Obsessive–Compulsive Disorder as an Epiphenomenon of Comorbid Bipolar Disorder? An Updated Systematic Review
Abstract
1. Introduction
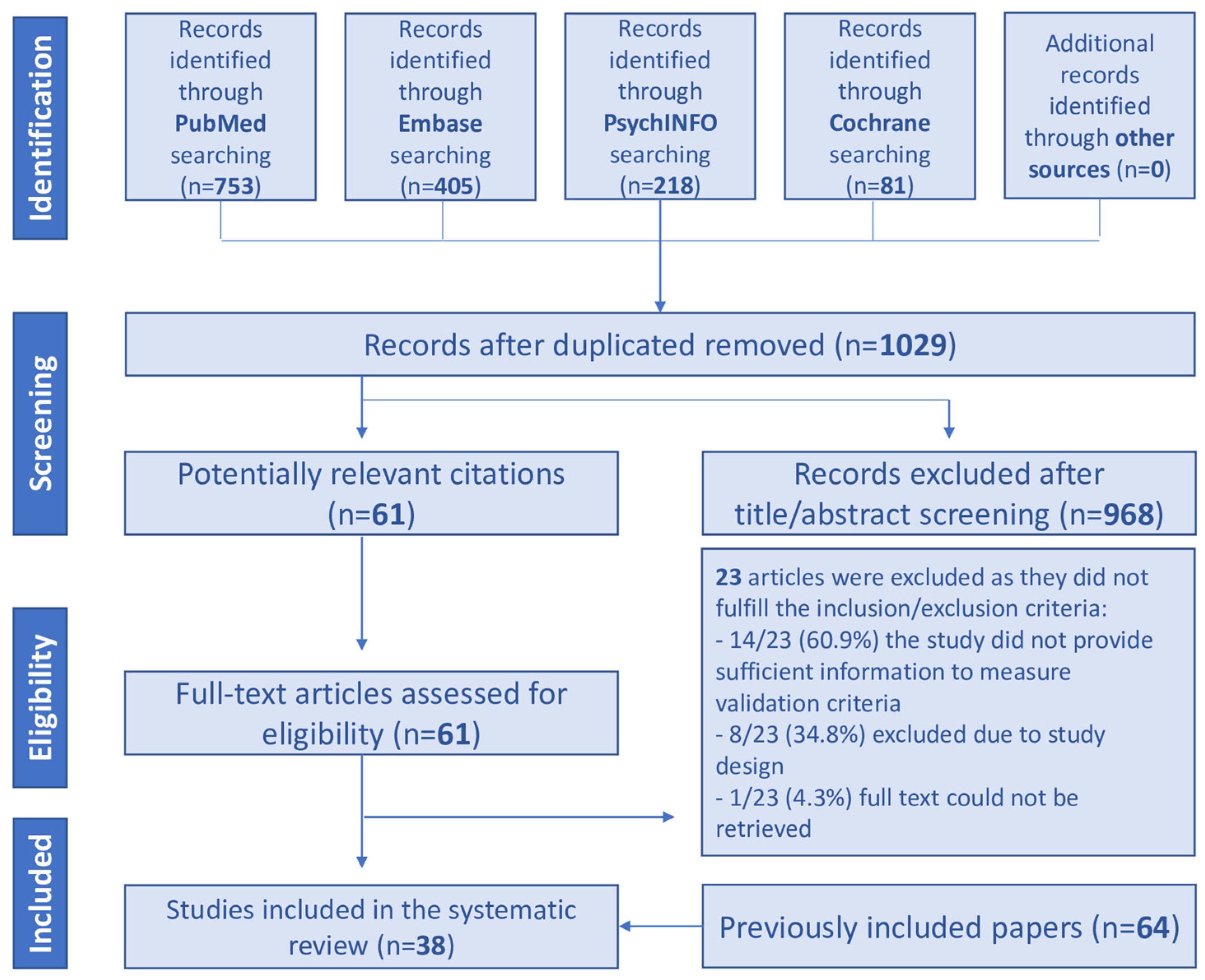
2. Materials and Methods
2.1. Information Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Outcome Measures
2.4. Study Selection and Data Extraction
2.5. Quality Assessment
3. Results
3.1. Included Studies
3.2. Comorbidity Rates
3.2.1. Comorbid OCD in BD Patients
3.2.2. Comorbid BD in OCD Patients
3.3. Phenomenology
3.4. Course of Illness
3.4.1. Age and Type of Onset
3.4.2. Course of Illness
3.4.3. Global Functioning and Quality of Life
3.4.4. Suicide Ideation and Attempts
3.4.5. Hospitalization
3.4.6. Substance and Alcohol Abuse
3.4.7. Other Psychiatric and General Medicine Comorbidities
3.4.8. Heredity
3.4.9. Biological Markers
3.4.10. Treatment
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feinstein, A.R. The pre-therapeutic classification of co-morbidity in chronic disease. J. Chronic Dis. 1970, 23, 455–468. [Google Scholar] [CrossRef]
- Jakovljević, M.; Ostojić, L. Comorbidity and multimorbidity in medicine today: Challenges and opportunities for bringing separated branches of medicine closer to each other. Psychiatr. Danub. 2013, 25 (Suppl. S1), 18–28. [Google Scholar] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition—Text Revision (DSM-5-TR); American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Amerio, A.; Stubbs, B.; Odone, A.; Tonna, M.; Marchesi, C.; Ghaemi, S. The prevalence and predictors of comorbid bipolar disorder and obsessive–compulsive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2015, 186, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Amerio, A.; Odone, A.; Marchesi, C.; Ghaemi, S.N. Treatment of comorbid bipolar disorder and obsessive-compulsive disorder: A systematic review. J. Affect. Disord. 2014, 166, 258–263. [Google Scholar] [CrossRef]
- Amerio, A.; Odone, A.; Liapis, C.C.; Ghaemi, S.N. Diagnostic validity of comorbid bipolar disorder and obsessive-compulsive disorder: A systematic review. Acta Psychiatr. Scand. 2014, 129, 343–358. [Google Scholar] [CrossRef]
- Amerio, A.; Maina, G.; Ghaemi, S.N. Updates in treating comorbid bipolar disorder and obsessive-compulsive disorder: A systematic review. J. Affect. Disord. 2019, 256, 433–440. [Google Scholar] [CrossRef]
- Amerio, A.; Stubbs, B.; Odone, A.; Tonna, M.; Marchesi, C.; Nassir Ghaemi, S. Bipolar I and II Disorders; A Systematic Review and Meta-Analysis on Differences in Comorbid Obsessive-Compulsive Disorder. Iran. J. Psychiatry Behav. Sci. 2016, 10, e3604. [Google Scholar] [CrossRef]
- De Prisco, M.; Tapoi, C.; Oliva, V.; Possidente, C.; Strumila, R.; Takami Lageborn, C.; Bracco, L.; Girone, N.; Macellaro, M.; Vieta, E.; et al. Clinical features in co-occuring obsessive-compulsive disorder and bipolar disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2024, 80, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Robins, E.; Guze, S.B. Establishment of Diagnostic Validity in Psychiatric Illness: Its Application to Schizophrenia. Am. J. Psychiatry 1970, 126, 983–987. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Geddes, J.R.; Goodwin, G.M.; Rendell, J.; Azorin, J.-M.; Cipriani, A.; Ostacher, M.J.; Morriss, R.; Alder, N.; Juszczak, E. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): A randomised open-label trial. Lancet 2010, 375, 385–395. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders Clinical Descriptions and Diagnostic Guidelines; WHO: Geneva, Switzerland, 1992.
- Angst, J.; Gamma, A.; Endrass, J.; Goodwin, R.; Ajdacic, V.; Eich, D.; Rössler, W. Obsessive-compulsive severity spectrum in the community: Prevalence, comorbidity, and course. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 156–164. [Google Scholar] [CrossRef]
- Angst, J.; Gamma, A.; Endrass, J.; Hantouche, E.; Goodwin, R.; Ajdacic, V.; Eich, D.; Rössler, W. Obsessive-compulsive syndromes and disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 255, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hantouche, E.G.; Angst, J.; Demonfaucon, C.; Perugi, G.; Lancrenon, S.; Akiskal, H.S. Cyclothymic OCD: A distinct form? J. Affect. Disord. 2003, 75, 1–10. [Google Scholar] [CrossRef]
- Mukaddes, N.M.; Fateh, R. High rates of psychiatric co-morbidity in individuals with Asperger’s disorder. World J. Biol. Psychiatry 2009, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bachen, E.A.; Chesney, M.A.; Criswell, L.A. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum. 2009, 61, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Abramovitch, A.; Anholt, G.E.; Cooperman, A.; van Balkom, A.J.L.M.; Giltay, E.J.; Penninx, B.W.; van Oppen, P. Body mass index in obsessive-compulsive disorder. J. Affect. Disord. 2019, 245, 145–151. [Google Scholar] [CrossRef]
- Adam, Y.; Meinlschmidt, G.; Gloster, A.T.; Lieb, R. Obsessive–compulsive disorder in the community: 12-month prevalence, comorbidity and impairment. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 339–349. [Google Scholar] [CrossRef]
- Carta, M.G.; Fineberg, N.; Moro, M.F.; Preti, A.; Romano, F.; Balestrieri, M.; Caraci, F.; Dell’Osso, L.; Disciascio, G.; Drago, F.; et al. The Burden of Comorbidity Between Bipolar Spectrum and Obsessive-Compulsive Disorder in an Italian Community Survey. Front. Psychiatry 2020, 11, 188. [Google Scholar] [CrossRef]
- Cederlöf, M.; Lichtenstein, P.; Larsson, H.; Boman, M.; Rück, C.; Landén, M.; Mataix-Cols, D. Obsessive-Compulsive Disorder, Psychosis, and Bipolarity: A Longitudinal Cohort and Multigenerational Family Study. Schizophr. Bull. 2015, 41, 1076–1083. [Google Scholar] [CrossRef]
- Chen, Y.W.; Dilsaver, S.C. Comorbidity for obsessive-compulsive disorder in bipolar and unipolar disorders. Psychiatry Res. 1995, 59, 57–64. [Google Scholar] [CrossRef]
- Faravelli, C.; Abrardi, L.; Bartolozzi, D.; Cecchi, C.; Cosci, F.; D’Adamo, D.; Lo Iacono, B.; Ravaldi, C.; Scarpato, M.A.; Truglia, E.; et al. The Sesto Fiorentino Study: Background, Methods and Preliminary Results. Psychother. Psychosom. 2004, 73, 216–225. [Google Scholar] [CrossRef]
- Fineberg, N.A.; Hengartner, M.P.; Bergbaum, C.; Gale, T.; Rössler, W.; Angst, J. Lifetime comorbidity of obsessive-compulsive disorder and sub-threshold obsessive-compulsive symptomatology in the community: Impact, prevalence, socio-demographic and clinical characteristics. Int. J. Psychiatry Clin. Pract. 2013, 17, 188–196. [Google Scholar] [CrossRef]
- Fireman, B.; Koran, L.M.; Leventhal, J.L.; Jacobson, A. The Prevalence of Clinically Recognized Obsessive-Compulsive Disorder in a Large Health Maintenance Organization. Am. J. Psychiatry 2001, 158, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-C.; Tsai, K.-J.; Wang, H.-K.; Sung, P.-S.; Wu, M.-H.; Hung, K.-W.; Lin, S.-H. Prevalence, incidence, and comorbidity of clinically diagnosed obsessive–compulsive disorder in Taiwan: A national population-based study. Psychiatry Res. 2014, 220, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.A.; Petukhova, M.; Kessler, R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; Abdin, E.; Vaingankar, J.; Shafie, S.; Chang, S.; Seow, E.; Chua, B.Y.; Jeyagurunathan, A.; Heng, D.; Kwok, K.W.; et al. Obsessive-Compulsive Disorder in Singapore: Prevalence, Comorbidity, Quality of Life and Social Support. Ann. Acad. Med. Singap. 2020, 49, 15–25. [Google Scholar] [CrossRef]
- Teh, W.L.; Abdin, E.; Vaingankar, J.; Shafie, S.; Yiang Chua, B.; Sambasivam, R.; Zhang, Y.; Shahwan, S.; Chang, S.; Mok, Y.M.; et al. Prevalence and correlates of bipolar spectrum disorders in Singapore: Results from the 2016 Singapore Mental Health Study (SMHS 2016). J. Affect. Disord. 2020, 274, 339–346. [Google Scholar] [CrossRef]
- Anholt, G.E.; Aderka, I.M.; van Balkom, A.J.L.M.; Smit, J.H.; Schruers, K.; van der Wee, N.J.A.; Eikelenboom, M.; De Luca, V.; van Oppen, P. Age of onset in obsessive–compulsive disorder: Admixture analysis with a large sample. Psychol. Med. 2014, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Baptista, T.; Galué, L.; Martínez, F. Bidirectional comorbidity between bipolarand obsessive-compulsive disorders: Symptoms frequency, treatment challenges and underexplored areas. Investig. Clin. 2020, 61, 189–195. [Google Scholar] [CrossRef]
- Benatti, B.; Dell’Osso, B.; Arici, C.; Hollander, E.; Altamura, A.C. Characterizing impulsivity profile in patients with obsessive–compulsive disorder. Int. J. Psychiatry Clin. Pract. 2014, 18, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Dafeeah, E.E.; Abdulla, M.A.; Abou-Saleh, M.T.; Ventriglio, A. Comorbid obsessive-compulsive disorder and bipolar disorder in a highly endogamous population: Which came first? Int. J. Cult. Ment. Health 2016, 9, 407–413. [Google Scholar] [CrossRef]
