The Effects of Post-Surgical Pregnancy on Weight Loss Trajectories after Bariatric Surgery: Are Initial Weight and Age Prognostic Factors? †
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Pre-Operative Management
2.3. Surgical Techniques and Bariatric Procedures
2.4. Post-Operative Management
2.5. Research Procedures and Data Collection
2.6. Definitions
2.7. Study Limitations
2.8. Study Strengths
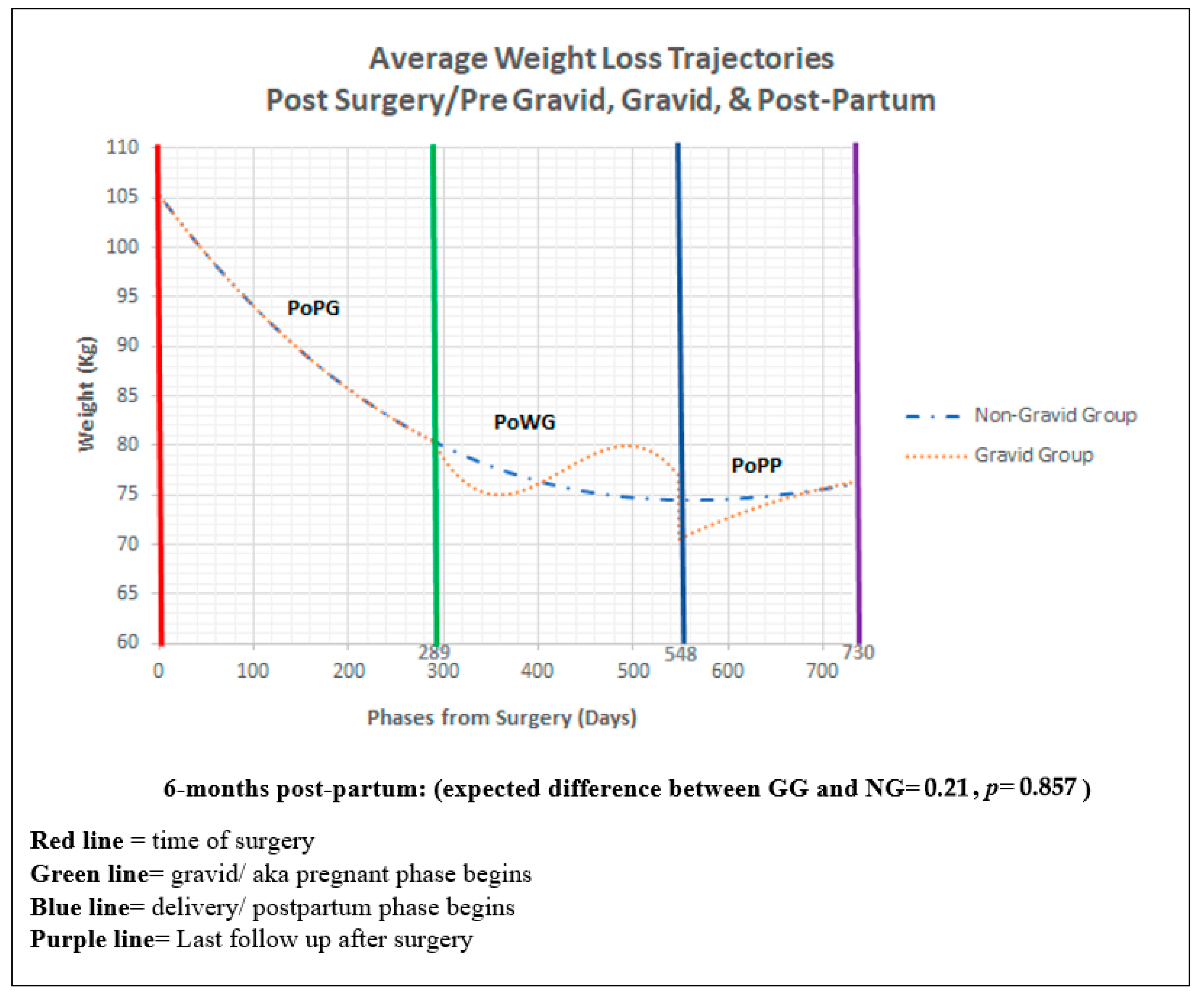
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 43, 1764–1774. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Syed, A.A. Pregnancy Following Bariatric Surgery—Medical Complications and Management. Obes. Surg. 2016, 26, 2523–2529. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, P.; Li, P.; Wang, G.; Yi, X.; Fu, Z.; Sun, X.; Cui, B.; Zhu, L.; Zhu, S. Bariatric Surgery Improves Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Obes. Surg. 2022, 32, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Heusschen, L.; Krabbendam, I.; van der Velde, J.M.; Deden, L.N.; Aarts, E.O.; Merién, A.E.; Emous, M.; Bleumink, G.S.; Lutgers, H.L.; Hazebroek, E.J. A Matter of Timing—Pregnancy after Bariatric Surgery. Obes. Surg. 2021, 31, 2072–2079. [Google Scholar] [CrossRef]
- Yau, P.O.; Parikh, M.; Saunders, J.K.; Chui, P.; Zablocki, T.; Welcome, A.U. Pregnancy after bariatric surgery: The effect of time-to-conception on pregnancy outcomes. Surg. Obes. Relat. Dis. 2017, 13, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.E.; Furtado, M.M. Pregnancy after Bariatric Surgery: What Are the Risks and Benefits? Curr. Surg. Rep. 2023, 11, 196–201. [Google Scholar] [CrossRef]
- Keen, C.L.; Clegg, M.S.; Hanna, L.A.; Lanoue, L.; Rogers, J.M.; Daston, G.P.; Oteiza, P.; Uriu-Adams, J.Y. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J. Nutr. 2003, 133 (Suppl. S2), 1597S–1605S. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Teitelman, M.; Grotegut, C.A.; Williams, N.N.; Lewis, J.D. The impact of bariatric surgery on menstrual patterns. Obes. Surg. 2006, 16, 1457–1463. [Google Scholar] [CrossRef]
- Navaee, M.; Kashanian, M.; Kabir, A.; Zamaninour, N.; Chamari, M.; Pazouki, A. Maternal and fetal/neonatal outcomes in pregnancy, delivery and postpartum following bariatric surgery and comparison with pregnant women with obesity: A study protocol for a prospective cohort. Reprod. Health 2024, 21, 8. [Google Scholar] [CrossRef]
- Vazhiyelethil, J.; Minisha, F.; Al Obaidly, S.; AlQubaisi, M.; Salama, H.; Ali, N.; Khenyab, N.; Mohan, S.; Pallivalappil, A.R.; Al-Dewik, N.; et al. Impact of bariatric surgery on maternal gestational weight gain and pregnancy outcomes in women with obesity: A population-based cohort study from Qatar. Qatar Med. J. 2024, 2024, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Holowko, N.; Näslund, I.; Ottosson, J.; Arkema, E.V.; Neovius, M.; Stephansson, O.; Johansson, K. Pregnancy Weight Gain after Gastric Bypass or Sleeve Gastrectomy. JAMA Netw. Open 2023, 6, e2346228. [Google Scholar] [CrossRef]
