Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Preparation of UCB-PL Gel
2.2.1. Collecting Cord Blood and Evaluating Suitability for Non-Transplant
2.2.2. UCB-PL Preparation
2.2.3. UCB-PL Gel Activation before Application
2.3. Ulcer Management—Application of UCB-PL Gel
2.4. Follow-Up and Evaluation of the Outcome
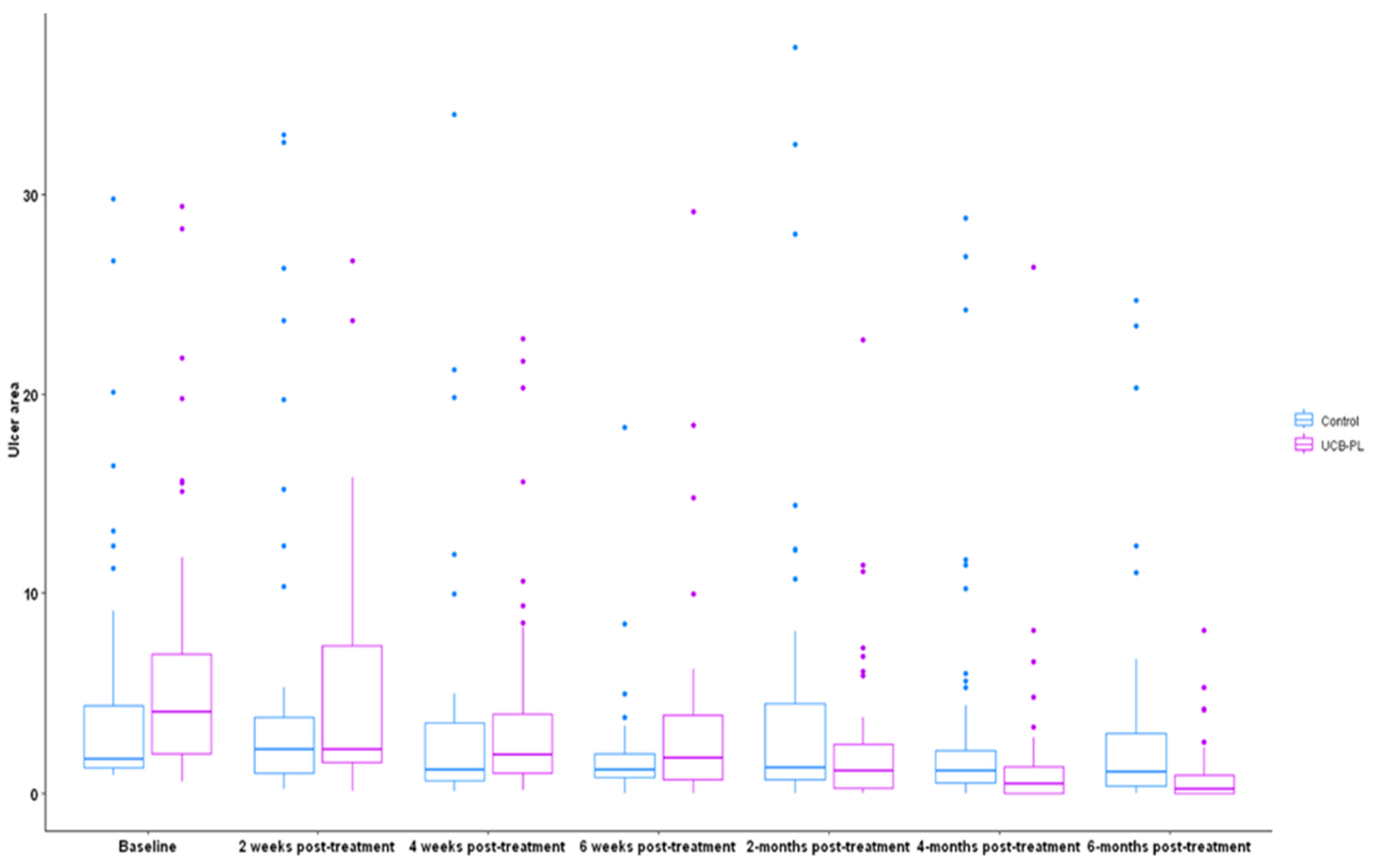
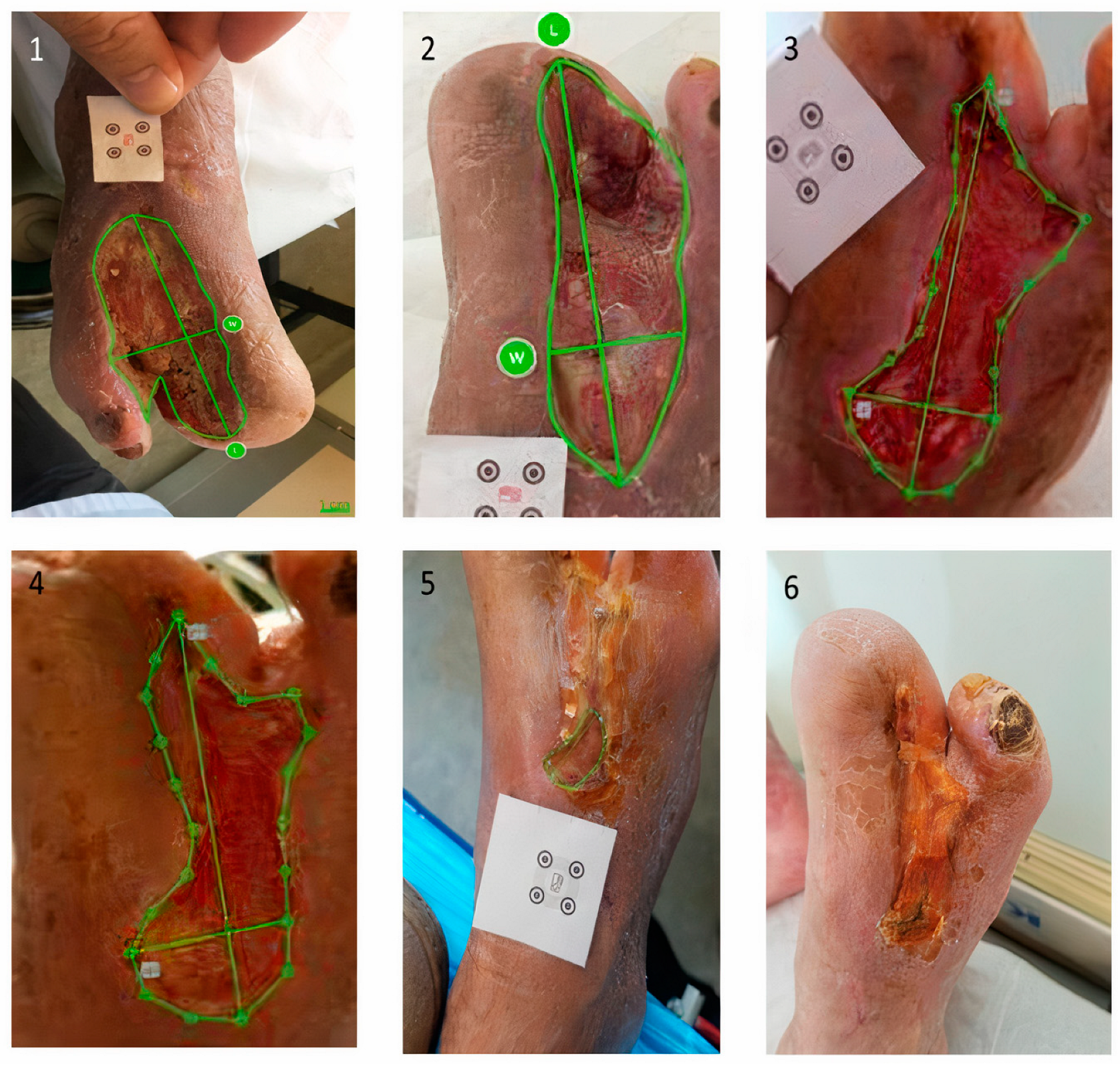
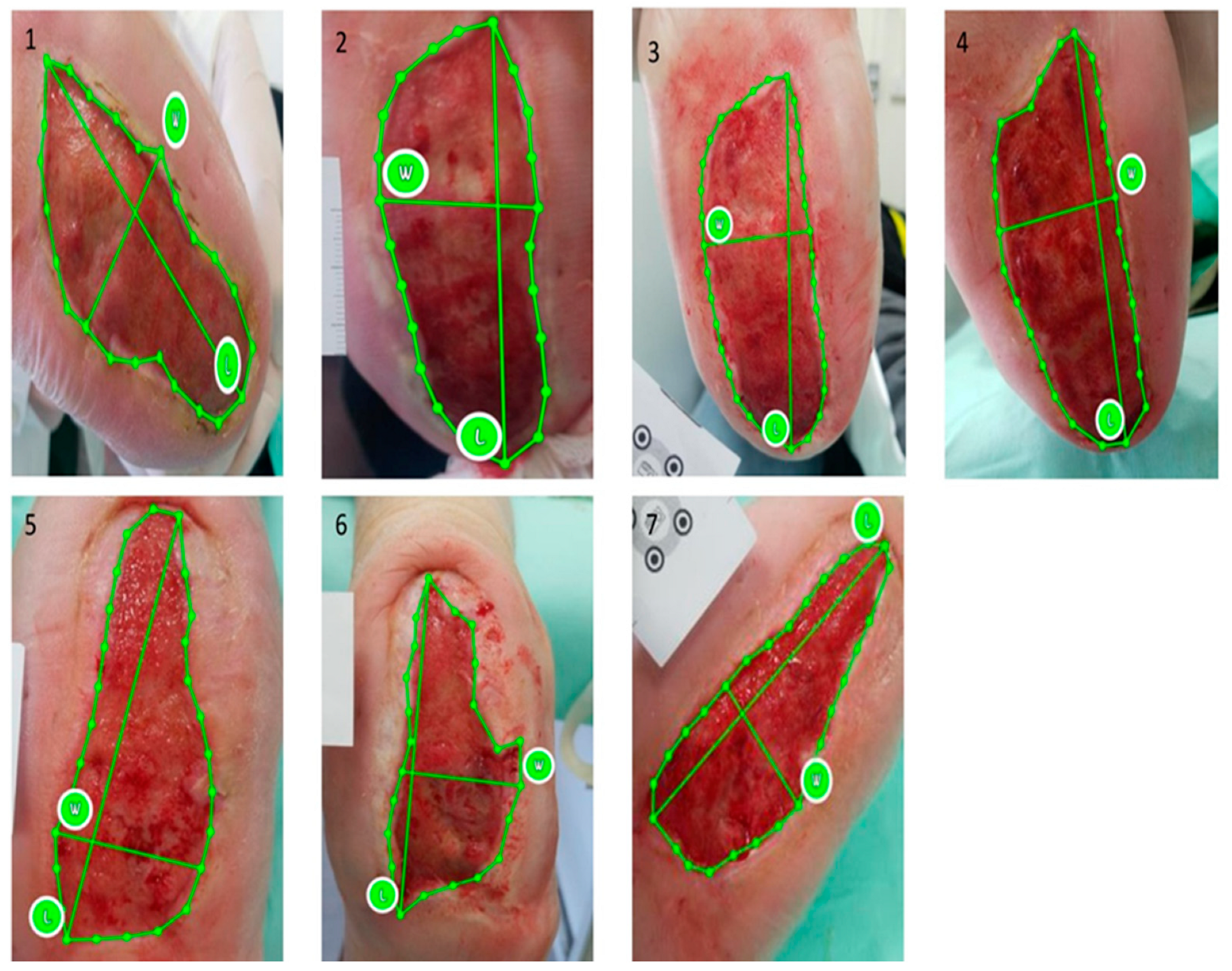
2.5. Ulcer Size Assessment—imitoMeasure Application
2.6. Endpoints
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
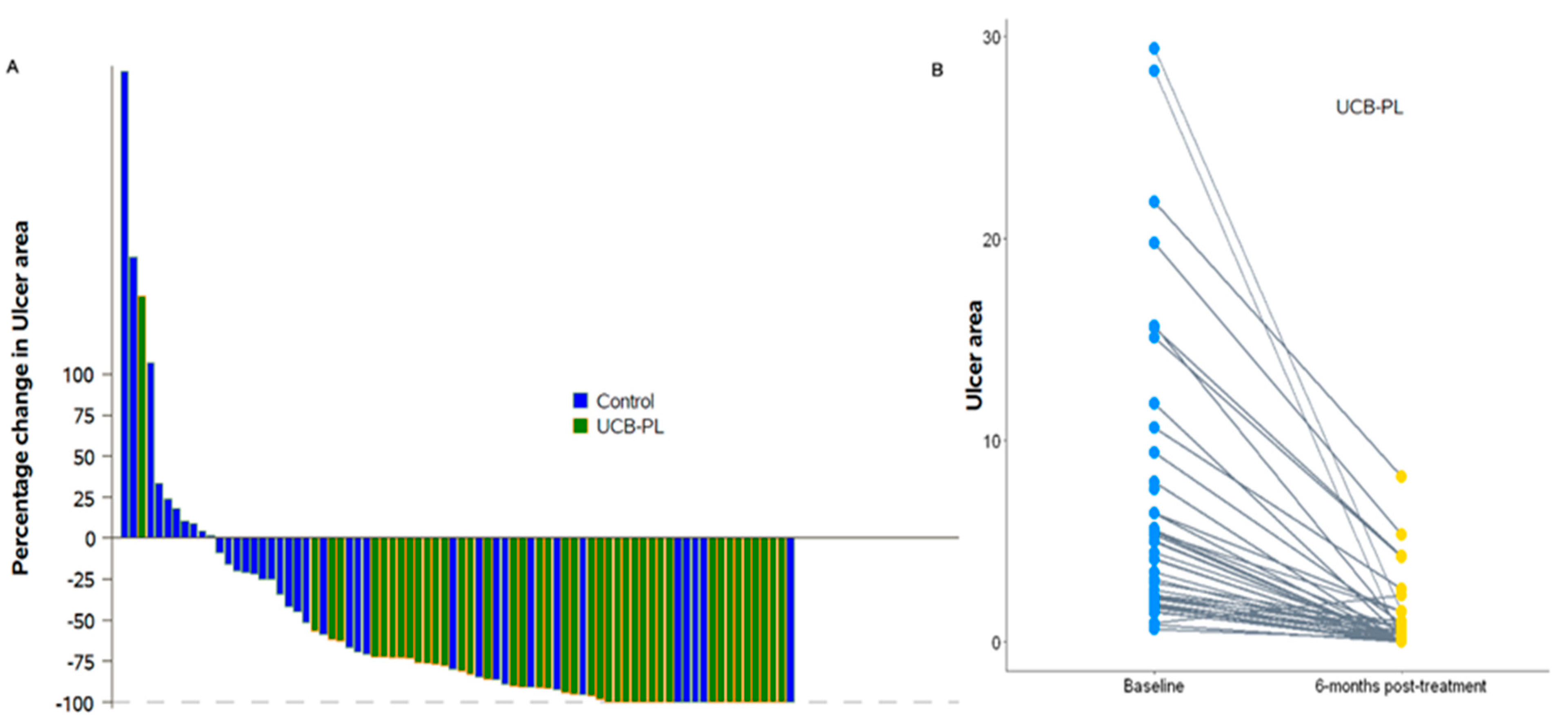
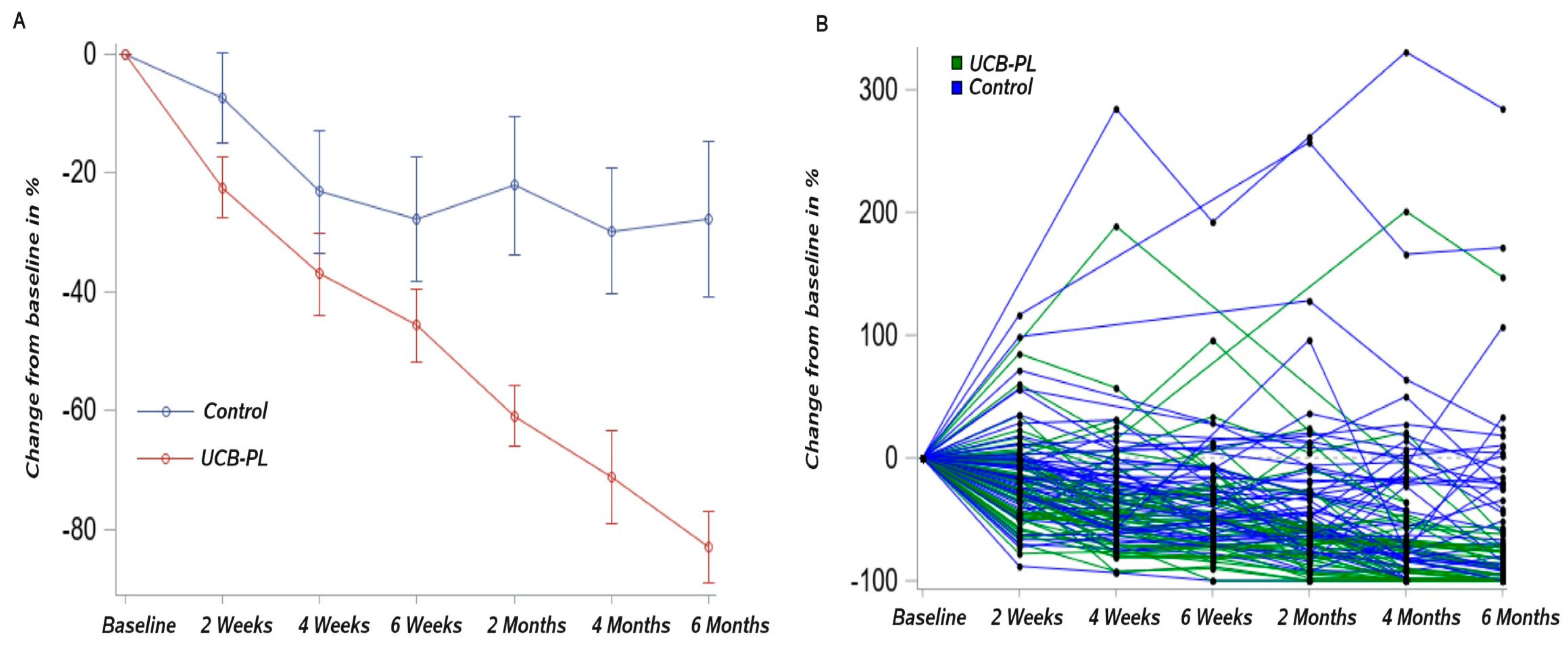
3.2. Outcome of Treatment
3.3. Complete Healing
3.4. Safety-Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J.M. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018, 41, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes. Diabetes Care 2022, 45 (Suppl. 1), S185–S194. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Reiber, G.E.; Vileikyte, L.; Boyko, E.J.; del Aguila, M.; Smith, D.G.; Lavery, L.A.; Boulton, A.J. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999, 22, 157–162. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Game, F.L.; Apelqvist, J.; Attinger, C.; Hartemann, A.; Hinchliffe, R.J.; Löndahl, M.; Price, P.E.; Jeffcoate, W.J.; International Working Group on the Diabetic Foot. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2016, 32 (Suppl. 1), 154–168. [Google Scholar] [CrossRef]
