Electrochemotherapy in Aggressive Hemangioma of the Spine: A Case Series and Narrative Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
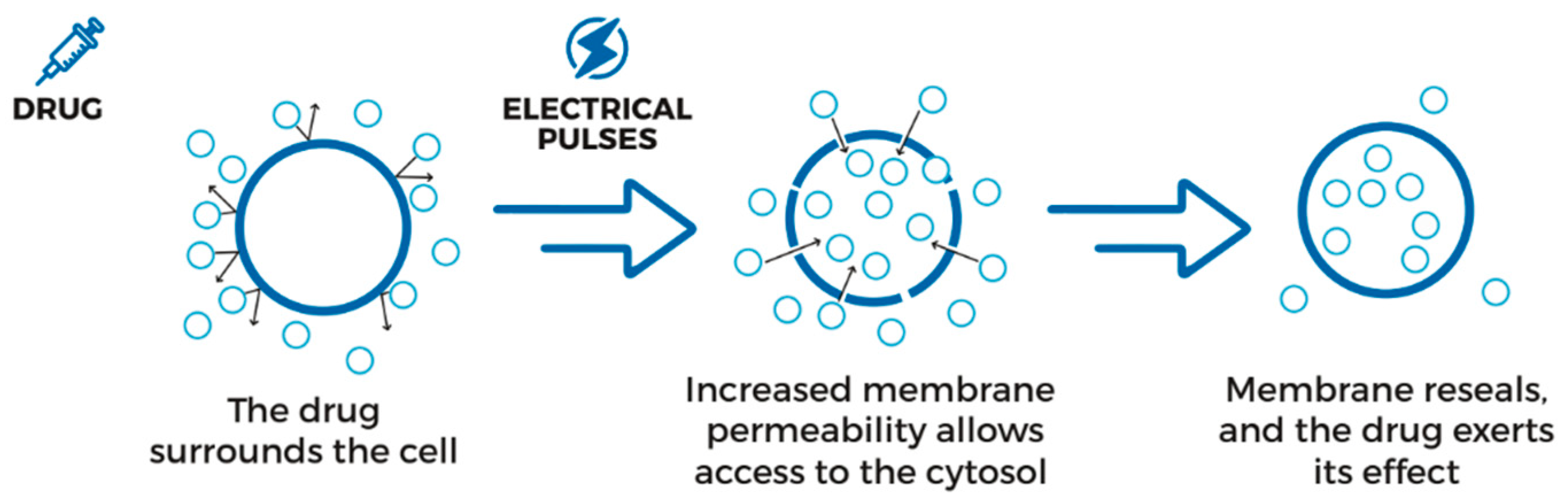
2.2. Electrochemotherapy Procedure
3. Results
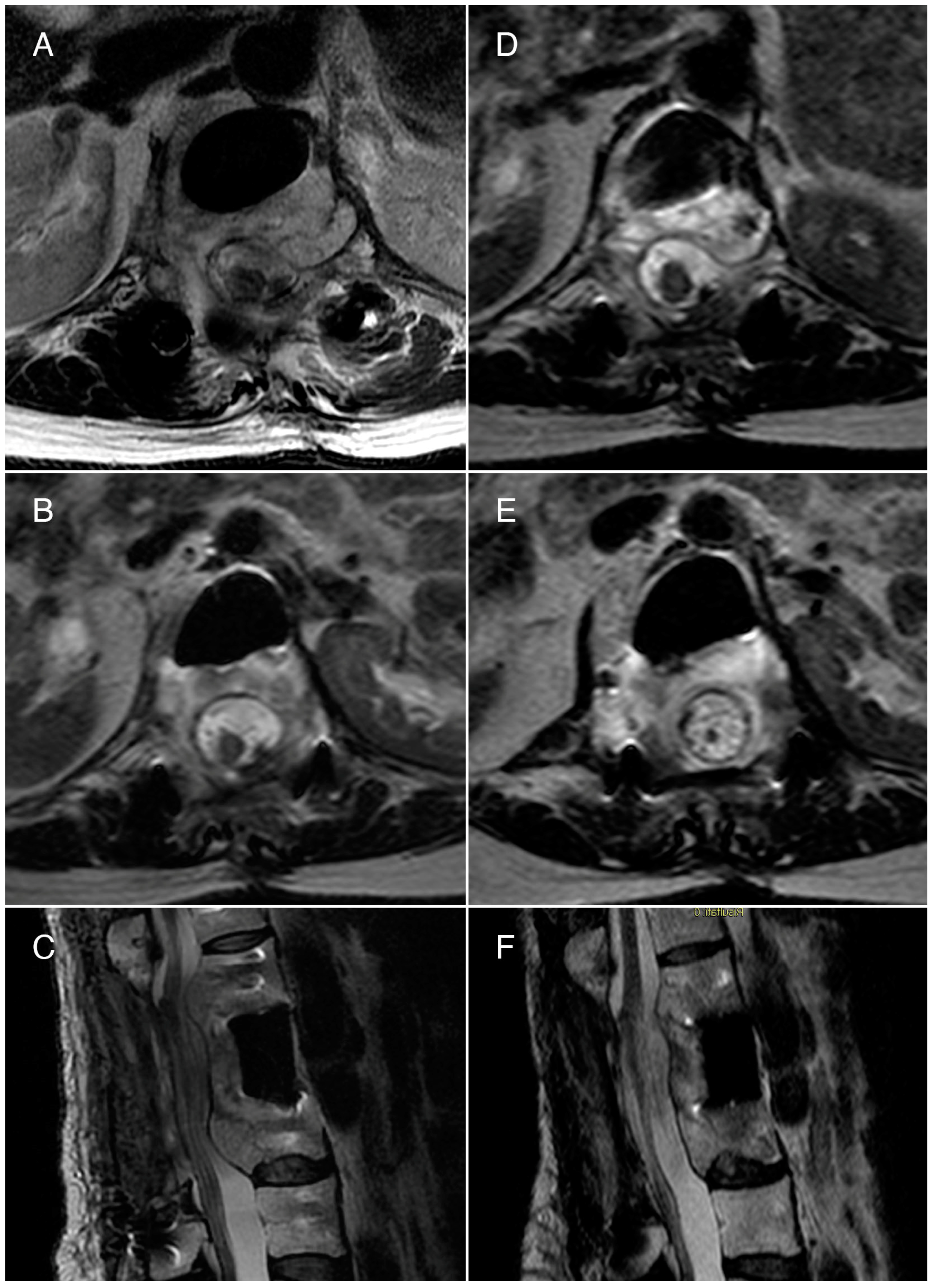
3.1. Case 1
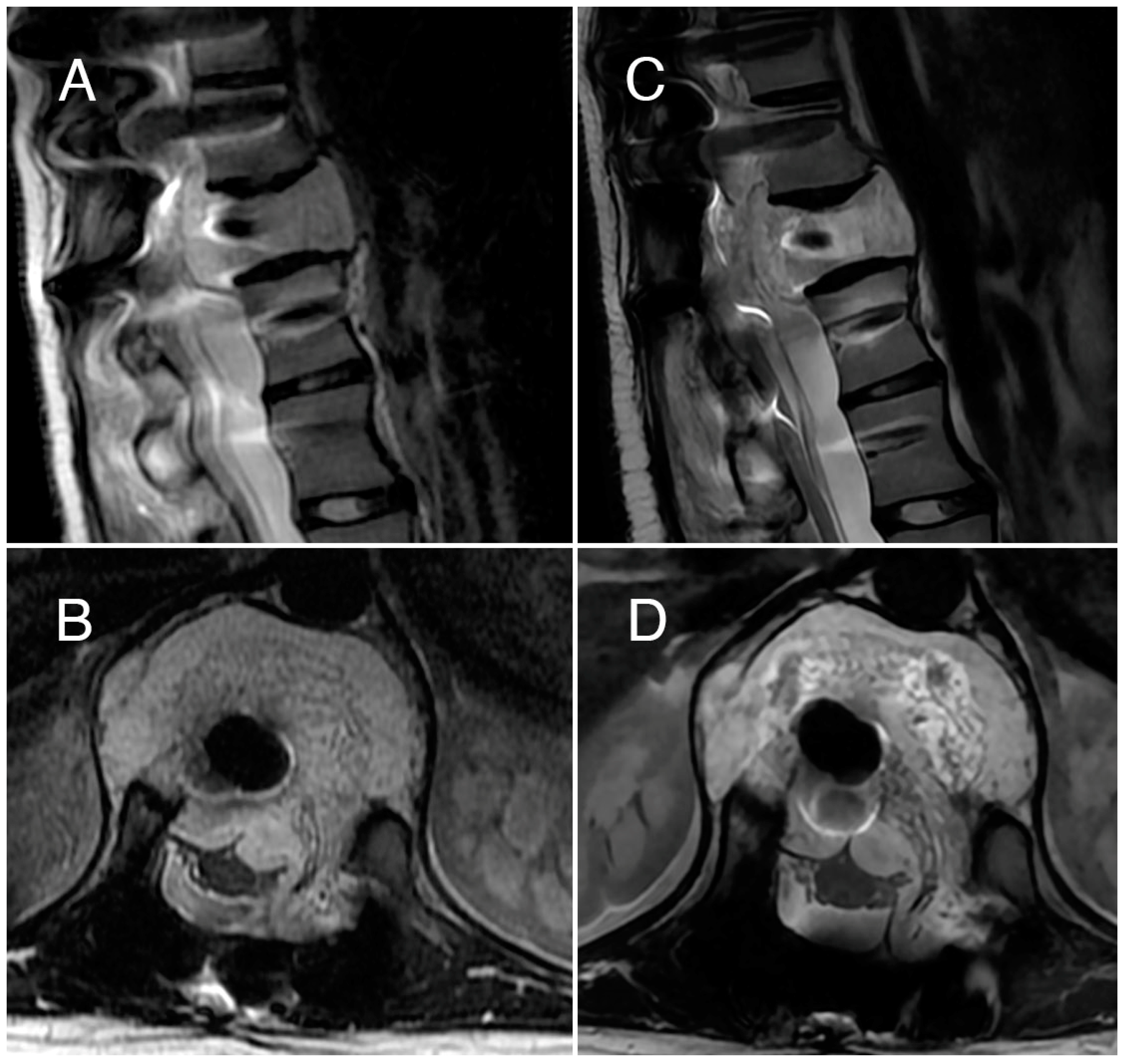
3.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Kamal, R.; Rathi, A.K. Vertebral Hemangioma—The Current Radiation Therapy Perspective. Rep. Pract. Oncol. Radiother. 2023, 28, 93–101. [Google Scholar] [CrossRef]
- George, A.; Mani, V.; Noufal, A. Update on the Classification of Hemangioma. J. Oral Maxillofac. Pathol. 2014, 18, 117. [Google Scholar] [CrossRef]
- Bruder, E.; Perez-Atayde, A.R.; Jundt, G.; Alomari, A.I.; Rischewski, J.; Fishman, S.J.; Mulliken, J.B.; Kozakewich, H.P.W. Vascular Lesions of Bone in Children, Adolescents, and Young Adults. A Clinicopathologic Reappraisal and Application of the ISSVA Classification. Virchows Arch. 2009, 454, 161–179. [Google Scholar] [CrossRef]
- Gaudino, S.; Martucci, M.; Colantonio, R.; Lozupone, E.; Visconti, E.; Leone, A.; Colosimo, C. A Systematic Approach to Vertebral Hemangioma. Skelet. Radiol. 2015, 44, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, S.A.; Mamourian, A.; Schmitt, J.E.; Cloran, F.; Vossough, A.; Pukenas, B.; Loevner, L.A.; Mohan, S. Utility of Fat-Suppressed Sequences in Differentiation of Aggressive vs Typical Asymptomatic Haemangioma of the Spine. Br. J. Radiol. 2016, 89, 20150557. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F. Muscoloskeletal Tumor Surgery; Churchill Livingstone: London, UK, 1983; Volume 1. [Google Scholar]
- Fox, M.W.; Onofrio, B.M. The Natural History and Management of Symptomatic and Asymptomatic Vertebral Hemangiomas. J. Neurosurg. 1993, 78, 36–45. [Google Scholar] [CrossRef]
- Acosta, F.L.; Sanai, N.; Chi, J.H.; Dowd, C.F.; Chin, C.; Tihan, T.; Chou, D.; Weinstein, P.R.; Ames, C.P. Comprehensive Management of Symptomatic and Aggressive Vertebral Hemangiomas. Neurosurg. Clin. N. Am. 2008, 19, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Robinson, Y.; Sheta, R.; Salci, K.; Willander, J. Blood Loss in Surgery for Aggressive Vertebral Haemangioma with and without Embolisation. Asian Spine J. 2015, 9, 483. