An Investigation of the Relationship between Henoch-Schönlein Purpura and Viral Infection in Korea Using the Health Insurance Database
Abstract
1. Introduction
2. Methods
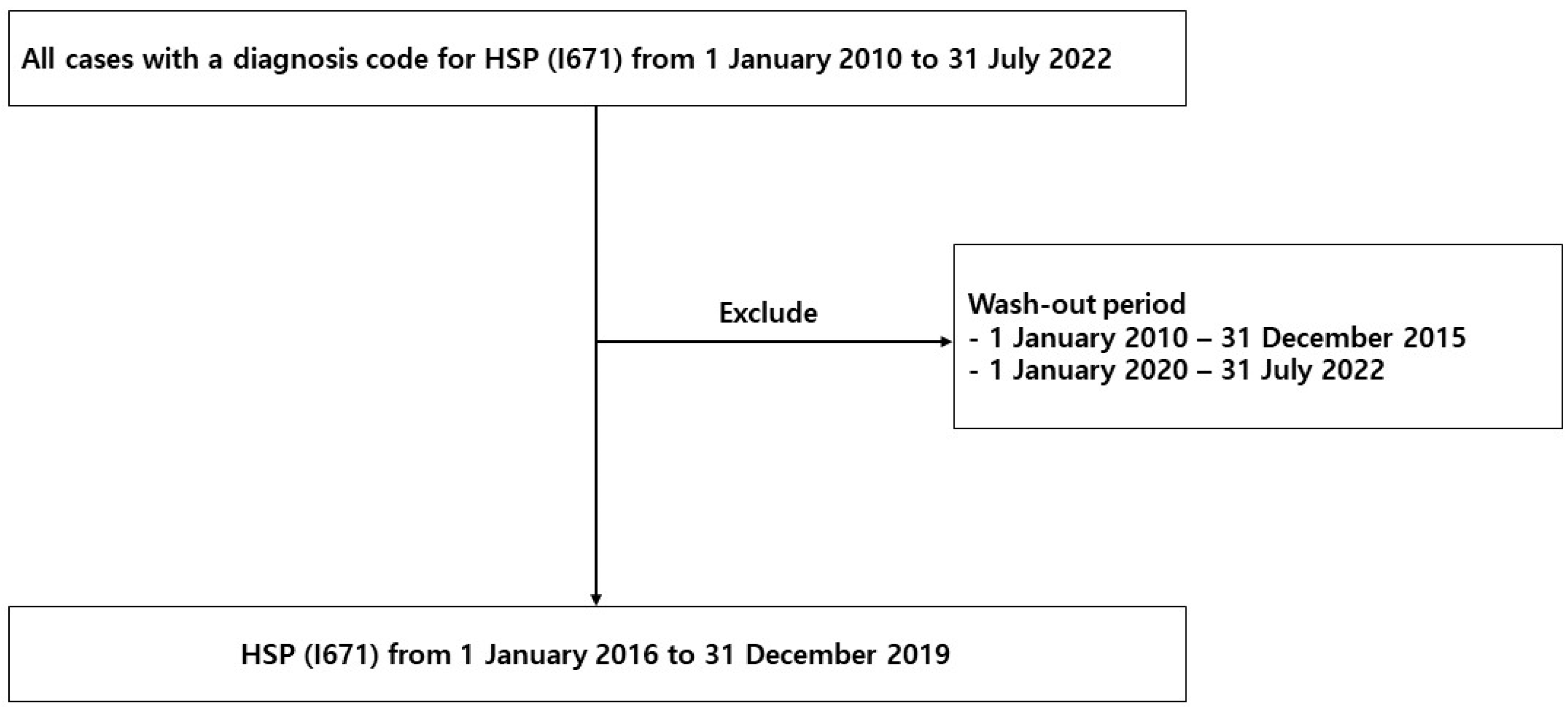
2.1. Study Population
2.2. Surveillance Data of the Virus
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Trend Analysis of HSP
3.3. Clinical Course of HSP
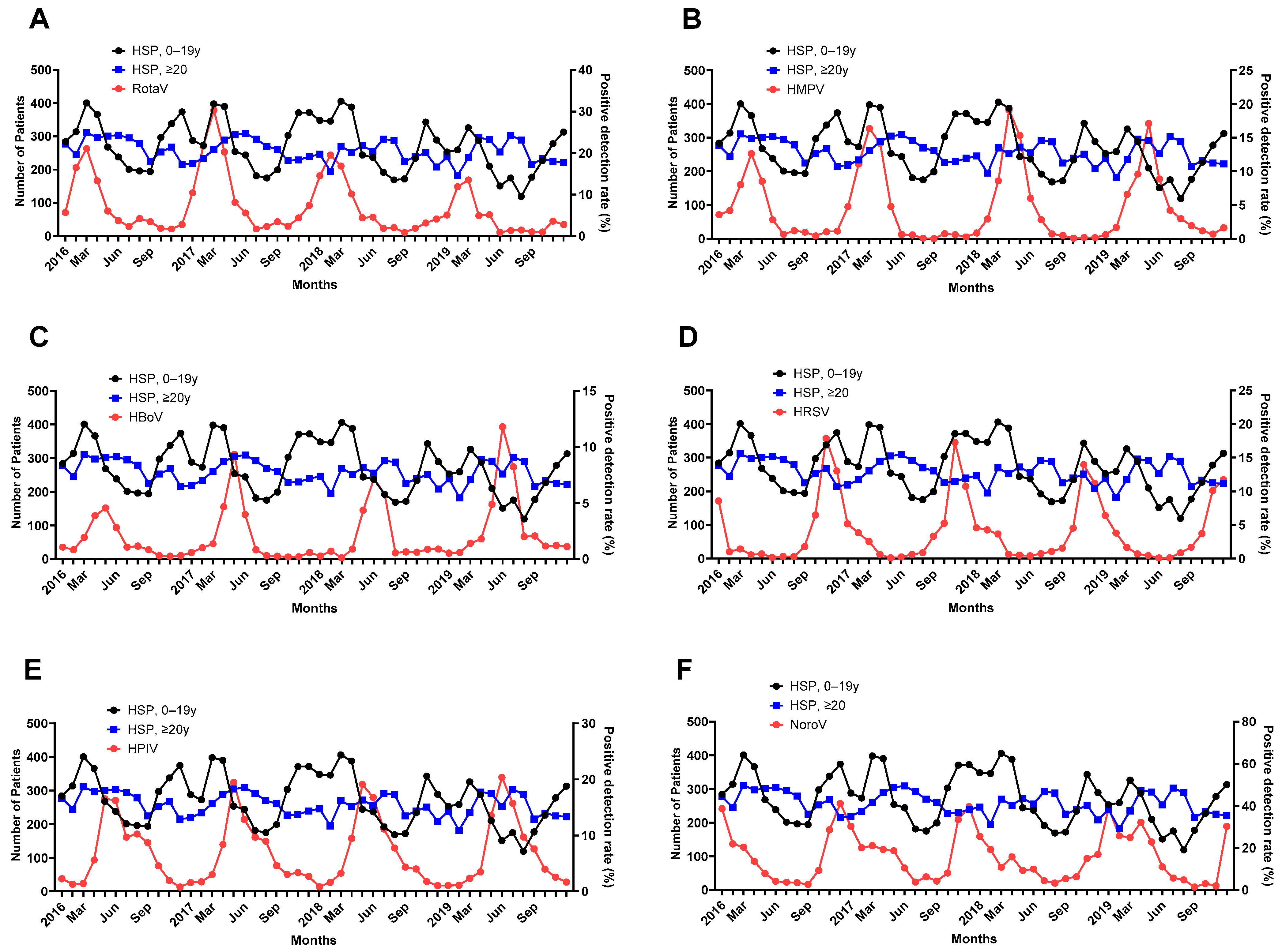
3.4. PDRs of the Virus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rook, A. William Heberden’s cases of anaphylactoid purpura. Arch. Dis. Child. 1958, 33, 271. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Janniger, C.K.; Schwartz, R.A. Pediatric Henoch-Schonlein purpura. Int. J. Dermatol. 2009, 48, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Piram, M.; Mahr, A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schonlein): Current state of knowledge. Curr. Opin. Rheumatol. 2013, 25, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Pistorio, A.; Iusan, S.M.; Bakkaloglu, A.; Herlin, T.; Brik, R.; Buoncompagni, A.; Lazar, C.; Bilge, I.; Uziel, Y.; et al. EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann. Rheum. Dis. 2010, 69, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Nikolaishvili, M.; Pazhava, A.; Di Lernia, V. Viral Infections May Be Associated with Henoch-Schonlein Purpura. J. Clin. Med. 2023, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Borocco, C.; Lafay, C.; Plantard, I.; Gottlieb, J.; Kone-Paut, I.; Galeotti, C. SARS-CoV-2-associated Henoch-Schonlein purpura in a 13-year-old girl. Arch. Pediatr. 2021, 28, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Casini, F.; Magenes, V.C.; De Sanctis, M.; Gattinara, M.; Pandolfi, M.; Cambiaghi, S.; Zuccotti, G.V.; Fabiano, V. Henoch-Schonlein purpura following COVID-19 vaccine in a child: A case report. Ital. J. Pediatr. 2022, 48, 158. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Ennas, S.; Pizzoferrato, M.; Bibbo, S.; Porcari, S.; Ianiro, G.; Cammarota, G. Henoch-schonlein purpura following exposure to SARS-CoV2 vaccine or infection: A systematic review and a case report. Intern. Emerg. Med. 2023. [Google Scholar] [CrossRef]