- Berkol, T.D.; Aytac, H.M. Comparison of Clinical Features of Bipolar Disorder Patients with and without Psychiatric Comorbidity. Eurasian J. Med. 2021, 53, 203–207. [Google Scholar] [CrossRef]
- Bogetto, F.; Venturello, S.; Albert, U.; Maina, G.; Ravizza, L. Gender-related clinical differences in obsessive-compulsive disorder. Eur. Psychiatry 1999, 14, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Boylan, K.R.; Bieling, P.J.; Marriott, M.; Begin, H.; Young, L.T.; MacQueen, G.M. Impact of Comorbid Anxiety Disorders on Outcome in a Cohort of Patients With Bipolar Disorder. J. Clin. Psychiatry 2004, 65, 1106–1113. [Google Scholar] [CrossRef]
- Bramante, S.; Quarato, F.; Mehanović, E.; Rigardetto, S.; Maina, G. The forbidden thoughts dimension and psychiatric comorbidities in a large sample of OCD patients: A possible link to bipolar I comorbid disorder. J. Obsessive. Compuls. Relat. Disord. 2021, 29, 100642. [Google Scholar] [CrossRef]
- Braverman, L.; Fuchs, C.; Weizman, A.; Poyurovsky, M. Elevated rate of OCD-spectrum and tic disorders in patients with bipolar depression and comorbid OCD. J. Obsessive. Compuls. Relat. Disord. 2021, 29, 100643. [Google Scholar] [CrossRef]
- Braverman, L.; Fuchs, C.; Weizman, A.; Poyurovsky, M. Rate of OCD and sub-threshold OCD in bipolar disorder patients with first depressive episode. Psychiatry Res. 2021, 302, 114010. [Google Scholar] [CrossRef]
- Cannon, D.M.; Ichise, M.; Fromm, S.J.; Nugent, A.C.; Rollis, D.; Gandhi, S.K.; Klaver, J.M.; Charney, D.S.; Manji, H.K.; Drevets, W.C. Serotonin Transporter Binding in Bipolar Disorder Assessed using [11C]DASB and Positron Emission Tomography. Biol. Psychiatry 2006, 60, 207–217. [Google Scholar] [CrossRef]
- Cassano, G.B.; Pini, S.; Saettoni, M.; Dell’Osso, L. Multiple Anxiety Disorder Comorbidity in Patients With Mood Spectrum Disorders With Psychotic Features. Am. J. Psychiatry 1999, 156, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Centorrino, F.; Hennen, J.; Mallya, G.; Egli, S.; Clark, T.; Baldessarini, R.J. Clinical outcome in patients with bipolar I disorder, obsessive compulsive disorder or both. Hum. Psychopharmacol. Clin. Exp. 2006, 21, 189–193. [Google Scholar] [CrossRef]
- Cosoff, S.J.; Hafner, R.J. The prevalence of comorbid anxiety in schizophrenia, schizoaffective disorder and bipolar disorder. Aust. N. Z. J. Psychiatry 1998, 32, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.; Hwang, M.Y.; Bromet, E.J. Obsessive-Compulsive and Panic Symptoms in Patients With First-Admission Psychosis. Am. J. Psychiatry 2002, 159, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Das, A. Anxiety Disorders in Bipolar I Mania: Prevalence, Effect on Illness Severity, and Treatment Implications. Indian J. Psychol. Med. 2013, 35, 53–59. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, R.; Aloi, M.; Bruni, A.; Gaetano, R.; Segura-Garcia, C.; De Fazio, P. Bipolar disorder and obsessive compulsive disorder: The comorbidity does not further impair the neurocognitive profile. J. Affect. Disord. 2018, 235, 1–6. [Google Scholar] [CrossRef]
- Di Salvo, G.; Pessina, E.; Aragno, E.; Martini, A.; Albert, U.; Maina, G.; Rosso, G. Impact of comorbid obsessive-compulsive disorder on suicidality in patients with bipolar disorder. Psychiatry Res. 2020, 290, 113088. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Pini, S.; Tundo, A.; Sarno, N.; Musetti, L.; Cassano, G.B. Clinical characteristics of mania, mixed mania, and bipolar depression with psychotic features. Compr. Psychiatry 2000, 41, 242–247. [Google Scholar] [CrossRef]
- Dilsaver, S.C.; Benazzi, F.; Akiskal, K.K.; Akiskal, H.S. Differential patterns of lifetime multiple anxiety disorder comorbidity between Latino adults with bipolar I and major depressive disorders. Bull. Menn. Clin. 2008, 72, 130–148. [Google Scholar] [CrossRef]
- Diniz, J.B.; Rosario-Campos, M.C.; Shavitt, R.G.; Curi, M.; Hounie, A.G.; Brotto, S.A.; Miguel, E.C. Impact of Age at Onset and Duration of Illness on the Expression of Comorbidities in Obsessive-Compulsive Disorder. J. Clin. Psychiatry 2004, 65, 22–27. [Google Scholar] [CrossRef]
- Domingues-Castro, M.S.; Torresan, R.C.; Shavitt, R.G.; Fontenelle, L.F.; Ferrão, Y.A.; Rosário, M.C.; Torres, A.R. Bipolar disorder comorbidity in patients with obsessive-compulsive disorder: Prevalence and predictors. J. Affect. Disord. 2019, 256, 324–330. [Google Scholar] [CrossRef]
- Edmonds, L.K.; Mosley, B.J.; Admiraal, A.J.; Olds, R.J.; Romans, S.E.; Silverstone, T.; Walsh, A.E.S. Familial Bipolar Disorder: Preliminary Results from the Otago Familial Bipolar Genetic Study. Aust. N. Z. J. Psychiatry 1998, 32, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Goes, F.S.; McCusker, M.G.; Bienvenu, O.J.; MacKinnon, D.F.; Mondimore, F.M.; Schweizer, B.; DePaulo, J.R.; Potash, J.B. Co-morbid anxiety disorders in bipolar disorder and major depression: Familial aggregation and clinical characteristics of co-morbid panic disorder, social phobia, specific phobia and obsessive-compulsive disorder. Psychol. Med. 2012, 42, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; LaSalle-Ricci, V.H.; Ronquillo, J.G.; Crawley, S.A.; Cochran, L.W.; Kazuba, D.; Greenberg, B.D.; Murphy, D.L. Obsessive–compulsive disorder symptom dimensions show specific relationships to psychiatric comorbidity. Psychiatry Res. 2005, 135, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Van den Bulke, D.; Bellivier, F.; Etain, B.; Rouillon, F.; Leboyer, M. Anxiety disorders in 318 bipolar patients: Prevalence and impact on illness severity and response to mood stabilizer. J. Clin. Psychiatry 2003, 64, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Issler, C.K.; Amaral, J.A.D.M.S.; Tamada, R.S.; Schwartzmann, A.M.; Shavitt, R.G.; Miguel, E.C.; Lafer, B. Clinical expression of obsessive-compulsive disorder in women with bipolar disorder. Rev. Bras. Psiquiatr. 2005, 27, 139–142. [Google Scholar] [CrossRef]
- Issler, C.K.; Monkul, E.S.; Amaral, J.A.D.M.S.; Tamada, R.S.; Shavitt, R.G.; Miguel, E.C.; Lafer, B. Bipolar disorder and comorbid obsessive-compulsive disorder is associated with higher rates of anxiety and impulse control disorders. Acta Neuropsychiatr. 2010, 22, 81–86. [Google Scholar] [CrossRef]
- Jakubovski, E.; Diniz, J.B.; Valerio, C.; Fossaluza, V.; Belotto-Silva, C.; Gorenstein, C.; Miguel, E.; Shavitt, R.G. Clinical predictors of long-term outcome in obsessive-compulsive disorder. Depress. Anxiety 2013, 30, 763–772. [Google Scholar] [CrossRef]
- Jeon, S.; Baek, J.H.; Yang, S.Y.; Choi, Y.; Ahn, S.W.; Ha, K.; Hong, K.S. Exploration of comorbid obsessive-compulsive disorder in patients with bipolar disorder: The clinic-based prevalence rate, symptoms nature and clinical correlates. J. Affect. Disord. 2018, 225, 227–233. [Google Scholar] [CrossRef]
- Kazhungil, F.; Cholakottil, A.; Kattukulathil, S.; Kottelassal, A.; Vazhakalayil, R. Clinical and familial profile of bipolar disorder with and without obsessive-compulsive disorder: An Indian study. Trends Psychiatry Psychother. 2017, 39, 270–275. [Google Scholar] [CrossRef][Green Version]
- Khan, Q.U.A.; Younus, S.; Hasan, H.; Khan, M.Z. Association of bipolar I disorder with obsessive compulsive disorder: A clinical study from Pakistan. Neurol. Psychiatry Brain Res. 2019, 33, 89–92. [Google Scholar] [CrossRef]
- Kemp, D.E.; Sylvia, L.G.; Calabrese, J.R.; Nierenberg, A.A.; Thase, M.E.; Reilly-Harrington, N.A.; Ostacher, M.J.; Leon, A.C.; Ketter, T.A.; Friedman, E.S.; et al. General medical burden in bipolar disorder: Findings from the LiTMUS comparative effectiveness trial. Acta Psychiatr. Scand. 2014, 129, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, F.; Hamidia, A.; Kheirkhah, F.; Moghadamnia, A.A.; Bijani, A.; Mirtabar, S.M.; Koutanaei, S.J. Comparison of Aripiprazole and Risperidone effectiveness in treating obsessive-compulsive disorder in patients with bipolar disorder: Double-blind, randomized clinical trial. Health Sci. Rep. 2023, 6, e1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Berk, L.; Kulkarni, J.; Dodd, S.; de Castella, A.; Fitzgerald, P.B.; Amminger, G.P.; Berk, M. Impact of comorbid anxiety disorders and obsessive-compulsive disorder on 24-month clinical outcomes of bipolar I disorder. J. Affect. Disord. 2014, 166, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, A.; Tükel, R.; Özyıldırım, İ.; Meteris, H.; Yazıcı, O. Impact of obsessive-compulsive disorder comorbidity on the sociodemographic and clinical features of patients with bipolar disorder. Compr. Psychiatry 2010, 51, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S. Comorbidity of obsessive compulsive disorder in bipolar disorder. J. Affect. Disord. 1995, 34, 117–120. [Google Scholar] [CrossRef]
- Krüger, S.; Bräunig, P.; Cooke, R.G. Comorbidity of obsessive-compulsive disorder in recovered inpatients with bipolar disorder. Bipolar Disord. 2000, 2, 71–74. [Google Scholar] [CrossRef] [PubMed]