- Elraiyah, T.; Tsapas, A.; Prutsky, G.; Domecq, J.P.; Hasan, R.; Firwana, B.; Nabhan, M.; Prokop, L.; Hingorani, A.; Claus, P.L.; et al. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J. Vasc. Surg. 2016, 63 (Suppl. 2), 46S–58S.e2. [Google Scholar] [CrossRef]
- Steed, D.L. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. J. Vasc. Surg. 1995, 21, 71–81. [Google Scholar] [CrossRef]
- Driver, V.R.; Hanft, J.; Fylling, C.P.; Beriou, J.M.; Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Manag. 2006, 52, 68–70. [Google Scholar]
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Regen. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Obolenskiy, V.N.; Ermolova, D.A.; Laberko, L.A.; Semenova, T.V. Efficacy of platelet rich plasma for the treatment of chronic wounds. EWMA J. 2014, 14, 37–41. [Google Scholar]
- Rebulla, P.; Pupella, S.; Santodirocco, M.; Greppi, N.; Villanova, I.; Buzzi, M.; De Fazio, N.; Grazzini, G.; Italian Cord Blood Platelet Gel Study Group (see Appendix 1). Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. = Trasfus. Sangue 2016, 14, 73–79. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Servatkhah, M.; Rafati, A.R. The in vitro effect of different cord blood platelet rich plasma concentrations on proliferation of dermal fibroblasts. Biosci. Biotechnol. Res. Asia 2016, 13, 1709–1713. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mahmoodi, M.; Rafati, A.R.; Manafi, F.; Mehrabani, D. The Role of Human Adult Peripheral and Umbilical Cord Blood Platelet-Rich Plasma on Proliferation and Migration of Human Skin Fibroblasts. World J. Plast. Surg. 2017, 6, 198–205. [Google Scholar] [PubMed]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef] [PubMed]