- Nishimura, N.; Shiomichi, Y.; Takeuchi, S.; Akamine, S.; Yoneda, R.; Yoshizawa, S. IgA vasculitis following COVID-19 vaccination. Mod. Rheumatol. Case Rep. 2023, 7, 122–126. [Google Scholar] [CrossRef]
- Tang, X.; Liu, F.; Li, Q.; Fu, H.; Wang, J.; Mao, J. De Novo Vasculitis after COVID-19 Vaccination. Curr. Rheumatol. Rev. 2023, 19, 151–158. [Google Scholar] [CrossRef]
- Ramdani, Y.; Largeau, B.; Jonville-Bera, A.P.; Maillot, F.; Audemard-Verger, A. COVID-19 Vaccination as a Trigger of IgA Vasculitis: A Global Pharmacovigilance Study. J. Rheumatol. 2023, 50, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Rista, E.; Strakosha, A.; Saliaj, K.; Ymeri, F.; Ikonomi, M. IgA Vasculitis Following COVID-19 Vaccination. Cureus 2023, 15, e33938. [Google Scholar] [CrossRef] [PubMed]
- Salem, Y.; Alam, Z.; Shalabi, M.M.; Hosler, G.A.; Acharya, S. IgA Vasculitis Associated with COVID-19. Cureus 2023, 15, e38725. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S. Special issue on the national health care system of South Korea. Soc. Work Public Health 2010, 25, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S. Introduction: Health of the health care system in Korea. Soc. Work Public Health 2010, 25, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.W.J. Investigating Causal Relations by Econometric Models and Cross-Spectral Methods. Econometrica 1969, 37, 424. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, Y.K.; Min, S.H.; Kim, S.W.; Lee, Y.H.; Lee, J.M. Seasonal Trends of Viral Prevalence and Incidence of Kawasaki Disease: A Korea Public Health Data Analysis. J. Clin. Med. 2021, 10, 3301. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, Y.K.; Min, S.H.; Kim, S.W.; Lee, Y.H.; Lee, J.M. Epidemiology and Viral Etiology of Pediatric Immune Thrombocytopenia through Korean Public Health Data Analysis. J. Clin. Med. 2021, 10, 1356. [Google Scholar] [CrossRef]