- LaSalle-Ricci, V.H.; Arnkoff, D.B.; Glass, C.R.; Crawley, S.A.; Ronquillo, J.G.; Murphy, D.L. The hoarding dimension of OCD: Psychological comorbidity and the five-factor personality model. Behav. Res. Ther. 2006, 44, 1503–1512. [Google Scholar] [CrossRef]
- Lensi, P.; Cassano, G.B.; Correddu, G.; Ravagli, S.; Kunovac, J.L.; Akiskal, H.S. Obsessive-compulsive disorder. Familial-developmental history, symptomatology, comorbidity and course with special reference to gender-related differences. Br. J. Psychiatry 1996, 169, 101–107. [Google Scholar] [CrossRef]
- Magalhães, P.V.S.; Kapczinski, N.S.; Kapczinski, F. Correlates and impact of obsessive-compulsive comorbidity in bipolar disorder. Compr. Psychiatry 2010, 51, 353–356. [Google Scholar] [CrossRef]
- Mahasuar, R.; Janardhan Reddy, Y.C.; Math, S.B. Obsessive-compulsive disorder with and without bipolar disorder. Psychiatry Clin. Neurosci. 2011, 65, 423–433. [Google Scholar] [CrossRef]
- Maina, G.; Albert, U.; Pessina, E.; Bogetto, F. Bipolar obsessive-compulsive disorder and personality disorders. Bipolar Disord. 2007, 9, 722–729. [Google Scholar] [CrossRef]
- Marazziti, D.; Dell’Osso, L.; Di Nasso, E.; Pfanner, C.; Presta, S.; Mungai, F.; Cassano, G.B. Insight in obsessive–compulsive disorder: A study of an Italian sample. Eur. Psychiatry 2002, 17, 407–410. [Google Scholar] [CrossRef]
- McElroy, S.L.; Strakowski, S.M.; Keck, P.E.; Tugrul, K.L.; West, S.A.; Lonczak, H.S. Differences and similarities in mixed and pure mania. Compr. Psychiatry 1995, 36, 187–194. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.L.; Altshuler, L.L.; Suppes, T.; Keck, P.E.; Frye, M.A.; Denicoff, K.D.; Nolen, W.A.; Kupka, R.W.; Leverich, G.S.; Rochussen, J.R.; et al. Axis I Psychiatric Comorbidity and Its Relationship to Historical Illness Variables in 288 Patients With Bipolar Disorder. Am. J. Psychiatry 2001, 158, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Bradler, K.; Slaney, C.; Garnham, J.; Ruzickova, M.; O’Donovan, C.; Hajek, T.; Alda, M. An admixture analysis of the age at index episodes in bipolar disorder. Psychiatry Res. 2011, 188, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ozdemiroglu, F.; Sevincok, L.; Sen, G.; Mersin, S.; Kocabas, O.; Karakus, K.; Vahapoglu, F. Comorbid obsessive–compulsive disorder with bipolar disorder: A distinct form? Psychiatry Res. 2015, 230, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Perugi, G.; Akiskal, H.S.; Pfanner, C.; Presta, S.; Gemignani, A.; Milanfranchi, A.; Lensi, P.; Ravagli, S.; Cassano, G.B. The clinical impact of bipolar and unipolar affective comorbidity on obsessive–compulsive disorder. J. Affect. Disord. 1997, 46, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Perugi, G.; Akiskal, H.S.; Gemignani, A.; Pfanner, C.; Presta, S.; Milanfranchi, A.; Lensi, P.; Ravagli, S.; Maremmani, I.; Cassano, G.B. Episodic course in obsessive-compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 240–244. [Google Scholar] [CrossRef]
- Perugi, G.; Akiskal, H.S.; Ramacciotti, S.; Nassini, S.; Toni, C.; Milanfranchi, A.; Musetti, L. Depressive comorbidity of panic, social phobic, and obsessive–compulsive disorders re-examined: Is there a bipolar ii connection? J. Psychiatr. Res. 1999, 33, 53–61. [Google Scholar] [CrossRef]
- Perugi, G.; Toni, C.; Frare, F.; Travierso, M.C.; Hantouche, E.; Akiskal, H.S. Obsessive-compulsive-bipolar comorbidity: A systematic exploration of clinical features and treatment outcome. J. Clin. Psychiatry 2002, 63, 1129–1134. [Google Scholar] [CrossRef]
- Pini, S.; Cassano, G.B.; Simonini, E.; Savino, M.; Russo, A.; Montgomery, S.A. Prevalence of anxiety disorders comorbidity in bipolar depression, unipolar depression and dysthymia. J. Affect. Disord. 1997, 42, 145–153. [Google Scholar] [CrossRef]
- Pini, S.; Dell’Osso, L.; Mastrocinque, C.; Marcacci, G.; Papasogli, A.; Vignoli, S.; Pallanti, S.; Cassano, G. Axis I comorbidity in bipolar disorder with psychotic features. Br. J. Psychiatry 1999, 175, 467–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahraian, A.; Jahromi, L.R.; Ghanizadeh, A.; Mowla, A. Memantine as an Adjuvant Treatment for Obsessive Compulsive Symptoms in Manic Phase of Bipolar Disorder. J. Clin. Psychopharmacol. 2017, 37, 246–249. [Google Scholar] [CrossRef]
- Sahraian, A.; Ehsaei, Z.; Mowla, A. Aripiprazole as an adjuvant treatment for obsessive and compulsive symptoms in manic phase of bipolar disorder: A randomized, double-blind, placebo-controlled clinical trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 267–271. [Google Scholar] [CrossRef]
- Sahraian, A.; Ghahremanpouri, B.; Mowla, A. Is quetiapine effective for obsessive and compulsive symptoms in patients with bipolar disorder? A randomized, double-blind, placebo-controlled clinical trial. CNS Spectr. 2022, 27, 634–638. [Google Scholar] [CrossRef]
- Saraf, G.; Paul, I.; Viswanath, B.; Narayanaswamy, J.C.; Math, S.B.; Reddy, Y.C.J. Bipolar disorder comorbidity in patients with a primary diagnosis of OCD. Int. J. Psychiatry Clin. Pract. 2016, 21, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Saunders, E.F.H.; Fitzgerald, K.D.; Zhang, P.; McInnis, M.G. Clinical features of bipolar disorder comorbid with anxiety disorders differ between men and women. Depress. Anxiety 2012, 29, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Shabani, A.; Alizadeh, A. The specific pattern of obsessive compulsive symptoms in patients with bipolar disorder. J. Res. Med. Sci. 2008, 13, 48–54. [Google Scholar]
- Shashidhara, M.; Sushma, B.R.; Viswanath, B.; Math, S.B.; Janardhan Reddy, Y.C. Comorbid obsessive compulsive disorder in patients with bipolar-I disorder. J. Affect. Disord. 2015, 174, 367–371. [Google Scholar] [CrossRef]
- Simon, N.M.; Smoller, J.W.; Fava, M.; Sachs, G.; Racette, S.R.; Perlis, R.; Sonawalla, S.; Rosenbaum, J.F. Comparing anxiety disorders and anxiety-related traits in bipolar disorder and unipolar depression. J. Psychiatr. Res. 2003, 37, 187–192. [Google Scholar] [CrossRef]
- Simon, N.M.; Otto, M.W.; Wisniewski, S.R.; Fossey, M.; Sagduyu, K.; Frank, E.; Sachs, G.S.; Nierenberg, A.A.; Thase, M.E.; Pollack, M.H. Anxiety disorder comorbidity in bipolar disorder patients: Data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am. J. Psychiatry 2004, 161, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Strakowski, S.M.; Tohen, M.; Stoll, A.L.; Faedda, G.L.; Goodwin, D.C. Comorbidity in mania at first hospitalization. Am. J. Psychiatry 1992, 149, 554–556. [Google Scholar] [PubMed]
- Strakowski, S.M.; Sax, K.W.; McElroy, S.L.; Keck, P.E.; Hawkins, J.M.; West, S.A. Course of Psychiatric and Substance Abuse Syndromes Co-Occurring With Bipolar Disorder After a First Psychiatric Hospitalization. J. Clin. Psychiatry 1998, 59, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Tamam, L.; Ozpoyraz, N. Comorbidity of Anxiety Disorder among Patients with Bipolar I Disorder in Remission. Psychopathology 2002, 35, 203–209. [Google Scholar] [CrossRef]
- Tasdemir, A.; Tamam, L.; Keskin, N.; Evlice, Y.E. Assessment of co-morbidity of adult separation anxiety in patients with bipolar disorder. Nord. J. Psychiatry 2016, 70, 93–102. [Google Scholar] [CrossRef]
- Timpano, K.R.; Rubenstein, L.M.; Murphy, D.L. Phenomenological features and clinical impact of affective disorders in OCD: A focus on the bipolar disorder and OCD connection. Depress. Anxiety 2012, 29, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Tonna, M.; Trinchieri, M.; Lucarini, V.; Ferrari, M.; Ballerini, M.; Ossola, P.; De Panfilis, C.; Marchesi, C. Pattern of occurrence of obsessive-compulsive symptoms in bipolar disorder. Psychiatry Res. 2021, 297, 113715. [Google Scholar] [CrossRef]
- Tükel, R.; Meteris, H.; Koyuncu, A.; Tecer, A.; Yazıcı, O. The clinical impact of mood disorder comorbidity on obsessive–compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 240–245. [Google Scholar] [CrossRef]
- Tükel, R.; Oflaz, S.B.; Özyıldırım, İ.; Aslantaş, B.; Ertekin, E.; Sözen, A.; Alyanak, F.; Atlı, H. Comparison of clinical characteristics in episodic and chronic obsessive–compulsive disorder. Depress. Anxiety 2007, 24, 251–255. [Google Scholar] [CrossRef]
- Zutshi, A.; Reddy, Y.C.J.; Thennarasu, K.; Chandrashekhar, C.R. Comorbidity of anxiety disorders in patients with remitted bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 428–436. [Google Scholar] [CrossRef]
- Zutshi, A.; Kamath, P.; Reddy, Y.C.J. Bipolar and nonbipolar obsessive-compulsive disorder: A clinical exploration. Compr. Psychiatry 2007, 48, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, P.G.; do Rosario, M.C.; Cesar, R.C.; Manfro, G.G.; Moriyama, T.S.; Bloch, M.H.; Shavitt, R.G.; Hoexter, M.Q.; Coughlin, C.G.; Leckman, J.F.; et al. Obsessive–compulsive symptoms are associated with psychiatric comorbidities, behavioral and clinical problems: A population-based study of Brazilian school children. Eur. Child Adolesc. Psychiatry 2016, 25, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hofer, P.D.; Wahl, K.; Meyer, A.H.; Miché, M.; Beesdo-Baum, K.; Wong, S.F.; Grisham, J.R.; Wittchen, H.-U.; Lieb, R. Obsessive-compulsive disorder and the risk of subsequent mental disorders: A community study of adolescents and young adults. Depress. Anxiety 2018, 35, 339–345. [Google Scholar] [CrossRef]