- Warkentin, P.I.; Foundation for the Accreditation of Cellular Therapy. Voluntary accreditation of cellular therapies: Foundation for the Accreditation of Cellular Therapy (FACT). Cytotherapy 2003, 5, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Michalopoulos, E.; Sarri, E.F.; Papadopoulou, E.; Theodoropoulou, V.; Katsimpoulas, M.; Stavropoulos-Giokas, C. Evaluation of the Regenerative Potential of Platelet-Lysate and Platelet-Poor Plasma Derived from the Cord Blood Units in Corneal Wound Healing Applications: An In Vitro Comparative Study on Corneal Epithelial Cells. Curr. Issues Mol. Biol. 2022, 44, 4415–4438. [Google Scholar] [CrossRef]
- Martin, P. Wound healing-aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Kakudo, N.; Kushida, S.; Ogura, N.; Hara, T.; Suzuki, K. The use of autologous platelet rich plasma in the treatment of intractable skin ulcer: A case series. Open J. Reg. Med. 2012, 1, 29–32. [Google Scholar] [CrossRef]
- Knighton, D.R.; Ciresi, K.F.; Fiegel, V.D.; Austin, L.L.; Butler, E.L. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann. Surg. 1986, 204, 322–330. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Driver, V.R.; Carman, D.; Lucero, B.; Borris-Hale, C.; Fylling, C.P.; Rappl, L.M.; Clausen, P.A. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: A prospective case series. Ostomy/Wound Manag. 2010, 56, 36–44. [Google Scholar]
- Elsaid, A.; El-Said, M.; Emile, S.; Youssef, M.; Khafagy, W.; Elshobaky, A. Randomized Controlled Trial on Autologous Platelet-Rich Plasma Versus Saline Dressing in Treatment of Non-healing Diabetic Foot Ulcers. World J. Surg. 2020, 44, 1294–1301. [Google Scholar] [CrossRef]