- Terano, C.; Hamada, R.; Tatsuno, I.; Hamasaki, Y.; Araki, Y.; Gotoh, Y.; Nakanishi, K.; Nakazato, H.; Matsuyama, T.; Iijima, K.; et al. Epidemiology of biopsy-proven Henoch-Schonlein purpura nephritis in children: A nationwide survey in Japan. PLoS ONE 2022, 17, e0270796. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Barankin, B.; Leong, K.F. Henoch-Schonlein Purpura in Children: An Updated Review. Curr. Pediatr. Rev. 2020, 16, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, P.; Telekesova, P.; Nemcova, D.; Hoza, J. Incidence of vasculitis in children in the Czech Republic: 2-year prospective epidemiology survey. J. Rheumatol. 2004, 31, 2295–2299. [Google Scholar] [PubMed]
- Gardner-Medwin, J.M.; Dolezalova, P.; Cummins, C.; Southwood, T.R. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002, 360, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Calvino, M.C.; Llorca, J.; Garcia-Porrua, C.; Fernandez-Iglesias, J.L.; Rodriguez-Ledo, P.; Gonzalez-Gay, M.A. Henoch-Schonlein purpura in children from northwestern Spain: A 20-year epidemiologic and clinical study. Medicine 2001, 80, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Hung, C.F.; Hsu, C.R.; Wang, L.C.; Chuang, Y.H.; Lin, Y.T.; Chiang, B.L. A nationwide survey on epidemiological characteristics of childhood Henoch-Schonlein purpura in Taiwan. Rheumatology 2005, 44, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, P.; Jain, A.; Amponsah, J.K.; Bhatt, P.; Parmar, N.; Donda, K.; Sharma, M.; Dave, M.; Chaudhari, R.; Vasylyeva, T.L.; et al. National Trends in the Epidemiology and Resource Use for Henoch-Schonlein Purpura (IgA Vasculitis) Hospitalizations in the United States from 2006 to 2014. Hosp. Pediatr. 2019, 9, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Al E’ed, A. Henoch-Schonlein purpura in Saudi Arabia: Characteristics and rare vital organ involvement. Minerva Pediatr. 2021, 73, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Xu, Y.; Liu, F.F.; Wu, Y.; Samadli, S.; Wu, Y.F.; Luo, H.H.; Zhang, D.D.; Hu, P. Association of the infectious triggers with childhood Henoch-Schonlein purpura in Anhui province, China. J. Infect. Public Health 2020, 13, 110–117. [Google Scholar] [CrossRef]