- Dilsaver, S.C.; Akiskal, H.S.; Akiskal, K.K.; Benazzi, F. Dose–response relationship between number of comorbid anxiety disorders in adolescent bipolar/unipolar disorders, and psychosis, suicidality, substance abuse and familiality. J. Affect. Disord. 2006, 96, 249–258. [Google Scholar] [CrossRef]
- Joshi, G.; Mick, E.; Wozniak, J.; Geller, D.; Park, J.; Strauss, S.; Biederman, J. Impact of obsessive-compulsive disorder on the antimanic response to olanzapine therapy in youth with bipolar disorder. Bipolar Disord. 2010, 12, 196–204. [Google Scholar] [CrossRef]
- Joshi, G.; Wozniak, J.; Petty, C.; Vivas, F.; Yorks, D.; Biederman, J.; Geller, D. Clinical characteristics of comorbid obsessive-compulsive disorder and bipolar disorder in children and adolescents. Bipolar Disord. 2010, 12, 185–195. [Google Scholar] [CrossRef]
- Masi, G.; Perugi, G.; Toni, C.; Millepiedi, S.; Mucci, M.; Bertini, N.; Akiskal, H.S. Obsessive-compulsive bipolar comorbidity: Focus on children and adolescents. J. Affect. Disord. 2004, 78, 175–183. [Google Scholar] [CrossRef]
- Masi, G.; MIllepiedi, S.; Mucci, M.; Bertini, N.; Milantoni, L.; Arcangeli, F. A Naturalistic Study of Referred Children and Adolescents With Obsessive-Compulsive Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 673–681. [Google Scholar] [CrossRef]
- Masi, G.; Perugi, G.; Millepiedi, S.; Toni, C.; Mucci, M.; Pfanner, C.; Berloffa, S.; Pari, C.; Akiskal, H.S. Bipolar Co-morbidity in Pediatric Obsessive-Compulsive Disorder: Clinical and Treatment Implications. J. Child Adolesc. Psychopharmacol. 2007, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Millepiedi, S.; Perugi, G.; Pfanner, C.; Berloffa, S.; Pari, C.; Mucci, M. Pharmacotherapy in Paediatric Obsessive-Compulsive Disorder. CNS Drugs 2009, 23, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Millepiedi, S.; Perugi, G.; Pfanner, C.; Berloffa, S.; Pari, C.; Mucci, M.; Akiskal, H.S. A Naturalistic Exploratory Study of the Impact of Demographic, Phenotypic and Comorbid Features in Pediatric Obsessive-Compulsive Disorder. Psychopathology 2010, 43, 69–78. [Google Scholar] [CrossRef]
- Masi, G.; Berloffa, S.; Mucci, M.; Pfanner, C.; D’Acunto, G.; Lenzi, F.; Liboni, F.; Manfredi, A.; Milone, A. A naturalistic exploratory study of obsessive-compulsive bipolar comorbidity in youth. J. Affect. Disord. 2018, 231, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.; Sinha, V.K.; Sarkhel, S.; Praharaj, S.K. Co-morbidity of Obsessive-compulsive Disorder and Other Anxiety Disorders with Child and Adolescent Mood Disorders. East Asian Arch. Psychiatry 2015, 25, 58–63. [Google Scholar] [PubMed]
- Reddy, Y.J.; Reddy, P.S.; Srinath, S.; Khanna, S.; Sheshadri, S.; Girimaji, S. Comorbidity in Juvenile Obsessive—Compulsive Disorder: A Report from India. Can. J. Psychiatry 2000, 45, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Tillman, R.; Geller, B.; Bolhofner, K.; Craney, J.L.; Williams, M.; Zimerman, B. Ages of Onset and Rates of Syndromal and Subsyndromal Comorbid DSM-IV Diagnoses in a Prepubertal and Early Adolescent Bipolar Disorder Phenotype. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 1486–1493. [Google Scholar] [CrossRef]
- Abramovitch, A.; McCormack, B.; Brunner, D.; Johnson, M.; Wofford, N. The impact of symptom severity on cognitive function in obsessive-compulsive disorder: A meta-analysis. Clin. Psychol. Rev. 2019, 67, 36–44. [Google Scholar] [CrossRef]
- Hantouche, E.G.; Kochman, F.; Demonfaucon, C.; Barrot, I.; Millet, B.; Lancrenon, S.; Akiskal, H.S. Bipolar obsessive-compulsive disorder: Confirmation of results of the “ABC-OCD” survey in 2 populations of patient members versus non-members of an association. L’Encephale 2002, 28, 21–28. [Google Scholar]
- Osland, S.; Arnold, P.D.; Pringsheim, T. The prevalence of diagnosed obsessive compulsive disorder and associated comorbidities: A population-based Canadian study. Psychiatry Res. 2018, 268, 137–142. [Google Scholar] [CrossRef]
- Gaetano, R.; de Filippis, R.; Segura-Garcia, C.; De Fazio, P. Impact of Bipolar Disorder and Obsessive-Comulsive Dioserder comorbidity on neurocognitive profile: A mini-review. Psychiatr. Danub. 2020, 32, 346–350. [Google Scholar] [CrossRef]
- Chen, Y.W.; Dilsaver, S.C. Comorbidity of panic disorder in bipolar illness: Evidence from the Epidemiologic Catchment Area Survey. Am. J. Psychiatry 1995, 152, 280–282. [Google Scholar]
- Kraepelin, E. Manic-Depressive Insanity and Paranoia; E & S Livingstone: Edinburgh, UK, 1921. [Google Scholar]
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; Salazar de Pablo, G.; Il Shin, J.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 2022, 27, 281–295. [Google Scholar] [CrossRef]
- van Oudheusden, L.J.B.; van de Schoot, R.; Hoogendoorn, A.; van Oppen, P.; Kaarsemaker, M.; Meynen, G.; van Balkom, A.J.L.M. Classification of comorbidity in obsessive–compulsive disorder: A latent class analysis. Brain Behav. 2020, 10, e01641. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Azzoni, A. Clinical management of obsessive-compulsive-bipolar comorbidity: A case series. Bipolar Disord. 2004, 6, 264–270. [Google Scholar] [CrossRef]
- Maina, G.; Albert, U.; Rosso, G.; Bogetto, F. Olanzapine or Lamotrigine Addition to Lithium in Remitted Bipolar Disorder Patients With Anxiety Disorder Comorbidity. J. Clin. Psychiatry 2008, 69, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Maina, G.; Pessina, E.; Teobaldi, E.; Barbaro, F.; Martini, A.; Albert, U.; Rosso, G. Aripiprazole Augmentation to Mood Stabilizers for Obsessive-Compulsive Symptoms in Bipolar Disorder. Medicina 2020, 57, 9. [Google Scholar] [CrossRef]
- Fluoxetine-induced mania in a patient with obsessive-compulsive disorder. Am. J. Psychiatry 1991, 148, 1403b–1404b. [CrossRef]
- Vieta, E.; Bernardo, M. Antidepressant-induced mania in obsessive-compulsive disorder. Am. J. Psychiatry 1992, 149, 1282–1283. [Google Scholar] [CrossRef]
- Tondo, L.; Vázquez, G.; Baldessarini, R.J. Mania associated with antidepressant treatment: Comprehensive meta-analytic review. Acta Psychiatr. Scand. 2009, 121, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Surtees, P.G.; Kendell, R.E. The Hierarchy Model of Psychiatric Symptomatology: An Investigation Based on Present State Examination Ratings. Br. J. Psychiatry 1979, 135, 438–443. [Google Scholar] [CrossRef]
- Bisol, L.; Lara, D. Improvement of Obsessive-compulsive Disorder with Divalproex and Lamotrigine in Two Patients with Bipolar II Disorder. Pharmacopsychiatry 2009, 42, 37–39. [Google Scholar] [CrossRef]
- Uguz, F. Successful treatment of comorbid obsessive-compulsive disorder with aripiprazole in three patients with bipolar disorder. Gen. Hosp. Psychiatry 2010, 32, 556–558. [Google Scholar] [CrossRef]
- Perlis, R.H.; Ostacher, M.J.; Patel, J.K.; Marangell, L.B.; Zhang, H.; Wisniewski, S.R.; Ketter, T.A.; Miklowitz, D.J.; Otto, M.W.; Gyulai, L.; et al. Predictors of Recurrence in Bipolar Disorder: Primary Outcomes From the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am. J. Psychiatry 2006, 163, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Maj, M. ‘Psychiatric comorbidity’: An artefact of current diagnostic systems? Br. J. Psychiatry 2005, 186, 182–184. [Google Scholar] [CrossRef] [PubMed]
| References | Study Design | Country | Study Population | Sample Size | Diagnosis Assessment | Outcomes | Quality * |
|---|---|---|---|---|---|---|---|
| Population-based studies: adults | |||||||
| Abramovitch et al., 2019 [21] | Prospective cohort study | Netherlands | 2.067 subjects recruited (mean age 36.86 ± 11.05): OCD (n = 316) | 316 | SCID-I, Y-BOCS; DSM-IV | PR | 26/31 |
| Adam et al., 2012 [22] | Cross-sectional study | Germany | 4181 subjects (age range = 18–65): OCD (n = 30) | 30 | Broad concept of OCD, DIA-X/M-CIDI; DSM-IV | PR | 25/31 |
| Angst et al., 2004 ** [15] | Prospective cohort study | Switzerland | 591 subjects recruited at age 19/20 and assessed over 20 years: OCD (n = 30) | 30 | Broad definition for BD and OCD; DSM-IV | PR | 26/31 |
| Angst et al., 2005 ** [16] | Prospective cohort study | Switzerland | 591 subjects recruited at age 19/20 and assessed over 20 years: OCD (n = 30), BD (n = 93) | 123 | Broad definition for BD and OCD; DSM-IV | HE, CI | 26/31 |
| Carta et al., 2020 [23] | Community survey | Italy | 2.267 subjects recruited (age > 18) | 44 | ANTAS-SCID, SF-12, MDQ; DSM-IV | PR | 22/31 |