- Suthar, M.; Gupta, S.; Bukhari, S.; Ponemone, V. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: A case series. J. Biomed. Sci. 2017, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, M.C.; Losi, P.; Briganti, E.; Sanguinetti, E.; Magera, A.; Al Kayal, T.; Feriani, R.; Di Stefano, R.; Soldani, G. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS ONE 2013, 8, e84753. [Google Scholar] [CrossRef]
- Losi, P.; Al Kayal, T.; Buscemi, M.; Foffa, I.; Cavallo, A.; Soldani, G. Bilayered Fibrin-Based Electrospun-Sprayed Scaffold Loaded with Platelet Lysate Enhances Wound Healing in a Diabetic Mouse Model. Nanomaterials 2020, 10, 2128. [Google Scholar] [CrossRef] [PubMed]
- Jafar, H.; Hasan, M.; Al-Hattab, D.; Saleh, M.; Ameereh, L.A.; Khraisha, S.; Younes, N.; Awidi, A. Platelet lysate promotes the healing of long-standing diabetic foot ulcers: A report of two cases and in vitro study. Heliyon 2020, 6, e03929. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Hunt, C.; Morrow, A.S.; Urtecho, M.; Amin, M.; Shah, S.; Hasan, B.; Abd-Rabu, R.; Ashmore, Z.; et al. The Effectiveness and Safety of Platelet-Rich Plasma for Chronic Wounds: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2021, 96, 2407–2417. [Google Scholar] [CrossRef]
- Moneib, H.A.; Youssef, S.S.; Aly, D.G.; Rizk, M.A.; Abdelhakeem, Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Dermatol. 2018, 17, 495–501. [Google Scholar] [CrossRef]