- Demirkesen, C. Approach to cutaneous vasculitides with special emphasis on small vessel vasculitis: Histopathology and direct immunofluorescence. Curr. Opin. Rheumatol. 2017, 29, 39–44. [Google Scholar] [CrossRef]
- Yang, Y.H.; Chuang, Y.H.; Wang, L.C.; Huang, H.Y.; Gershwin, M.E.; Chiang, B.L. The immunobiology of Henoch-Schonlein purpura. Autoimmun. Rev. 2008, 7, 179–184. [Google Scholar] [CrossRef]
- Hwang, H.H.; Lim, I.S.; Choi, B.S.; Yi, D.Y. Analysis of seasonal tendencies in pediatric Henoch-Schonlein purpura and comparison with outbreak of infectious diseases. Medicine 2018, 97, e12217. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Dong, L.; Feng, S.; Wang, Z. Parainfluenza infection is associated with Henoch-Schönlein purpura in children. Pediatr. Infect. Dis. 2016, 8, 110–114. [Google Scholar] [CrossRef]
- Weiss, P.F.; Klink, A.J.; Luan, X.; Feudtner, C. Temporal association of Streptococcus, Staphylococcus, and parainfluenza pediatric hospitalizations and hospitalized cases of Henoch-Schonlein purpura. J. Rheumatol. 2010, 37, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Keim, D.E.; Keller, E.W.; Hirsch, M.S. Mucocutaneous lymph-node syndrome and parainfluenza 2 virus infection. Lancet 1977, 2, 303. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Azimi, P. Kawasaki disease associated with Klebsiella pneumoniae bacteremia and parainfluenza type 3 virus infection. Pediatr. Infect. Dis. 1985, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, M.; Lancrei, H.M.; Brosh-Nissimov, T.; Yeshayahu, Y. Purpurona: A Novel Report of COVID-19-Related Henoch-Schonlein Purpura in a Child. Pediatr. Infect. Dis. J. 2021, 40, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, B.; Keeven, N.; Dang, M.; Keller, E.; Nagpal, R. A Child with COVID-19 and Immunoglobulin A Vasculitis. Pediatr. Ann. 2021, 50, e44–e48. [Google Scholar] [CrossRef]