| Cederlöf et al., 2014 [24] | Prospective cohort study | Sweden | OCD (n = 19.814) and BD (n = 48.180) patients included in the Swedish Patient Register between January 1969 and December 2009: BD-OCD (n = 76), OCD-BD (n = 787) | 76/787 | NS; ICD-10 | CI, HE, PR, TR | 23/31 |
| Chen et al., 1995 [25] | Cross-sectional study | USA | Pt. with BD, unipolar disorder or any Axis I disorder other than bipolar or unipolar disorder (n = 6622, age > 18): BD (n = 167) | 167 | DIS; DSM-III | PR, CI | 25/31 |
| Faravelli et al., 2004 [26] | Cross-sectional study | Italy | 2500 subjects randomly selected from the lists of 15 GPs: BD (n = 19, age > 14), OCD (n = 57, age > 14) | 76 | SCID; DSM-IV | PR | 24/31 |
| Fineberg et al., 2013 [27] | Prospective cohort study | Switzerland | 591 subjects recruited at age 19/20 and assessed over 30 years: OCD (n = 30) | 30 | SCL-90; CGI; DSM-IV | PR, PH, CI | 23/31 |
| Fireman et al., 2001 [28] | Cross-sectional study | USA | 1.728.480 subjects (age > 6): adults with OCD (n = 1078), children and adolescents with OCD (198) | 1078/198 | NS; DSM-IV | PR | 24/31 |
| Huang et al., 2014 [29] | Prospective cohort study | Taiwan | OCD patients, age > 18, with comorbid diagnosis: 1763; OCD patients, age ≤ 18, with comorbid diagnosis: 277 | 1763/277 | NS; ICD-9-CM | PR | 23/31 |
| Merikangas et al., 2007 [30] | Cross-sectional study | USA | 9282 subjects recruited (age ≥ 18): adults with any BD (n = 408) | 408 | CIDI, SCID; DSM-IV | PR | 25/31 |
| Subramaniam et al., 2020 [31] | Cross-sectional study | Singapore | 6126 recruited subjects (4258 completed the study): OCD (n = 217, age ≥ 18) | 217 | WHO-CIDI, YMRS, QIDS-SR, SDS, SF-12, MCS, PCS, MSPSS; DSM-IV | PR | 24/31 |
| Teh et al., 2020 [32] | Cross-sectional study | Singapore | 6126 recruited subjects (4258 completed the study): BD (n = 94, age ≥ 18) | 94 | WHO-CIDI version 3.0, YMRS, QIDS-SR, SDS; DSM-IV | PR | 23/31 |
| Hospital-based studies: adults | |||||||
| Anholt et al., 2014 [33] | Cross-sectional study | Netherlands | 419 recruited subjects (377 included in the study, mean age 36.3 ± 11.2) | 377 | SCID-I, Y-GTSS, BAI, BDI, Y-BOCS, AQ; DSM-IV | CI | 23/31 |
| Baptista et al., 2020 [34] | Cross-sectional study | Venezuela | BD (n = 40, mean age 46.6 ± 13.5), OCD (n = 42, mean age 35.2 ± 13.5) | 82 | Y-BOCS, YMRS, MDQ; DSM-IV | PR | 22/31 |
| Benatti et al., 2014 [35] | Cross-sectional study | Italy | OCD (n = 75, mean age 41.62 ± 10.78) | 75 | SCID-I, -II, BIS, Y-BOCS; DSM-IV | PR, PH, TR | 22/31 |
| Bener et al., 2016 [36] | Cohort study | Qatar | BD (n = 396, mean age 41.4 ± 12.28) | 396 | SCID, WMH-CIDI, Y-BOCS, HAM-D, YMRS; DSM-IV | PR, PH, CI, HE | 21/31 |
| Berkol et al., 2021 [37] | Retrospective cohort study | Turkey | BD (n = 255, mean age 40.7 ± 12.6), of which BD-I (n = 244), BD-II (n = 11) | 255 | SCID-I/CV; DSM-IV | CI, HE, PH, PR, TR | 20/31 |
| Bogetto et al., 1999 [38] | Case control study | Italy | OCD (n = 160, mean age: males 32.1 ± 13.0, females 36.9 ± 11.4) | 160 | NS; DSM-IV | PR | 21/31 |
| Boylan et al., 2004 [39] | Prospective cohort study | Canada | BD (n = 138, age range = 16–65) | 138 | SCID; DSM-IV | PR | 22/31 |
| Bramante et al., 2021 [40] | Cross-sectional study | Italy | OCD (n = 601, mean age 35.0 ± 12.5 | 601 | SCID-I, Y-BOCS, HAM-A, HAM-D, SCID-PQ; DSM-5 | PR, PH | 22/31 |
| Braverman et al., 2021a ** [41] | Cross-sectional study | Israel | BD (n = 73, mean age 32.9 ± 10.4): BD without OCD (n = 37, mean age 33.1 ± 10.4), BD-OCD (n = 19, mean age 3.6 ± 10.4), BD-OCD subthreshold (n = 17, mean age 33.1 ± 10.6) | 73 | SCID I/P, HAM-D, Y-BOCS, YMRS; DSM-5 | PR, PH | 21/31 |
| Braverman et al., 2021b ** [42] | Cross-sectional study | Israel | BD (n = 70): BD-OCD (n = 27, mean age 33.3 ± 10.3), BD (n = 43, mean age 33.5 ± 10.7) | 70 | SCID I/P, HAM-D, Y-BOCS, YMRS, DSM-5 | PR, PH | 21/31 |
| Cannon et al., 2006 [43] | Case control study | USA | BD (n = 18, mean age = 30 ± 9) | 18 | NS; DSM-IV | BM | 23/31 |
| Cassano et al., 1999 [44] | Cross-sectional study | Italy | Pt. with psychotic symptoms consecutively hospitalized (n = 77, mean age = 33.5 ± 10.3): BD-I (n = 48), schizoaffective disorder, bipolar type (n = 11), unipolar depression (n = 18) | 48 | SCID-P; DSM-III-R | PR | 23/31 |
| Centorrino et al., 2006 [45] | Case control study | USA | Adults (n = 62) with BD, OCD, or BD-OCD | 62 | NS; DSM-IV | CI | 20/31 |
| Cosoff et al., 1998 [46] | Case control study | Australia | Subjects with a psychotic disorder (n = 100, mean age: men = 34.8 ± 10.0, women = 34.9 ± 9.6): BD (n = 20) | 20 | SCID-P; DSM-III-R | PR | 20/31 |
| Craig et al., 2002 [47] | Cross-sectional study | USA | 450 subjects: BD with psychosis (n = 138, mean age range = 15–60) | 138 | SCID; DSM-III-R | PR | 22/31 |
| Das et al., 2013 [48] | Prospective cohort study | India | BD-I mania (n = 84, age 30.85 ± 8.76) | 84 | YMRS, Brief Psychiatric Rating Scale, HAM-A, SADS-L; DSM-IV-TR | PR | 26/31 |
| de Filippis et al., 2018 [49] | Cross-sectional study | Italy | 68 recruited subjects: BD (n = 22, mean age 47.9 ± 11.8), BD-OCD (n = 26, 47.38 ± 13.2), 20 OCD (42.5 ± 15.5) | 68 | MINI, YMRS, HAM-D, MMSE, Y-BOCS, CGI, RCFT, IGT, WCST, TMT, HSct; DSM-IV-TR | PR, PH, TR | 20/31 |
| Di Salvo et al., 2020 [50] | Cross-sectional study | Italy | BD (n = 990, mean age 49.0 ± 15.6): BD without OCD (n = 789, mean age 50.7 ± 15.5), BD-OCD (n = 201, mean age 42.3 ± 14.1) | 990 | DSM-IV-TR, DSM-5 | PR, CI | 22/31 |
| Dell’Osso et al., 2000 [51] | Case control study | Italy | BD with psychotic features (n = 125, mean age: 33.3 ± 11.1) | 125 | SCID-P; DSM-III-R | PH | 21/31 |
| Dilsaver et al., 2008 [52] | Case control study | USA | 187 Latino pt. enrolled consecutively from 2001 to 2003: BD-I (n = 69, mean age = 34.9 ± 11.8) | 69 | SCID-CV; DSM-IV | PR | 20/31 |
| Diniz et al., 2004 [53] | Cross-sectional study | Brazil | OCD (n = 161, mean age = 30 ± 10)) | 161 | SCID; DSM-IV | PR | 21/31 |
| Domingues-Castro et al., 2019 [54] | Cross-sectional study | Brazil | OCD (n = 955, mean age 35.8 ± 12.5): OCD without BD (n = 881, mean age 35.9 ± 12.6), OCD-BD (n = 74, mean age 34.9 ± 10.7 | 955 | SCID-I, Y-BOCS, DY-BOCS, OCD Natural History Questionnaire, BDI, BAI, BABS, USP Sensory Phenomena Scale, Suicidality Assessment; DSM-IV-TR | CI, HE, PH, PR, TR | 22/31 |
| Edmonds et al., 1998 [55] | Case control study | New Zealand | BD (n = 55, mean age = 41.6), first-degree relatives (n = 67, mean age = 50.3) | 122 | DIGS; DSM-IV | PR | 20/31 |
| Goes et al., 2012 [56] | Cross-sectional study | USA | BD (n = 1416, mean age = 42.0), first-degree relatives with BD (n = 850) | 1416/850 | DIGS; DSM-IV | CI | 23/31 |
| Hantouche et al., 2003 [17] | Case control study | France | OCD (n = 628, mean age CYC-OCD = 35 ± 12, mean age NC-OCD = 36 ± 14) | 628 | NS; DSM-IV | PR | 24/31 |
| Hasler et al., 2005 [57] | Cross-sectional study | USA | OCD (n = 317, age > 18) | 317 | SCID; DSM-IV | PH | 23/31 |
| Henry et al., 2003 [58] | Cross-sectional study | France | BD (n = 318, mean age = 53.3 ± 15.1) | 318 | DIGS; DSM-IV | PR | 23/31 |
| Issler et al., 2005 [59] | Case control study | Brazil | OCD-BD (n = 15, mean age = 38.9 ± 10.7) | 15 | SCID-P; DSM-IV | PH, CI | 14/31 |
| Issler et al., 2010 [60] | Case control study | Brazil | BD (n = 30, mean age: BD = 41.8 ± 10.5, OCD-BD = 38.9 ± 10.7) | 30 | SCID-P; DSM-IV | CI | 17/31 |
| Jakubovski et al., 2013 [61] | Prospective cohort study | Brazil | OCD (n = 196, mean age = 33.6 ± 10.6) | 196 | SCID-I, Y-BOCS DY-BOCS, BDI, BAI; DSM-IV-TR | PR | 26/31 |
| Jeon et al., 2017 [62] | Cross-sectional study | South Korea | BD (n = 314, mean age = 34.9 ± 11.6) | 314 | SCID, Y-BOCS, OCI-R-K | PR, CI, PH, TR | 21/31 |
| Kazhungil et al., 2017 [63] | Cross-sectional study | India | BD (n = 90): BD-OCD (n = 20, mean age = 41.03 ± 11.77), BD with obsessive–compulsive symptoms (n = 32, mean age = 39.36 ± 11.24) | 90 | Structured Clinical Interview for DSM-IV Axis I Disorders, GAF, HDRS, YMRS, Y-BOCS, FIGS | PR, CI, HE, PH | 20/31 |
| Khan et al., 2019 [64] | Cross-sectional study | Pakistan | BD-I (n = 469) | 469 | DSM-IV-TR | PR, CI, HE, PH, TR | 21/31 |
| Kemp et al., 2014 [65] | Cross-sectional study (LiTMUS) | USA | BD (n = 264, mean age = 39.2 ± 12.4) | 264 | SCID, MADRS, YMRS, QIDS-SR, the Quality of Life, Enjoyment, Q-LES-Q, LIFE-RIFT, CIRS; DSM-IV | PR, CI | 20/31 |
| Khorshidian et al., 2023 [66] | RCT | Iran | 64 pt. recruited; 61 completed the study (31 in the risperidone group and 30 in the aripiprazole group; mean age was 38.9 in the risperidone group and 40.1 in the aripiprazole group) | 64 | DSM-5, HDRS, YMRS, Y-BOCS | PR, TR | 28/31 |
| Kim et al., 2014 [67] | Prospective cohort study | Australia | BD (n = 174): BD-OCD (n = 24, mean age = 39.8 ± 12.0) | 174 | MINI, YMRS, HAM-D; DSM-IV | PR, CI, TR | 26/31 |