- Helmy, Y.; Farouk, N.; Ali Dahy, A.; Abu-Elsoud, A.; Fouad Khattab, R.; Elshahat Mohammed, S.; Abdullbary Gad, L.; Altramsy, A.; Hussein, E.; Farahat, A. Objective assessment of Platelet-Rich Plasma (PRP) potentiality in the treatment of Chronic leg Ulcer: RCT on 80 patients with Venous ulcer. J. Cosmet. Dermatol. 2021, 20, 3257–3263. [Google Scholar] [CrossRef]
- Elbarbary, A.H.; Hassan, H.A.; Elbendak, E.A. Autologous platelet-rich plasma injection enhances healing of chronic venous leg ulcer: A prospective randomised study. Int. Wound J. 2020, 17, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Han, S.K.; Kim, W.K. Treatment of diabetic foot ulcers using a blood bank platelet concentrate. Reconstr. Surg. 2010, 125, 944–952. [Google Scholar] [CrossRef]
- Picardi, A.; Lanti, A.; Cudillo, L.; Cerretti, R.; Dentamaro, T.; De Angelis, G.; Ferraro, A.; Di Veroli, A.; Adorno, G.; Arcese, W.; et al. Platelet gel for treatment of mucocutaneous lesions related to graft-versus-host disease after allogeneic hematopoietic stem cell transplant. Transfusion 2010, 50, 501–506. [Google Scholar] [CrossRef]
- Parazzi, V.; Lazzari, L.; Rebulla, P. Platelet gel from cord blood: A novel tool for tissue engineering. Platelets 2010, 21, 549–554. [Google Scholar] [CrossRef]
- Losi, P.; Barsotti, M.C.; Foffa, I.; Buscemi, M.; De Almeida, C.V.; Fabbri, M.; Gabbriellini, S.; Nocchi, F.; Ursino, S.; Urciuoli, P.; et al. In vitro human cord blood platelet lysate characterisation with potential application in wound healing. Int. Wound J. 2020, 17, 65–72. [Google Scholar] [CrossRef]