- Riscassi, S.; Kalapurackal, M.A.; Battisti, L.; Eisendle, K.; Raffeiner, B.; Mercolini, F. Vasculitis in a Child with COVID-19: A Novel Presentation of Henoch-Schonlein Purpura. Klin. Padiatr. 2022, 234, 116–118. [Google Scholar] [CrossRef]
- Messova, A.; Pivina, L.; Muzdubayeva, Z.; Sanbayev, D.; Urazalina, Z.; Adams, A. COVID-19 and New Onset IgA Vasculitis: A Systematic Review of Case Reports. J. Emerg. Nurs. 2022, 48, 348–365. [Google Scholar] [CrossRef]
- Hines, A.M.; Murphy, N.; Mullin, C.; Barillas, J.; Barrientos, J.C. Henoch-Schonlein purpura presenting post COVID-19 vaccination. Vaccine 2021, 39, 4571–4572. [Google Scholar] [CrossRef]
- Grossman, M.E.; Appel, G.; Little, A.J.; Ko, C.J. Post-COVID-19 vaccination IgA vasculitis in an adult. J. Cutan. Pathol. 2022, 49, 385–387. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. [Google Scholar] [CrossRef]
- Maye, J.A.; Chong, H.P.; Rajagopal, V.; Petchey, W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. 2021, 14, e247188. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Odajima, K.; Niimura, Y.; Fujii, M.; Sone, M.; Asakawa, S.; Arai, S.; Yamazaki, O.; Tamura, Y.; Saito, K.; et al. IgA vasculitis with transient glomerular hematuria, diarrhea, and pericarditis following COVID-19 mRNA vaccination in a young patient with possible pre-existing ulcerative colitis. CEN Case Rep. 2023, 12, 84–90. [Google Scholar] [CrossRef]
- Kaya Akca, U.; Atalay, E.; Cuceoglu, M.K.; Balik, Z.; Sener, S.; Ozsurekci, Y.; Basaran, O.; Batu, E.D.; Bilginer, Y.; Ozen, S. Impact of the COVID-19 pandemic on the frequency of the pediatric rheumatic diseases. Rheumatol. Int. 2022, 42, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.B.; Wu, J.G.; Cheng, Y.; Li, J.J. Epidemiology and Clinical Characteristics of Henoch-Schönlein Purpura Associated with Epstein-Barr Virus Infection. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021064. [Google Scholar] [CrossRef]