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Akbari, S.; Rasekhi, A.; Kazeminezhad, A.; Motlagh, M.A.S.; Taherpour, S. A Less Aggressive Approach to the Treatment of Aggressive Vertebral Hemangioma of the Thoracic Spine: A Case Report and Literature Review. Int. J. Surg. Case Rep. 2023, 105, 108027. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.G.; Yuan, H.S.; Yang, S.M.; Li, J.; Wei, F.; Liu, C.; Dang, L.; Liu, Z.J. Diagnosis and Treatment of Vertebral Hemangiomas with Neurologic Deficit: A Report of 29 Cases and Literature Review. Spine J. 2014, 14, 944–954. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Angelini, A.; Vottis, C.; Pala, E.; Calabrò, T.; Papagelopoulos, P.J.; Ruggieri, P. Modern Palliative Treatments for Metastatic Bone Disease: Awareness of Advantages, Disadvantages, and Guidance. Clin. J. Pain 2016, 32, 337–350. [Google Scholar] [CrossRef]
- Hay, J.; Shahzeidi, S.; Laurent, G. Mechanism of Bleomycin Induced Lung Damage. Arch. Toxicol. 1990, 65, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Muir, T.; Bertino, G.; Groselj, A.; Ratnam, L.; Kis, E.; Odili, J.; McCafferty, I.; Wohlgemuth, W.A.; Cemazar, M.; Krt, A.; et al. Bleomycin Electrosclerotherapy (BEST) for the Treatment of Vascular Malformations. An International Network for Sharing Practices on Electrochemotherapy (InspECT) Study Group Report. Radiol. Oncol. 2023, 57, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, L.; Cevolani, L.; De Terlizzi, F.; Saenz, L.; Alì, N.; Bianchi, G.; Donati, D.M. Electrochemotherapy Is Effective in the Treatment of Bone Metastases. Curr. Oncol. 2022, 29, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Gaudy, C.; Richard, M.-A.; Folchetti, G.; Bonerandi, J.J.; Grob, J.-J. Randomized Controlled Study of Electrochemotherapy in the Local Treatment of Skin Metastases of Melanoma. J. Cutan. Med. Surg. 2006, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bik, L.; Sangers, T.; Greveling, K.; Prens, E.; Haedersdal, M.; Van Doorn, M. Efficacy and Tolerability of Intralesional Bleomycin in Dermatology: A Systematic Review. J. Am. Acad. Dermatol. 2020, 83, 888–903. [Google Scholar] [CrossRef]