- Tadini, G.; Guez, S.; Pezzani, L.; Marconi, M.; Greppi, N.; Manzoni, F.; Rebulla, P.; Esposito, S. Preliminary evaluation of cord blood platelet gel for the treatment of skin lesions in children with dystrophic epidermolysis bullosa. Blood Transfus. = Trasfus. Sangue 2015, 13, 153–158. [Google Scholar] [CrossRef]
- Samarkanova, D.; Rodríguez, L.; Vives, J.; Coll, R.; Tahull, E.; Azqueta, C.; Valdivia, E.; Codinach, M.; Farssac, E.; Gaitan, J.; et al. Cord blood-derived platelet concentrates as starting material for new therapeutic blood components prepared in a public cord blood bank: From product development to clinical application. Blood Transfus. = Trasfus. Sangue 2020, 18, 208–216. [Google Scholar] [CrossRef]
- Volpe, P.; Marcuccio, D.; Stilo, G.; Alberti, A.; Foti, G.; Volpe, A.; Princi, D.; Surace, R.; Pucci, G.; Massara, M. Efficacy of cord blood platelet gel application for enhancing diabetic foot ulcer healing after lower limb revascularization. Semin. Vasc. Surg. 2017, 30, 106–112. [Google Scholar] [CrossRef]
- Guarro, G.; Cozzani, F.; Rossini, M.; Bonati, E.; Del Rio, P. Wounds morphologic assessment: Application and reproducibility of a virtual measuring system, pilot study. Acta Bio-Medica Atenei Parm. 2021, 92, e2021227. [Google Scholar] [CrossRef]
- Do Khac, A.; Jourdan, C.; Fazilleau, S.; Palayer, C.; Laffont, I.; Dupeyron, A.; Verdun, S.; Gelis, A. mHealth App for Pressure Ulcer Wound Assessment in Patients With Spinal Cord Injury: Clinical Validation Study. JMIR mHealth uHealth 2021, 9, e26443. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 96) | UCB-PL Group (n = 47) | Control Group (n = 49) | p-Value | |