| Koyuncu et al., 2010 [68] | Case control study | Turkey | BD (n = 214, mean age: BD = 34.8 ± 10.3, OCD-BD = 36.2 ± 15.9) | 214 | SCID; DSM-IV | PR, HE, CI | 20/31 |
| Kruger et al., 1995 [69] | Cross-sectional study | Germany | Major affective disorder (n = 149, mean age = 49 ± 12): BD (n = 44) | 37 | DIS; DSM-III | PR | 21/31 |
| Kruger et al., 2000 [70] | Case control study | Germany | BD-I or BD-II (n = 143, mean age = 44) | 143 | SCID; DSM-III-R | PR, CI | 22/31 |
| LaSalle-Ricci et al., 2006 [71] | Cross-sectional study | USA | OCD (n = 204, age > 18) | 204 | SCID-P for DSM-IV | PH | 20/31 |
| Lensi et al., 1996 [72] | Case control study | Italy | OCD (n = 263, mean age = 33.1) | 263 | NS; DSM-III-R | PR | 23/31 |
| Magalhaes et al., 2010 [73] | Case control study | Brazil | BD (n = 259, mean age = 41) | 259 | SCID; DSM-IV | PR, CI | 23/31 |
| Mahasuar et al., 2011 [74] | Case control study | India | OCD (n = 91, mean age: OCD = 29.36 ± 8.31, BD-OCD = 28.39 ± 7.10) | 91 | SCID for DSM-IV | PH, HE, CI | 19/31 |
| Maina et al., 2007 [75] | Case control study | Italy | OCD (n = 204, mean age = 34.7 ± 12.1) | 204 | SCID; DSM-IV | PR, PH, CI | 21/31 |
| Marazziti et al., 2002 [76] | Cross-sectional study | Italy | OCD (n = 117, mean age = 30 ± 9.3) | 117 | SCID-P; DSM-IV | PR, PH | 21/31 |
| McElroy et al., 1995 [77] | Case control study | USA | BD (n = 71, mean age = 35 ± 16) | 71 | SCID-P; DSM-III-R | PH | 23/31 |
| McElroy et al., 2001 [78] | Case control study | USA | BD-I or BD-II (n = 288, mean age = 42.8 ± 11.3) | 288 | SCID-P; DSM-IV | PR | 23/31 |
| Ortiz et al., 2011 [79] | Cross-sectional study | Canada | BD (n = 379, mean age = 25.1 ± 10.6) | 379 | SADS-L; DSM-IV | CI | 23/31 |
| Ozdemiroglu et al., 2017 [80] | Cross-sectional study | Turkey | BD (n = 48, mean age = 42.3 ± 12.4), OCD (n = 61, mean age = 37.7 ± 12.9), BD-OCD (n = 32, mean age = 36.2 ± 12.0) | 141 | SCID-I, Y-BOCS, YMRS, BDI, BIS-11; DSM-IV | PR, CI, HE, PE, PH, TR | 21/31 |
| Perugi et al., 1997 [81] | Case control study | Italy | OCD (n = 315, mean age: BD-OCD = 32.8 ± 12.2, OCD = 32.5 ± 12.6) | 315 | NS; DSM-III-R | PR, PH, CI | 22/31 |
| Perugi et al., 1998 [82] | Case control study | Italy | OCD (n = 135, mean age = 38.4 ± 13.3) | 135 | NS; DSM-III-R | PR, HE, CI | 21/31 |
| Perugi et al., 1999 [83] | Case control study | Italy | 269 pt. enrolled consecutively from 1993 to 1995: OCD (n = 79, mean age = 30.4 ± 11.8) | 79 | SCID-Up-R; DSM-III-R | PR, TR | 20/31 |
| Perugi et al., 2002 [84] | Case control study | Italy | OCD-MDE (n = 68, mean age = 34.2 ± 12.5); BD-OCD (n = 38, mean age = 35.9 ± 12.2) | 68 | SCID; DSM-IV | PR, PH, CI, HE, TR | 20/31 |
| Pini et al., 1997 [85] | Case control study | Italy | Current episode of depression (n = 87): bipolar depression (n = 24, mean age = 37.9 ± 12.0), unipolar depression (n = 38, mean age = 47.0 ± 15.0), dysthymia (n = 25, mean age = 43.0 ± 12.0) | 24 | SCID-P; DSM-III-R | PR | 20/31 |
| Pini et al., 1999 [86] | Cross-sectional study | Italy | BD (n = 125, age > 16) | 125 | SCID-P; DSM-III-R | PR | 21/31 |
| Sahraian et al., 2017 [87] | RCT | Iran | 58 BD pt. recruited; 38 completed the study (19 in the memantine group and 19 in the placebo group; mean age 34.21 ± 10.18 and 32.26 ± 10.30, respectively) | 58 | Y-BOCS; DSM-IV-TR | PR, TR | 28/31 |
| Sahraian et al., 2018 [88] | RCT | Iran | 56 BD pt. recruited; 46 completed the study (23 in aripiprazole group and 23 in placebo group; mean age 34.10 ± 11.60 and 39.4 ± 14.20, respectively) | 56 | Y-BOCS, YMRS; DSM-IV | PR, TR | 28/31 |
| Sahraian et al., 2021 [89] | RCT | Iran | 79 BD pt. recruited; 40 completed the study (24 in quetiapine group, and 20 in placebo group; mean age 36.45 ± 13.83 and 38.45 ± 14.60, respectively) | 79 | HDRS, YMRS, Y-BOCS | PR, TR | 28/31 |
| Saraf et al., 2017 [90] | Cross-sectional study | India | OCD (n = 171, mean age = 28.89 ± 9.52) | 171 | MINI, Y-BOCS, CGI, GAF, DSM-IV | PR, CI, PH | 20/31 |
| Saunders et al., 2012 [91] | Cross-sectional study | USA | BD, type I or Schizoaffective Disorder, Bipolar type (n = 736, mean age = 42 ± 12) | 736 | DIGS; DSM-IV | PR | 19/31 |
| Shabani et al., 2008 [92] | Case control study | Iran | OCD 78: BD-OCD (n = 39, mean age = 26.6 ± 7.23), OCD (n = 39, mean age = 30.1 ± 6.52) | 78 | SCID-CV; DSM-IV | PH | 20/31 |
| Shashidhara et al., 2015 [93] | Retrospective cohort study | India | BD (n = 396, mean age = 31.87 ± 11.1) | 396 | MINI, SCID-II, FIGS, Y-BOCS, YMRS, HAM-D, GAF, CGI-S; DSM-IV | PR, CI, HE, PH, TR | 20/31 |
| Simon et al., 2003 [94] | Case control study | USA | 236 subjects: BD (n = 122, mean age = 40.8 ± 12.2) | 122 | SCID; DSM-IV | PR | 23/31 |
| Simon et al., 2004 [95] | Cross-sectional study | USA | BD (n = 475, mean age = 41.7 ± 12.8) | 475 | MINI; DSM-IV | PR, CI | 23/31 |
| Strakowski et al., 1992 [96] | Cross-sectional study | USA | BD (n = 41, mean age = 40.4 ± 11.7) | 41 | SCID; DSM-III-R | PR | 22/31 |
| Strakowski et al., 1998 [97] | Cross-sectional study | USA | BD, manic or mixed with psychosis (n = 77, mean age= 25 ± 6) | 77 | SCID-P; DSM-III-R | PR, CI | 22/31 |
| Tamam et al., 2002 [98] | Cross-sectional study | Turkey | BD-I in remission (n = 70, mean age = 33.4 ± 10.3) | 70 | SCID-CV; DSM-IV | PR | 20/31 |
| Tasdemir et al., 2015 [99] | Cross-sectional study | Turkey | BD (n = 70, mean age = 38.7 ± 11.1) | 70 | SCID, HAM-A, SCI-SAS, BDFQ, SASI, ASA; DSM-IV-TR | PR, CI | 21/31 |
| Timpano et al., 2012 [100] | Case control study | USA | OCD (n = 605, mean age = 39.2) | 605 | SCID-P; DSM-IV | PR, PH, CI | 20/31 |
| Tonna et al., 2021 [101] | Cross-sectional study | Italy | BD (n = 154), OCD (n = 11) (mean age 46 ± 11.74): euthymic (n = 62), hypomanic/manic (n = 34), depressive (n = 43), mixed (n = 26) state | 165 | Y-BOCS, HAM-D, YMRS, RRS; DSM-5 | PR, PH, TR | 22/31 |
| Tukel et al., 2006 [102] | Case control study | Turkey | OCD (n = 115, age > 18) | 117 | SCID-CV for DSM-IV | PH, CI | 21/31 |
| Tukel et al., 2007 [103] | Case control study | Turkey | OCD (n = 128, mean age = 29.3 ± 10.8) | 128 | SCID-CV; DSM-IV | PR, CI | 21/31 |
| Zutshi et al., 2006 [104] | Case control study | India | BD in remission (n = 80, mean age = 30.06 ± 7.77) | 80 | SCID-CV; DSM-IV | PR | 22/31 |
| Zutshi et al., 2007 [105] | Case control study | India | OCD (n = 106, mean age: BD-OCD = 27.93 ± 6.71, OCD = 26.47 ± 7.38) | 106 | SCID-CV; DSM-IV | PH, HE, CI | 20/31 |
| Population-based studies: children, adolescents | |||||||
| Alvarenga et al., 2015 [106] | Cross-sectional study | Brazil | 2512 children (6–12 years old, mean age 8.86 ± 1.84): OCD (n = 77), OCS (n = 448) | 77/448 | FHS, DAWBA, SDQ, CBCL; DSM-IV | PR | 23/31 |
| Hofer et al., 2017 [107] | Prospective cohort study | Germany | 3.021 subjects recruited age 14–24, assessed for up to 10 years: OCD (n = 210) | 210 | DIA-X/M-CIDI, DIA-X/M-CID OCD module; DSM-IV | PR | 23/31 |
| Hospital-based studies: children, adolescents | |||||||
| Dilsaver et al., 2006 [108] | Case control study | USA | Latino adolescents (n = 313): BD (n = 115, mean age = 14.6 ± 1.5) | 115 | SCID-CV; DSM-IV | PR, CI | 18/31 |
| Joshi, Mick et al., 2010 [109] | Open label trial | USA | BD enrolled in the olanzapine trials (n = 52, mean age = 8.4 ± 3.1) | 52 | K-SADS-E; DSM-IV | TR | 20/31 |
| Joshi, Wozniak et al., 2010 [110] | Case control study | USA | OCD (n = 125, age range = 6–17), BD (n = 82, age range = 6–17) | 207 | K-SADS-E; DSM-III-R | PR, PH, CI | 19/31 |
| Masi et al., 2004 [111] | Case control study | Italy | BD, OCD, BD-OCD (n = 102, mean age = 14.2 ± 3.2) | 102 | DICA-R; DSM-IV | PH, CI | 21/31 |
| Masi et al., 2005 [112] | Prospective cohort study | Italy | OCD (n = 94, mean age = 13.6 ± 2.8) | 94 | DICA-R; DSM-IV | PR | 20/31 |
| Masi et al., 2007 [113] | Prospective cohort study | Italy | OCD (n = 120, mean age = 13.7 ± 2.8) | 120 | K-SADS-PL or DICA-R; DSM-IV | PR, PH, TR, CI | 21/31 |
| Masi et al., 2009 *** [114] | Case control study | Italy | OCD (n = 257, mean age = 13.6 ± 2.7) | 257 | K-SADS-PL; DSM-IV | PR, TR | 22/31 |
| Masi et al., 2010 *** [115] | Cross-sectional study | Italy | OCD (n = 257, mean age = 13.6 ± 2.7) | 257 | K-SADS-PL; DSM-IV | PR, PH | 22/31 |
| Masi et al., 2018 [116] | Prospective cohort study | Italy | BD (n = 172, mean age = 13.7 ± 2.9), OCD (n = 169, mean age = 13.2 ± 2.7), BD-OCD (n = 88, mean age = 14.2 ± 2.6) | 429 | K-SADS-PL, CGI, C-GAS; DSM-IV, DSM-IV-TR, DSM-5 | PR, CI, PH, TR | 23/31 |
| Paul et al., 2015 [117] | Cross-sectional study | India | Patients aged <18 (n = 100): BD (n = 85, mean age 15.57 ± 1.75) | 85 | K-SADSPL, CY-BOCS, YMRS, CDI, HAM-A, BPRS-C; DSM-IV-TR | PR, CI, PH | 22/31 |
| Reddy et al., 2000 [118] | Cross-sectional study | India | OCD (n = 54, age = 16 or less) | 54 | DICA-R; DSM-III-R | PR | 15/31 |
| Tillman et al., 2003 [119] | Case control study | USA | BD (n = 93, mean age = 10.9 ± 2.6) | 93 | WASH-U-K-SADS; DSM-IV | PR | 20/31 |