- Murakami, H.; Takahashi, S.; Kawakubo, Y.; Kinukawa, N.; Funaki, S.; Harada, K. Adolescent with Henoch-Schonlein purpura glomerulonephritis and intracranial hemorrhage possibly secondary to the reactivation of latent CMV. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2008, 50, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Komeda, Y.; Watanabe, T.; Kudo, M. Purpura-free small intestinal IgA vasculitis complicated by cytomegalovirus reactivation. BMJ Case Rep. 2020, 13. [Google Scholar] [CrossRef]
- Mizerska-Wasiak, M.; Winiarska, M.; Nogal, K.; Cichon-Kawa, K.; Panczyk-Tomaszewska, M.; Maldyk, J. IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis. Pediatr. Rep. 2021, 13, 416–420. [Google Scholar] [CrossRef]
- Shin, J.I.; Lee, J.S. Hepatitis B virus infection and Henoch-Schonlein purpura. J. Dermatol. 2007, 34, 156, author reply 157. [Google Scholar] [CrossRef]
- Ayoub, E.M.; McBride, J.; Schmiederer, M.; Anderson, B. Role of Bartonella henselae in the etiology of Henoch-Schonlein purpura. Pediatr. Infect. Dis. J. 2002, 21, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Nakanishi, K.; Yoshizawa, N.; Iijima, K.; Yoshikawa, N. Group A streptococcal antigen in the glomeruli of children with Henoch-Schonlein nephritis. Am. J. Kidney Dis. 2003, 41, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Suzuki, S.; Shirakawa, T.; Masuda, M.; Nakamura, H.; Iijima, K.; Yoshikawa, N. Haemophilus parainfluenzae antigen and antibody in children with IgA nephropathy and Henoch-Schonlein nephritis. Am. J. Kidney Dis. 2000, 36, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hetland, L.E.; Susrud, K.S.; Lindahl, K.H.; Bygum, A. Henoch-Schönlein Purpura: A Literature Review. Acta Derm. Venereol. 2017, 97, 1160–1166. [Google Scholar] [CrossRef]
- Dudley, J.; Smith, G.; Llewelyn-Edwards, A.; Bayliss, K.; Pike, K.; Tizard, J. Randomised, double-blind, placebo-controlled trial to determine whether steroids reduce the incidence and severity of nephropathy in Henoch-Schonlein Purpura (HSP). Arch. Dis. Child. 2013, 98, 756–763. [Google Scholar] [CrossRef]