|---|---|---|---|---|
| Age, years (n = 75) | 63 (35–85) | 70 (48–75) | 61 (35–85) | 0.32 |
| Sex | 0.43 | |||
| Male Female, | 70 (72.9) 26 (27.1) | 36 (76.6) 11 (23.4) | 34 (69.4) 15 (30.6) | |
| Type of diabetes | 0.21 | |||
| Type 1 DM Type 2 DM | 17 (17.7) 79 (82.3) | 6 (12.8) 41 (87.2) | 11 (22.4) 38 (77.6) | |
| Diabetic Nephropathy | 0.29 | |||
| Yes | 24 (25.0) | 14 (29.8) | 10 (20.4) | |
| No | 72 (75.0) | 33 (70.2) | 39 (79.6) | |
| Diabetic Retinopathy | 0.29 | |||
| Yes | 24 (25.0) | 14 (29.8) | 10 (20.4) | |
| No | 72 (75.0) | 33 (70.2) | 39 (79.6) | |
| Diabetic Neuropathy | 0.84 | |||
| Yes | 46 (47.9) | 23 (48.9) | 23 (46.9) | |
| No | 50 (52.1) | 24 (51.1) | 26 (53.1) | |
| Intervention prior to randomization | 0.72 | |||
| Yes | 23 (24.0) | 12 (25.5) | 11 (22.4) | |
| No | 73 (76.0) | 35 (74.5) | 38 (77.6) | |
| Smoke (n = 56) | 0.33 | |||
| Yes | 22 (39.3) | 10 (33.3) | 12 (46.2) | |
| No | 34 (60.7) | 20 (66.7) | 14 (53.8) | |
| HbA1C (%) (n = 45) | 8.2 (5.4–13.8) | 8.2 (5.7–13.4) | 7.8 (5.4–13.8) | 0.64 |
| DM duration, years (n = 49) | 27.0 (1.00–60.0) | 20.0 (2.0–40.0) | 27.0 (1.00–60.0) | >0.999 |
| ABI (n = 59) | 1.06 (0.33–2.2) | 1.03 (0.33–2.2) | 1.06 (0.37–1.9) | 0.66 |
| NSS (n = 65) | 0.00 (0.00–9.0) | 0.00 (0.00–9.0) | 1.5 (0.00–9.0) | 0.93 |
| NDS (n = 63) | 9.0 (0.00–10.0) | 9.0 (0.00–10.0) | 8.0 (2.0–10.0) | 0.15 |
| Type of ulcer | 0.66 | |||
| Neuropathic ulcer | 51 (53.1) | 22 (46.8) | 29 (59.2) | |
| Ischemic Ulcer | 25 (26.0) | 13 (27.7) | 12 (24.5) | |