| BD Type | BD | BD-I | BD-II | |
|---|---|---|---|---|
| References | N | % | % | % |
| Population-based studies | ||||
| Cederlöf et al., 2014 [24] | 76 | 0.26 | ||
| Chen et al., 1995 [25] | 167 | 21.0 | ||
| Faravelli et al., 2004 [26] | 19 | 11.1 | ||
| Merikangas et al., 2007 [30] | 408 | 13.6 | 25.2 | 20.8 |
| Teh et al., 2020 [32] | 105 | 27.8 | ||
| Hospital-based studies: adults | ||||
| Baptista et al., 2020 [34] | 40 | 20 | 15.3 | 28.5 |
| Bener et al., 2016 [36] | 396 | 23.2 | ||
| Berkol et al., 2021 [37] | 255 | 21.25 | ||
| Boylan et al., 2004 [39] | 138 | 8.7 | ||
| Braverman et al., 2021a [41] | 73 | 26 | ||
| Braverman et al., 2021b [42] | 70 | 38.6 | ||
| Cassano et al., 1999 [44] | 48 | |||
| Cosoff et al., 1998 [46] | 20 | 30 | ||
| Craig et al., 2002 [47] | 138 | 3.8 | ||
| Das et al., 2013 [48] | 84 | 7.1 | 100 | |
| de Filippis et al., 2018 [49] | 48 | 54.2 | ||
| Di Salvo et al., 2020 [50] | 201 | 20.3 | 38.3 | 59.7 |
| Dilsaver et al., 2008 [52] | 69 | 62.3 | ||
| Edmonds et al., 1998 [55] | 55 | 1.8 | ||
| Henry et al., 2003 [58] | 318 | 3 | ||
| Jeon et al., 2017 [62] | 314 | 15.9 | ||
| Kazhungil et al., 2017 [63] | 90 | 35 | 100 | |
| Kemp et al., 2014 [65] | 264 | 10.2 | ||
| Khan et al., 2019 [64] | 469 | 7.5 | 100 | |
| Khorshidian et al., 2023 [66] | 64 | 100 | ||
| Kim et al., 2014 [67] | 174 | 13.8 | 100 | |
| Koyuncu et al., 2010 [68] | 214 | 16.3 | 11.9 | 23.1 |
| Kruger et al., 1995 [69] | 37 | 35.1 | ||
| Kruger et al., 2000 [70] | 143 | 7 | 100 | |
| Magalhaes et al., 2010 [73] | 259 | 12.4 | ||
| Masi et al., 2018 [116] | 52 | 33.8 | ||
| McElroy et al., 2001 [78] | 288 | 9 | 9 | 10 |
| Ozdemiroglu et al., 2017 [80] | 80 | 40 | ||
| Pini et al., 1997 [85] | 24 | |||
| Pini et al., 1999 [86] | 125 | |||
| Sahraian et al., 2017 [87] | 58 | 100 | 100 | |
| Sahraian et al., 2018 [88] | 56 | 100 | 100 | |
| Sahraian et al., 2021 [89] | 79 | 100 | 100 | |
| Saunders et al., 2012 [91] | 736 | 6 | ||
| Shashidhara et al., 2015 [93] | 396 | 7.6 | 100 | |
| Simon et al., 2003 [94] | 122 | 13.4 | ||
| Simon et al., 2004 [95] | 475 | 9.9 | 10.9 | 7.0 |
| Strakowski et al., 1992 [96] | 41 | 7.3 | ||
| Strakowski et al., 1998 [97] | 77 | 16 | ||
| Tamam et al., 2002 [98] | 70 | |||
| Tasdemir et al., 2015 [99] | 70 | 13.2 | ||
| Tonna et al., 2021 [101] | 165 | 93.3 | 81.2 | 18.8 |
| Zutshi et al., 2006 [104] | 80 | |||
| Hospital-based studies: children, adolescents | ||||
| Dilsaver et al., 2006 [108] | 115 | 46.9 | ||
| Joshi, Wozniak et al., 2010 [109] | 82 | 20.7 | ||
| Paul et al., 2015 [117] | 85 | 2.4 | ||
| Tillman et al., 2003 [119] | 93 | 24.7 | ||
| BD Type | BD | BD-I | BD-II | |
|---|---|---|---|---|
| References | N | % | % | % |
| Population-based studies | ||||
| Abramovitch et al., 2019 [21] | 316 | 3.2 | ||
| Adam et al., 2012 [22] | 30 | 10.0 | ||
| Angst et al., 2004 [15] | 30 | 53.3 | 30.0 | |
| Cederlöf et al., 2014 [24] | 787 | 4.16 | ||
| Fineberg et al., 2013 [27] | 30 | 40 | ||
| Fireman et al., 2001 [28] | 1078 (adults) 198 (children and adolescents) | 6.0 5.0 | ||
| Huang et al., 2014 [29] | 1763 277 | 3.2 0.3 | ||
| Osland et al., 2018 [122] | 267 | 18.2 | ||
| Subramaniam et al., 2020 [31] | 217 | 8.8 | ||
| Hospital-based studies: adults | ||||
| Baptista et al., 2020 [34] | 42 | 35.7 | 19 | 16.7 |
| Benatti et al., 2014 [35] | 75 | 9.3 | ||
| Bogetto et al., 1999 [38] | 160 | 6.8 | ||
| Bramante et al., 2021 [40] | 601 | 17.1 | 6 | 11.1 |
| de Filippis et al., 2018 [49] | 46 | 43.5 | ||
| Diniz et al., 2004 [53] | 161 | 9.0 | ||
| Domingues-Castro et al., 2019 [54] | 74 | 7.8 | 42 | 53 |
| Hantouche et al., 2003 [17] | 628 | 11.0 | 3.0 | 8.0 |
| Jakubovski et al., 2013 [61] | 196 | 4.6 | ||
| Lensi et al., 1996 [72] | 263 | 1.5 | 12.1 | |
| Marazziti et al., 2002 [76] | 117 | 20.5 | 5.0 | 15.3 |
| Masi et al., 2018 [116] | 36 | 34.2 | ||
| Ozdemiroglu et al., 2017 [80] | 93 | 34.4 | ||
| Perugi et al., 1997 [81] | 345 | 15.7 | 2.0 | 13.6 |
| Perugi et al., 1998 [82] | 135 | 19.2 | 1.4 | 17.7 |
| Perugi et al., 1999 [83] | 79 | 21.5 | 3.8 | 17.7 |
| Perugi et al., 2002 [84] | 68 | 55.8 | 17.6 | 38.2 |
| Saraf et al., 2017 [90] | 171 | 4 | ||
| Timpano et al., 2012 [100] | 605 | 13.1 | 8.4 | 4.6 |
| Tukel et al., 2007 [103] | 128 | 7.0 | ||
| Population-based studies: children, adolescents | ||||
| Alvarenga et al., 2015 [106] | 77 | 1.3 | ||
| Hofer et al., 2017 [107] | 210 | 4.5 | ||
| Hospital-based studies: children, adolescents | ||||
| Joshi, Wozniak et al., 2010 [109] | 125 | 15.2 | ||
| Masi et al., 2005 [112] | 94 | 24.4 | ||
| Masi et al., 2007 [113] | 120 | 35.8 | 11.7 | 16.7 |
| Masi et al., 2009 [114] | 257 | 34.2 | ||
| Masi et al., 2010 [115] | 257 | 34.2 | ||
| Reddy et al., 2000 [118] | 54 | 1.8 | ||
| References | Results |
|---|---|
| Phenomenology | |
| Bener et al., 2016 [36] | Lower mean HAM-D score in BD-OCD pt. compared to BD pt. (3.18 ± 0.59 vs. 3.74 ± 0.51, p = 0.016). No statistically significant difference in terms of YMRS score between BD-OCD pt. and BD pt. |
| Bramante et al., 2021 [40] | OCD pt. with forbidden thoughts symptoms presented higher rates of comorbid BD-I compared to OCD pt. without forbidden thoughts symptoms (7.6% vs. 2.9%, p = 0.023). |
| Braveman et al., 2021b [42] | Pt. with bipolar depression and comorbid OCD presented a significantly higher rate of body dysmorphic, hoarding, excoriation, and tic disorders compared to pt. with bipolar depression. No differences were found in the rate of trichotillomania. |
| de Filippis et al., 2018 [49] | No statistically significant difference in cognitive function among BD-OCD comorbidity and single diagnosis, including decision-making and cognitive flexibility, was found. |
| Dell’Osso et al., 2000 [51] | Patients with depression exhibited a greater prevalence of OCD compared to those experiencing mania. |
| Domingues-Castro et al., 2019 [54] | Compared to OCD pt., OCD-BD pt. more frequently presented “poor insight” (OR 1.98, 95% CI = 1.08–3.63), sensory phenomena (OR 1.90, 95% CI = 1.05–3.45), and higher severity of anxiety and depressive symptoms. |
| Hasler et al., 2005 [57] | Obsessions related to symmetry and compulsions involving repeating, counting, and ordering/arranging were linked to bipolar disorder (OR = 1.5, 95%; CI = 1.1–2.2). |
| Issler et al., 2005 [59] | A greater occurrence of aggression, symmetry, contamination, hoarding, and miscellaneous obsessions, as well as cleaning, checking, ordering, and various other compulsions, was observed in patients with comorbid BD and OCD compared to those without the comorbidity. |
| Jeon et al., 2017 [62] | In 65.4% of BD-OCD pt., obsessive–compulsive symptoms worsened or were confined to depressive episodes. Moreover, contamination obsession and checking compulsion were the most common types of obsessive–compulsive symptoms. |
| Joshi, Wozniak et al., 2010 [109] | Elevated frequencies of hoarding/saving obsessions (58% vs. 23%) and compulsions (63% vs. 20%) were observed in patients with comorbid BD and OCD compared to those without BD-OCD. |
| Kazhungil et al., 2017 [63] | A total of 25% of BD-OCD pt. predominantly had obsessions, 37.5% predominantly had compulsions, and 37.5% had mixed obsessions and compulsions. No significant differences between groups in HDRS and YMRS were found. |
| Khan et al., 2019 [64] | A majority of the BD-OCD pt. had OCD symptoms during a manic phase (40%) or in remission (37.1%), with contamination as the main theme (42.8%). |
| LaSalle-Ricci et al., 2006 [71] | A diagnosis of BD-I exhibited a significant and positive correlation with hoarding tendencies. |
| Mahasuar et al., 2011 [74] | Patients with comorbid OCD-BD demonstrated fewer pathological doubts (50% vs. 75%), an increased prevalence of pathological slowness (32% vs. 9%) and reassurance seeking (33% vs. 11%), and poorer insight into obsessive–compulsive symptoms (Y-BOCS-11: 1.11 ± 1.00 vs. 0.52 ± 0.71) compared to those without OCD-BD. |
| Maina et al., 2007 [75] | Elevated frequencies of hoarding (33.3% vs. 10.9%), sexual obsessions (42.9% vs. 19.7%), and repeating compulsions (71.4% vs. 42.6%) were observed in patients with comorbid OCD-BD compared to those without OCD-BD. |
| Marazziti et al., 2002 [76] | Patients affected by comorbid OCD-BD who had a positive history of repeated manic or hypomanic episodes exhibited a lower level of insight compared to patients with OCD alone. |
| Masi et al., 2004 [111] | Elevated frequencies of ordering compulsions (30% vs. 5.7%) were observed in patients with BD-OCD compared to those without BD-OCD. |
| Masi et al., 2007 [113] | Increased frequencies of hoarding obsessions and compulsions were observed in patients with OCD-BD compared to those without OCD-BD (14% vs. 2.6%). |
| Masi et al., 2010 [115] | An increased prevalence of BD was noted in patients with OCD who exhibited hoarding compulsions. |