- Stentebjerg, L.L.; Andersen, L.L.T.; Renault, K.; Støving, R.K.; Jensen, D.M. Pregnancy and perinatal outcomes according to surgery to conception interval and gestational weight gain in women with previous gastric bypass. J. Matern.-Fetal Neonatal Med. 2017, 30, 1182–1188. [Google Scholar] [CrossRef]
- Berglind, D.; Willmer, M.; Näslund, E.; Tynelius, P.; Sørensen, T.I.A.; Rasmussen, F. Differences in gestational weight gain between pregnancies before and after maternal bariatric surgery correlate with differences in birth weight but not with scores on the body mass index in early childhood. Pediatr. Obes. 2014, 9, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Grandfils, S.; Demondion, D.; Kyheng, M.; Duhamel, A.; Lorio, E.; Pattou, F.; Deruelle, P. Impact of gestational weight gain on perinatal outcomes after a bariatric surgery. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 401–405. [Google Scholar] [CrossRef]
- Woźniewska, P.; Diemieszczyk, I.; Groth, D.; Szczerbiński, Ł.; Choromańska, B.; Błachnio-Zabielska, A.; Krętowski, A.; Hady, H.R. The influence of patient’s age on metabolic and bariatric results of laparoscopic sleeve gastrectomy in 2-year observation. BMC Surg. 2020, 20, 323. [Google Scholar] [CrossRef]
- Toth, M.J.; Tchernof, A. Lipid metabolism in the elderly. Eur. J. Clin. Nutr. 2000, 54, S121–S125. [Google Scholar] [CrossRef]
- Scozzari, G.; Passera, R.; Benvenga, R.; Toppino, M.; Morino, M. Age as a long-term prognostic factor in bariatric surgery. Ann. Surg. 2012, 256, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.E.; Santander, C.; Court, I.; Bravo, J. Correlation between age and weight loss after bariatric surgery. Obes. Surg. 2013, 23, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Okuyan, E.; Akgul, O.K.; Tureyici, L.; Atac, H.; Cetin, M.; Günakan, E.; Karakaya, F. The relationship between maternal thyroid volume, TSH levels, and Healthy Eating Index scores in BMI-matched pregnant women with hyperemesis gravidarum. J. Matern. Fetal Neonatal Med. 2023, 36, 2236270. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009; Volume 184. [Google Scholar] [CrossRef]
- Catalano, P.M.; Mele, L.; Landon, M.B.; Ramin, S.M.; Reddy, U.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M., Jr.; et al. Inadequate weight gain in overweight and obese pregnant women: What is the effect on fetal growth? Am. J. Obstet. Gynecol. 2014, 211, 137.e1–137.e7. [Google Scholar] [CrossRef]
- Akhter, Z.; Rankin, J.; Ceulemans, D.; Ngongalah, L.; Ackroyd, R.; Devlieger, R.; Vieira, R.; Heslehurst, N. Pregnancy after bariatric surgery and adverse perinatal outcomes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002866. [Google Scholar] [CrossRef]
- Kjær, M.M.; Nilas, L. Timing of pregnancy after gastric bypass-a national register-based cohort study. Obes. Surg. 2013, 23, 1281–1285. [Google Scholar] [CrossRef]
