Neuromuscular Diseases and Complaints by Quantitative Muscle MRI. J. Clin. Med. 2024, 13, 1958. https://doi.org/10.3390/jcm13071958
Schlaffke L, Rehmann R, Güttsches A-K, Vorgerd M, Meyer-Frießem CH, Dinse HR, Enax-Krumova E, Froeling M, Forsting J. Evaluation of Neuromuscular Diseases and Complaints by Quantitative Muscle MRI. Journal of Clinical Medicine. 2024; 13(7):1958. https://doi.org/10.3390/jcm13071958
Chicago/Turabian StyleSchlaffke, Lara, Robert Rehmann, Anne-Katrin Güttsches, Matthias Vorgerd, Christine H. Meyer-Frießem, Hubert R. Dinse, Elena Enax-Krumova, Martijn Froeling, and Johannes Forsting. 2024. "Evaluation of Neuromuscular Diseases and Complaints by Quantitative Muscle MRI" Journal of Clinical Medicine 13, no. 7: 1958. https://doi.org/10.3390/jcm13071958
APA StyleSchlaffke, L., Rehmann, R., Güttsches, A.-K., Vorgerd, M., Meyer-Frießem, C. H., Dinse, H. R., Enax-Krumova, E., Froeling, M., & Forsting, J. (2024). Evaluation of Neuromuscular Diseases and Complaints by Quantitative Muscle MRI. Journal of Clinical Medicine, 13(7), 1958. https://doi.org/10.3390/jcm13071958