- Ozen, S.; Marks, S.D.; Brogan, P.; Groot, N.; de Graeff, N.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis-the SHARE initiative. Rheumatology 2019, 58, 1607–1616. [Google Scholar] [CrossRef]
- Stone, H.K.; Mitsnefes, M.; Dickinson, K.; Burrows, E.K.; Razzaghi, H.; Luna, I.Y.; Gluck, C.A.; Dixon, B.P.; Dharnidharka, V.R.; Smoyer, W.E.; et al. Clinical course and management of children with IgA vasculitis with nephritis. Pediatr. Nephrol. 2023, 38, 3721–3733. [Google Scholar] [CrossRef]

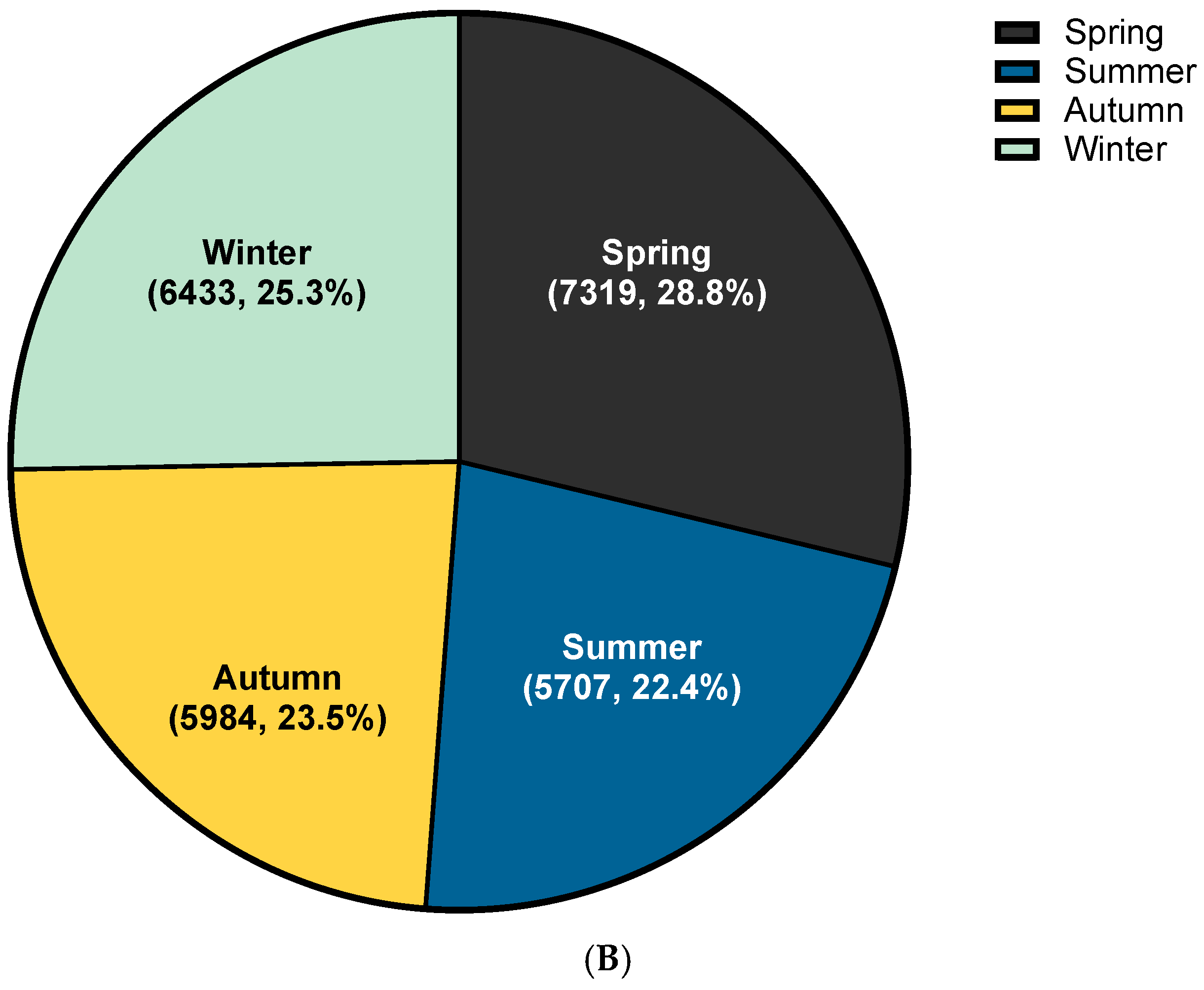

| Variable | n (%) | Annual Incidence * | ||
|---|---|---|---|---|
| Total number of patients | 25,443 | |||
| Age (years) | 28.1 ± 25.2 | 49.2 | ||
| Age group | ||||
| 0–19 years | 13,063 (51.3) | 130.0 | ||
| 0~4 years | 3893 (15.3) | 176.6 | ||
| 5~9 years | 6327 (24.9) | 267.9 | ||
| 10~14 years | 1855 (7.3) | 78.9 | ||
| 15~19 years | 988 (3.9) | 31.6 | ||
| 20–39 years | 3933 (15.5) | 27.5 | ||
| 40–59 years | 4401 (17.3) | 10.6 | ||
| ≥60 years | 4046 (15.9) | 39.9 | ||
| Sex | ||||
| Male | 11,623 (45.7) | 45.0 | ||
| Female | 13,820 (54.3) | 53.4 | ||
| Location | ||||
| Seoul | 7936 (23.1) | 79.9 | ||
| Pusan | 2181 (6.4) | 62.3 | ||
| Incheon | 1763 (5.1) | 71.0 | ||
| Daegu | 1922 (5.6) | 65.3 | ||
| Gwangju | 1519 (4.4) | 103.4 | ||
| Daejeon | 1500 (4.4) | 99.1 | ||
| Ulsan | 522 (1.5) | 44.5 | ||
| Gyeonggi | 1742 (22.8) | 716.7 | ||
| Gangwon | 1243 (3.6) | 9.8 | ||
| Chungbuk | 886 (2.6) | 57.1 | ||
| Chungnam | 1424 (4.1) | 89.5 | ||
| Jeonbuk | 1185 (3.5) | 56.5 | ||
| Jeonnam | 784 (2.3) | 42.0 | ||
| Gyeongbuk | 1061 (3.1) | 55.7 | ||
| Gyeongnam | 1959 (5.7) | 72.5 | ||
| Jeju | 629 (1.8) | 18.6 | ||
| Sejong | 3 (0.0) | 0.5 | ||
| Type of insurance | ||||
| Medical insurance | 24,533 (96.4) | 47.5 | ||
| Medical aid | 860 (3.4) | 1.7 | ||
| Free | 50 (0.2) | 0.1 |
| Treatment | n (%) | |
|---|---|---|
| IVIG | 200 (0.8) | |
| Steroid | 9153 (36.0) | |
| Duration of steroid medication | ||
| 5 days | 3629 (39.7) | |
| 7 days | 2758 (30.1) | |
| 10 days | 2014 (22.0) | |
| 14 days | 1430 (15.6) | |
| Hospitalization | 7358 (28.9) | |
| Hospital visit (days) | 4.30 ± 5.55 | |