- Falcone, V.; Stopp, T.; Feichtinger, M.; Kiss, H.; Eppel, W.; Husslein, P.W.; Prager, G.; Göbl, C.S. Pregnancy after bariatric surgery: A narrative literature review and discussion of impact on pregnancy management and outcome. BMC Pregnancy Childbirth 2018, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Jans, G.; Matthys, C.; Bogaerts, A.; Lannoo, M.; Verhaeghe, J.; Van der Schueren, B.; Devlieger, R. Maternal micronutrient deficiencies and related adverse neonatal outcomes after bariatric surgery: A systematic review. Adv. Nutr. 2015, 6, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.D.; Dewan, D.M. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology 1993, 79, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Wax, J.R.; Pinette, M.G.; Cartin, A. Roux-en-Y gastric bypass-associated bowel obstruction complicating pregnancy—An obstetrician’s map to the clinical minefield. Am. J. Obstet. Gynecol. 2013, 208, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Różańska-Walędziak, A.; Bartnik, P.; Kacperczyk-Bartnik, J.; Czajkowski, K.; Walędziak, M.; Kwiatkowski, A. Pregnancy after bariatric surgery—A narrative literature review. Wideochirurgia I Inne Techniki Maloinwazyjne 2021, 16, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Carelli, A.M.; Ren, C.J.; Youn, H.A.; Friedman, E.B.; Finger, A.E.; Lok, B.H.; Kurian, M.S.; Fielding, G.A. Impact of laparoscopic adjustable gastric banding on pregnancy, maternal weight, and neonatal health. Obes. Surg. 2011, 21, 1552–1558. [Google Scholar] [CrossRef] [PubMed]

| NG = 488 | GG = 20 | |
|---|---|---|
| Age (years), mean | 37 | 33 |
| Primary procedure, no. (%) | ||
| RYGB | 227 (46.5) | 10 (50.0) |
| SG | 261 (53.5) | 10 (50.0) |
| Pre-existing comorbidities, no. (%) | ||
| Hypertension | 150 (30.7) | 2 (10.0) |
| Hyperlipidemia | 202 (41.3) | 5 (25.0) |
| DM | 131 (26.8) | 2 (10.0) |
| GERD | 149 (30.5) | 6 (30.0) |
| OSA | 115 (23.5) | 1 (5.0) |
| Smoker within 1 year before surgery | 15 (3.0) | 0 |
| Medication use, no. (%) | ||
| Insulin | 53 (10.8) | 0 |
| DM | 152 (31.1) | 5 (25.0) |
| Orlistat | 141 (28.8) | 11 (55.0) |
| Steroid/Immunosuppressant | 16 (3.2) | 0 |
| Anticoagulation | 14 (2.8) | 1 (5.0) |
| ASA status | ||
| ASA I | 9 (1.8) | 0 |
| ASA II | 166 (34.2) | 6 (30.0) |
| ASA III | 311 (63.9) | 14 (70.0) |
| ASA IV | 2 (0.4) | 0 |
| Model Section Code | Model Section Name (Group) | Trajectory Parameter Name | Estimate | Std. Error | df | t | p | 95% CI of Estimate | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | |||||||||
| P1 | Phase 1 (post-surgery/pre-gravid) | Common | Wt at Day 0 (Intercept) | 105.396 | 0.199 | 723.235 | 530.785 | <0.001 | 105.007 | 105.786 |
| Days post-op (linear) | −0.129 | 0.001 | 6626.618 | −127.108 | <0.001 | −0.131 | −0.127 | |||
| Days post-op (quadratic) | 1.65 × 10−4 | 2.10 × 10−6 | 6566.227 | 78.753 | <0.001 | 1.61 × 10−4 | 1.69 × 10−4 | |||
| Days post-op (cubic) | −5.97 × 10−8 | 1.10 × 10−9 | 6540.807 | −54.491 | <0.001 | −6.19 × 10−8 | −5.76 × 10−8 | |||
| P2 | Phase 2 (post-surgery while gravid) | GG | Days Gravid (linear) | -0.126 | 0.043 | 6649.979 | −2.944 | 0.003 | −0.210 | −0.042 |
| Days Gravid (quadtratic) | 0.002 | 0.001 | 6562.778 | 2.837 | 0.005 | 4.81 × 10−4 | 0.003 | |||