- Hu, E.Y.; Bhagavatula, S.K.; Shi, A.; Merriam, P.; Levesque, V.M.; Shyn, P.B. Image-Guided Percutaneous Ablation of Hepatic Epithelioid Hemangioendothelioma. Abdom. Radiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ayoobi Yazdi, N.; Mehrabinejad, M.-M.; Dashti, H.; Pourghorban, R.; Nassiri Toosi, M.; Rokni Yazdi, H. Percutaneous Sclerotherapy with Bleomycin and Ethiodized Oil: A Promising Treatment in Symptomatic Giant Liver Hemangioma. Radiology 2021, 301, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard Operating Procedures of the Electrochemotherapy: Instructions for the Use of Bleomycin or Cisplatin Administered Either Systemically or Locally and Electric Pulses Delivered by the CliniporatorTM by Means of Invasive or Non-Invasive Electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Cindrič, H.; Kos, B.; Tedesco, G.; Cadossi, M.; Gasbarrini, A.; Miklavčič, D. Electrochemotherapy of Spinal Metastases Using Transpedicular Approach—A Numerical Feasibility Study. Technol. Cancer Res. Treat. 2018, 17, 153303461877025. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated Standard Operating Procedures for Electrochemotherapy of Cutaneous Tumours and Skin Metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Campanacci, L.; Bianchi, G.; Cevolani, L.; Errani, C.; Ciani, G.; Facchini, G.; Spinnato, P.; Tognù, A.; Massari, L.; Cornelis, F.H.; et al. Operating Procedures for Electrochemotherapy in Bone Metastases: Results from a Multicenter Prospective Study on 102 Patients. Eur. J. Surg. Oncol. 2021, 47, 2609–2617. [Google Scholar] [CrossRef]
- Laredo, J.D.; Reizine, D.; Bard, M.; Merland, J.J. Vertebral Hemangiomas: Radiologic Evaluation. Radiology 1986, 161, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, C.L.; Varga, P.P.; Gokaslan, Z.L.; Boriani, S.; Luzzati, A.; Rhines, L.; Fisher, C.G.; Chou, D.; Williams, R.P.; Dekutoski, M.B.; et al. Spinal Hemangiomas: Results of Surgical Management for Local Recurrence and Mortality in a Multicenter Study. Spine 2015, 40, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Liu, C.; Yang, S.M.; Jiang, L.; Liu, Z.J.; Liu, X.G.; Yuan, H.S.; Wei, F.; Yu, M. Aggressive Vertebral Hemangioma of the Thoracic Spine without Typical Radiological Appearance. Eur. Spine J. 2012, 21, 1994–1999. [Google Scholar] [CrossRef]