| Duration of hospitalization (days) | 7.87 ± 8.82 | |
| A. Diagnostic Data and Virus Data after 1 Month | ||||||||||||
| Age Group (Years) | HAdV | HPIV | HRSV | IFV | HCoV | HRV | HBoV | HMPV | Rotavirus | Norovirus | Adenovirus | Astrovirus |
| 0–19.9 | 0.982 | ≤0.001 | 0.063 | 0.798 | 0.068 | 0.982 | ≤0.001 | 0.007 | 0.191 | 0.889 | 0.514 | 0.231 |
| ≥20 | 0.038 | 0.044 | 0.002 | 0.694 | 0.26 | 0.828 | 0.001 | 0.005 | 0.006 | 0.801 | 0.534 | 0.526 |
| B. Diagnostic Data and Virus Data after 2 Months | ||||||||||||
| Age Group (Years) | HAdV | HPIV | HRSV | IFV | HCoV | HRV | HBoV | HMPV | Rotavirus | Norovirus | Adenovirus | Astrovirus |
| 0–19.9 | 0.509 | 0.002 | 0.271 | 0.176 | 0.053 | 0.3 | 0.002 | 0.086 | 0.002 | 0.288 | 0.833 | 0.174 |
| ≥20 | 0.064 | 0.544 | 0.014 | 0.64 | 0.221 | 0.119 | 0.004 | 0.002 | 0.001 | 0.033 | 0.666 | 0.872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Jo, S.M.; Kim, S.W.; Lee, J.M.; Baek, H.S. An Investigation of the Relationship between Henoch-Schönlein Purpura and Viral Infection in Korea Using the Health Insurance Database. J. Clin. Med. 2024, 13, 1290. https://doi.org/10.3390/jcm13051290
Park SH, Jo SM, Kim SW, Lee JM, Baek HS. An Investigation of the Relationship between Henoch-Schönlein Purpura and Viral Infection in Korea Using the Health Insurance Database. Journal of Clinical Medicine. 2024; 13(5):1290. https://doi.org/10.3390/jcm13051290
Chicago/Turabian StylePark, So Hyeon, Su Min Jo, Sang Won Kim, Jae Min Lee, and Hee Sun Baek. 2024. "An Investigation of the Relationship between Henoch-Schönlein Purpura and Viral Infection in Korea Using the Health Insurance Database" Journal of Clinical Medicine 13, no. 5: 1290. https://doi.org/10.3390/jcm13051290
APA StylePark, S. H., Jo, S. M., Kim, S. W., Lee, J. M., & Baek, H. S. (2024). An Investigation of the Relationship between Henoch-Schönlein Purpura and Viral Infection in Korea Using the Health Insurance Database. Journal of Clinical Medicine, 13(5), 1290. https://doi.org/10.3390/jcm13051290