| Days Gravid (cubic) | −3.99 × 10−6 | 1.64 × 10−6 | 6533.897 | −2.428 | 0.015 | −7.22 × 10−6 | −7.70 × 10−7 | |||
| P3 | Phase 3 (Post-partum) | GG | Wt change at Post-partum (Intercept) | −6.386 | 4.121 | 6511.170 | −1.550 | 0.121 | −14.463 | 1.692 |
| Days Post-partum (linear) | 0.170 | 0.099 | 6518.712 | 1.716 | 0.086 | −0.024 | 0.365 | |||
| Days Post-partum (quadratic) | 0.001 | 0.001 | 6510.628 | 1.873 | 0.061 | −6.47 × 10−5 | 0.003 | |||
| Days Post-partum (cubic) | 4.08 × 10−6 | 1.65 × 10−6 | 6534.923 | 2.476 | 0.013 | 8.50 × 10−7 | 7.30 × 10−6 | |||
| M | Phase 1 Trajectory Controls/Moderators | Baseline Age | Wt at Day 0 (Intercept) by Baseline Age | 0.031 | 0.016 | 657.887 | 1.932 | 0.054 | 0.000 | 0.063 |
| Days post-op (linear) by Baseline Age | 0.001 | 4.67 × 10−5 | 6661.029 | 17.322 | <0.001 | 0.001 | 0.001 | |||
| Days post-op (quadratic) by Baseline Age | −5.59 × 10−7 | 4.63 × 10−8 | 6586.543 | −12.068 | <0.001 | −6.50 × 10−7 | −4.69 × 10−7 | |||
| Baseline Weight | Wt at Day 0 (Intercept) by Baseline Weight | 0.962 | 0.011 | 647.513 | 89.521 | <0.001 | 0.941 | 0.983 | ||
| Days post-op (linear) by Baseline Weight | −0.001 | 2.83 × 10−5 | 6647.054 | −24.113 | <0.001 | −0.001 | −6.26 × 10−4 | |||
| Days post-op (quadratic) by Baseline Weight | 4.70 × 10−7 | 2.54 × 10−8 | 6592.478 | 18.471 | <0.001 | 4.20 × 10−7 | 5.19 × 10−7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barajas-Gamboa, J.S.; Khan, M.S.I.; Dang, J.T.; Romero-Velez, G.; Diaz Del Gobbo, G.; Abdallah, M.; Pantoja, J.P.; Abril, C.; Guerron, A.D.; Lee-St. John, T.; et al. The Effects of Post-Surgical Pregnancy on Weight Loss Trajectories after Bariatric Surgery: Are Initial Weight and Age Prognostic Factors? J. Clin. Med. 2024, 13, 1264. https://doi.org/10.3390/jcm13051264
Barajas-Gamboa JS, Khan MSI, Dang JT, Romero-Velez G, Diaz Del Gobbo G, Abdallah M, Pantoja JP, Abril C, Guerron AD, Lee-St. John T, et al. The Effects of Post-Surgical Pregnancy on Weight Loss Trajectories after Bariatric Surgery: Are Initial Weight and Age Prognostic Factors? Journal of Clinical Medicine. 2024; 13(5):1264. https://doi.org/10.3390/jcm13051264
Chicago/Turabian StyleBarajas-Gamboa, Juan S., Mohammed Sakib Ihsan Khan, Jerry T. Dang, Gustavo Romero-Velez, Gabriel Diaz Del Gobbo, Mohammed Abdallah, Juan Pablo Pantoja, Carlos Abril, Alfredo D. Guerron, Terrence Lee-St. John, and et al. 2024. "The Effects of Post-Surgical Pregnancy on Weight Loss Trajectories after Bariatric Surgery: Are Initial Weight and Age Prognostic Factors?" Journal of Clinical Medicine 13, no. 5: 1264. https://doi.org/10.3390/jcm13051264
APA StyleBarajas-Gamboa, J. S., Khan, M. S. I., Dang, J. T., Romero-Velez, G., Diaz Del Gobbo, G., Abdallah, M., Pantoja, J. P., Abril, C., Guerron, A. D., Lee-St. John, T., Corcelles, R., Rodriguez, J., Kroh, M., & Uy-Kroh, M. J. (2024). The Effects of Post-Surgical Pregnancy on Weight Loss Trajectories after Bariatric Surgery: Are Initial Weight and Age Prognostic Factors? Journal of Clinical Medicine, 13(5), 1264. https://doi.org/10.3390/jcm13051264