- Tomasian, A.; Wallace, A.N.; Jennings, J.W. Benign Spine Lesions: Advances in Techniques for Minimally Invasive Percutaneous Treatment. Am. J. Neuroradiol. 2017, 38, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Tomasian, A.; Jennings, J.W. Vertebral Hemangioma: Percutaneous Minimally Invasive Image-Guided Radiofrequency Ablation. J. Vasc. Interv. Radiol. 2020, 31, 1949–1952.e1. [Google Scholar] [CrossRef]
- Doppman, J.L.; Oldfield, E.H.; Heiss, J.D. Symptomatic Vertebral Hemangiomas: Treatment by Means of Direct Intralesional Injection of Ethanol. Radiology 2000, 214, 341–348. [Google Scholar] [CrossRef]
- Premat, K.; Clarençon, F.; Cormier, É.; Mahtout, J.; Bonaccorsi, R.; Degos, V.; Chiras, J. Long-Term Outcome of Percutaneous Alcohol Embolization Combined with Percutaneous Vertebroplasty in Aggressive Vertebral Hemangiomas with Epidural Extension. Eur. Radiol. 2017, 27, 2860–2867. [Google Scholar] [CrossRef]
- Srinivasan, G.; Moses, V.; Padmanabhan, A.; Ahmed, M.; Keshava, S.N.; Krishnan, V.; Joseph, B.V.; Raju, K.P.; Rajshekhar, V. Utility of Spinal Angiography and Arterial Embolization in Patients Undergoing CT Guided Alcohol Injection of Aggressive Vertebral Hemangiomas. Neuroradiology 2021, 63, 1935–1945. [Google Scholar] [CrossRef]
- Goyal, M.; Mishra, N.K.; Sharma, A.; Gaikwad, S.B.; Mohanty, B.K.; Sharma, S. Alcohol Ablation of Symptomatic Vertebral Hemangiomas. Am. J. Neuroradiol. 1999, 20, 1091–1096. [Google Scholar]
- Sharma, D.; Jain, V.; Rath, G.P. Asystole during Percutaneous Ethanol Injection of Symptomatic Vertebral Haemangioma. Anaesth. Intensive Care 2006, 34, 656–658. [Google Scholar] [CrossRef]
- Wang, B.; Meng, N.; Zhuang, H.; Han, S.; Yang, S.; Jiang, L.; Wei, F.; Liu, X.; Liu, Z. The Role of Radiotherapy and Surgery in the Management of Aggressive Vertebral Hemangioma: A Retrospective Study of 20 Patients. Med. Sci. Monit. 2018, 24, 6840–6850. [Google Scholar] [CrossRef]