| Neuropathic/Ischemic Ulcer | 12 (12.5) | 7 (14.9) | 5 (10.2) | |
| Ulcer after amputation n | 8 (8.3) | 5 (10.6) | 3 (6.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambadiari, V.; Kountouri, A.; Psahoulia, F.; Koliou, G.-A.; Lazaris, A.; Michalopoulos, E.; Mallis, P.; Korakas, E.; Eleftheriadou, I.; Balampanis, K.; et al. Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer. J. Clin. Med. 2024, 13, 1310. https://doi.org/10.3390/jcm13051310
Lambadiari V, Kountouri A, Psahoulia F, Koliou G-A, Lazaris A, Michalopoulos E, Mallis P, Korakas E, Eleftheriadou I, Balampanis K, et al. Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer. Journal of Clinical Medicine. 2024; 13(5):1310. https://doi.org/10.3390/jcm13051310
Chicago/Turabian StyleLambadiari, Vaia, Aikaterini Kountouri, Fοteini Psahoulia, Georgia-Angeliki Koliou, Andreas Lazaris, Efstathios Michalopoulos, Panagiotis Mallis, Emmanouil Korakas, Ioanna Eleftheriadou, Konstantinos Balampanis, and et al. 2024. "Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer" Journal of Clinical Medicine 13, no. 5: 1310. https://doi.org/10.3390/jcm13051310
APA StyleLambadiari, V., Kountouri, A., Psahoulia, F., Koliou, G.-A., Lazaris, A., Michalopoulos, E., Mallis, P., Korakas, E., Eleftheriadou, I., Balampanis, K., Sarris, M., Tsirigotis, P., Geroulakos, G., Stavropoulos-Giokas, C., Dimitriadis, G. D., & Tentolouris, N. (2024). Treatment with Umbilical Cord Blood Platelet Lysate Gel Improves Healing of Diabetic Foot Ulcer. Journal of Clinical Medicine, 13(5), 1310. https://doi.org/10.3390/jcm13051310