- Miszczyk, L.; Tukiendorf, A. Radiotherapy of Painful Vertebral Hemangiomas: The Single Center Retrospective Analysis of 137 Cases. Int. J. Radiat. Oncol. 2012, 82, e173–e180. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, V.S.; Chi, J.H.; Groff, M.W. Surgical Treatment of Aggressive Vertebral Hemangiomas. Neurosurg. Focus 2016, 41, E7. [Google Scholar] [CrossRef] [PubMed]
- Teferi, N.; Chowdhury, A.; Mehdi, Z.; Challa, M.; Eschbacher, K.; Bathla, G.; Hitchon, P. Surgical Management of Symptomatic Vertebral Hemangiomas: A Single Institution Experience and Literature Review. Spine J. 2023, 23, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Koci, T.; Mehringer, C.M.; Tsai, F.Y.; Fraser, K.W.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hieshima, G.B. Transarterial Embolization of Vertebral Hemangioma. J. Vasc. Interv. Radiol. 1993, 4, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Tsoneva, I.; Semkova, S.; Bakalova, R.; Zhelev, Z.; Nuss, P.; Staneva, G.; Nikolova, B. Electroporation, Electrochemotherapy and Electro-Assisted Drug Delivery in Cancer. A State-of-the-Art Review. Biophys. Chem. 2022, 286, 106819. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of Cytotoxicity by Electropermeabilization: An Improved Method for Screening Drugs. Anti-Cancer Drugs 1998, 9, 319–326. [Google Scholar] [CrossRef]
- Cemazar, M.; Parkins, C.S.; Holder, A.L.; Chaplin, D.J.; Tozer, G.M.; Sersa, G. Electroporation of Human Microvascular Endothelial Cells: Evidence for an Anti-Vascular Mechanism of Electrochemotherapy. Br. J. Cancer 2001, 84, 565–570. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Campos, W.K.; Campanacci, L.; Boriani, S. Electrochemotherapy to Metastatic Spinal Melanoma: A Novel Treatment of Spinal Metastasis? Spine 2015, 40, E1340–E1346. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, F.; Tselikas, L.; Yevich, S.; Bonnet, B.; Roux, C.; Kobe, A.; Besse, B.; Berthelot, K.; Gaudin, A.; Mir, L.M.; et al. Electrochemotherapy in Radiotherapy-Resistant Epidural Spinal Cord Compression in Metastatic Cancer Patients. Eur. J. Cancer 2023, 186, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Salamanna, F.; Ronchetti, M.; Cavani, F.; Gasbarrini, A.; Boriani, S.; Fini, M. Feasibility of Electroporation in Bone and in the Surrounding Clinically Relevant Structures: A Preclinical Investigation. Technol. Cancer Res. Treat. 2016, 15, 737–748. [Google Scholar] [CrossRef]
- Fini, M.; Tschon, M.; Ronchetti, M.; Cavani, F.; Bianchi, G.; Mercuri, M.; Alberghini, M.; Cadossi, R. Ablation of Bone Cells by Electroporation. J. Bone Jt. Surg. Br. 2010, 92-B, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zheng, J.; Yan, M.; Ding, W.; Xu, K.; Fan, Q.; Li, Z. The Effect of Irreversible Electroporation on the Femur: Experimental Study in a Rabbit Model. Sci. Rep. 2015, 5, 18187. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Salati, S.; Petrillo, A.; Di Bernardo, E.; Grassi, R.; Palaia, R.; Danti, G.; La Porta, M.; Cadossi, M.; et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. Int. J. Environ. Res. Public Health 2021, 18, 5592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, G.; Noli, L.E.; Griffoni, C.; Ghermandi, R.; Facchini, G.; Peta, G.; Papalexis, N.; Asunis, E.; Pasini, S.; Gasbarrini, A. Electrochemotherapy in Aggressive Hemangioma of the Spine: A Case Series and Narrative Literature Review. J. Clin. Med. 2024, 13, 1239. https://doi.org/10.3390/jcm13051239
Tedesco G, Noli LE, Griffoni C, Ghermandi R, Facchini G, Peta G, Papalexis N, Asunis E, Pasini S, Gasbarrini A. Electrochemotherapy in Aggressive Hemangioma of the Spine: A Case Series and Narrative Literature Review. Journal of Clinical Medicine. 2024; 13(5):1239. https://doi.org/10.3390/jcm13051239
Chicago/Turabian StyleTedesco, Giuseppe, Luigi Emanuele Noli, Cristiana Griffoni, Riccardo Ghermandi, Giancarlo Facchini, Giuliano Peta, Nicolas Papalexis, Emanuela Asunis, Stefano Pasini, and Alessandro Gasbarrini. 2024. "Electrochemotherapy in Aggressive Hemangioma of the Spine: A Case Series and Narrative Literature Review" Journal of Clinical Medicine 13, no. 5: 1239. https://doi.org/10.3390/jcm13051239
APA StyleTedesco, G., Noli, L. E., Griffoni, C., Ghermandi, R., Facchini, G., Peta, G., Papalexis, N., Asunis, E., Pasini, S., & Gasbarrini, A. (2024). Electrochemotherapy in Aggressive Hemangioma of the Spine: A Case Series and Narrative Literature Review. Journal of Clinical Medicine, 13(5), 1239. https://doi.org/10.3390/jcm13051239