| Masi et al., 2018 [116] | BD-OCD pt. presented a higher occurrence of BD-II compared to BD pt. Hoarding symptoms were more represented in BD-OCD pt. compared to OCD pt. |
| McElroy et al., 1995 [77] | Patients with episodes of mixed mania were found to be more prone to having comorbid OCD compared to those experiencing pure euphoric mania (21% vs. 4%). |
| Ozdemiroglu et al., 2017 [80] | Bipolarity did not have a specific effect on the phenomenology of OC symptoms. |
| Perugi et al., 1997 [81] | Elevated frequencies of sexual (41.1% vs. 20.3%) and religious obsessions (23.5% vs. 9.8%) were observed, along with a lower rate of checking rituals (56.8% vs. 73.4%), in patients with BD-OCD compared to those without BD-OCD. |
| Perugi et al., 2002 [84] | Patients with comorbid BD and OCD exhibited elevated frequencies of sexual obsessions (55.3% vs. 26.7%) and a decreased rate of ordering rituals (18.4% vs. 50.0%) compared to those without BD-OCD. |
| Shabani et al., 2008 [92] | Patients with BD-OCD demonstrated elevated frequencies of sexual obsessions (87.2% vs. 0.0%), religious obsessions (38.5% vs. 15.4%), and aggressive obsessions (35.9% vs. 7.7%), along with a reduced rate of contamination obsessions (64.1% vs. 84.6%), compared to those without BD-OCD. Additionally, BD-OCD patients exhibited a higher prevalence of miscellaneous compulsions (53.8% vs. 25.6%, p = 0.01) and a lower prevalence of washing compulsions (48.7% vs. 84.6%) compared to non-BD-OCD patients. |
| Shashidhara et al., 2015 [93] | BD-OCD pt. had significantly lower incidence of psychotic symptoms compared to BD pt. (53.6% vs. 30%, p = 0.01). |
| Timpano et al., 2012 [100] | Patients with OCD-BD exhibited more severe obsessive–compulsive symptoms, as indicated by a higher Y-BOCS total score (22.7 ± 9.8 vs. 18.2 ± 8.5), compared to those without OCD-BD. |
| Tonna et al., 2021 [101] | The severity of OCS was associated with the severity of depressive symptoms. The highest level of severity of OCS was observed in the mixed group, and the lowest scores were observed in the hypomanic/manic group. |
| Tukel et al., 2006 [102] | Patients with comorbid BD and OCD demonstrated elevated frequencies of symmetry/exactness obsessions (73.1% vs. 40.8%) and ordering/arranging compulsions (61.5% vs. 36.7%), along with lower rates of somatic obsessions (7.7% vs. 14.3%), compared to those without BD-OCD. |
| Zutshi et al., 2007 [105] | Patients suffering from BD-OCD showed less severe obsessive–compulsive symptoms, as indicated by a lower Y-BOCS total score (14.43 ± 7.27 vs. 25.53 ± 7.54), and lower rates of washing (32% vs. 62%), repeating (7% vs. 54%), and ordering (3% vs. 27%) compulsions compared to those without BD-OCD. |
| Heredity | |
| Angst et al., 2005 [16] | There were no statistically significant differences in family history regarding OCD, depression, and mania between individuals with obsessive–compulsive symptoms with or without comorbid BD. |
| Bener et al., 2016 [36] | A significantly higher proportion of BD pt. with (7.6%) and without OCD (17.8%) had a family history of BD (p = 0.030). |
| Cederlöf et al., 2014 [24] | OCD-unaffected first-, second-, and third-degree relatives of probands with OCD had a significantly increased risk for BD; the magnitude of this risk decreased as the genetic distance increased. |
| Domingues-Castro et al., 2019 [54] | OCD-BD pt. presenting comorbidity had more family history of affective symptoms compared to OCD pt. (depressive symptoms, OR 2.03, 95% CI = 1.13–3.65; agitation/euphoria symptoms OR 3.94, 95% CI = 2.37–6.54). |
| Kazhungil et al., 2017 [63] | In BD-OCD pt., first- and second-degree relatives had higher rates of BD-OCD and OCD but not of BD. |
| Khan et al., 2019 [64] | BD-I-OCD pt. had a higher incidence of OCD (2.9% vs. 0.2%, p = 0.008), as well as BD, in the family (20% vs. 9.2%). |
| Koyuncu et al., 2010 [68] | The frequency of OCD was higher in first-degree relatives of patients with comorbid BD and OCD compared to those without BD-OCD (45.7% vs. 5.7%). There were no statistically significant differences in the family history of BD. |
| Mahasuar et al., 2011 [74] | There were statistically non-significant trends indicating a higher prevalence of family history for mood disorders in patients with comorbid OCD and BD and a lower prevalence of family history for OCD compared to those without OCD-BD. |
| Ozdemiroglu et al., 2017 [80] | No difference in family history of OCD and BD between BD-OCD and BD pt. |
| Perugi et al., 2002 [84] | There were statistically non-significant trends suggesting a higher prevalence of family history for mood disorders in patients with BD-OCD and a lower prevalence of family history for OCD compared to those without BD-OCD. |
| Perugi et al., 1998 [83] | There was a positive correlation between episodic OCD and a family history of mood disorders compared to patients with continuous OCD (54.1% vs. 34.7%). |
| Shashidhara et al., 2015 [93] | Higher rates of OCD in first-degree relatives of BD-OCD pt. compared to BD pt. (6.7% vs. 0.3%, p = 0.02) were observed. |
| Zutshi et al., 2007 [105] | There was a higher prevalence of family history for mood disorders in BD-OCD compared to patients without BD-OCD (36% vs. 6%). Additionally, there was a lower prevalence of family history for OCD in BD-OCD patients compared to non-BD-OCD patients (0.0% vs. 21%). |
| Biological markers | |
| Cannon et al., 2006 [43] | There was a higher binding potential of 5-HTT in OCD-BD compared to patients without OCD-BD. This was observed in the insula, posterior cingulate cortex, subgenual anterior cingulate cortex, and dorsal cingulate cortex. |
| Treatment | |
| Benatti et al., 2014 [35] | In OCD-BD pt., 86% were on atypical antipsychotic treatment (alone: 71%; in association with lithium/valproate: 15%) and 14% were on antidepressant treatment in association with lithium or valproate. |
| Domingues-Castro et al., 2019 [54] | OCD-BD pt. were more commonly treated with psychotherapy compared to OCD pt. (OR 1.80, 95% CI = 1.03–3.12). |
| Jeon et al., 2017 [62] | A drug-induced (hypo)manic switch was observed in more than 60% of the BD-OCD pt. who were previously exposed to antidepressants. None of the BD-OCD pt. were taking antidepressants for OCD in the specialty clinics at that time. |
| Joshi, Mick et al., 2010 [109] | OCD-BD exhibited a lower treatment response compared to patients without OCD-BD, as evidenced by a smaller mean reduction in YMRS scores (−5.9 ± 13.1 vs. −13.7 ± 18.8), a lower percentage of ≥30% reduction (25% vs. 63%), and a lower proportion achieving a CGI-S Improvement score ≤ 2 (25% vs. 68%). However, no statistically significant differences were reported in the rates of dropouts or adverse effects. |
| Kazhungil et al., 2017 [63] | The number of failed trials of mood stabilizers, antipsychotics, and antidepressants used in their lifetime did not differ between BD-OCD pt. and BD pt. |
| Khan et al., 2019 [64] | A total of 60% of BD-OCD pt. were on an antidepressant/anxiolytic, with a mood stabilizer or antipsychotic or both, as compared to 45.8% of BD-I pt. who received antidepressants/anxiolytics. A higher number of BD-OCD pt. received SSRIs/antidepressants/anxiolytics. |
| Khorshidian et al., 2023 [66] | Both aripiprazole and risperidone were effective as an adjunctive therapy, with valproate sodium used for treating OCD in patients with BD without any serious and life-threatening adverse effect. Aripiprazole was more effective than Risperidone in treating OCD in BD (p < 0.001). |
| Kim et al., 2014 [67] | BD-OCD pt. were significantly associated with lower remission rates if treated with olanzapine ± other mood stabilizer compared to a mood stabilizer alone. |
| Masi et al., 2007 [113] | All patients with OCD received treatment with selective serotonin reuptake inhibitors (SRIs) such as sertraline, fluvoxamine, fluoxetine, paroxetine, citalopram, or clomipramine, either in mono- or polytherapy (89.2%), along with mood stabilizers (valproic acid, lithium) in 37.5% and antipsychotics (risperidone, olanzapine, quetiapine) in 30.8%. Patients with OCD-BD were more likely to receive mood stabilizers compared to those without OCD-BD (86.0% vs. 10.4%). Additionally, 69.8% of OCD-BD patients were treated with SRIs. There was a higher rate of non-responders to pharmacological treatment in OCD-BD patients compared to those without OCD-BD (54.7% vs. 25.6%). |
| Masi et al., 2009 [114] | Patients suffering from OCD-BD were more likely to receive polypharmacy compared to those taking selective serotonin reuptake inhibitors (SRIs) alone (51.1% vs. 5.6%). |
| Masi et al., 2018 [116] | BD-OCD pt. more frequently received psychotherapy and second-generation antipsychotics compared to BD and OCD pt., and they presented the poorest outcome in terms of response to treatments. |
| Ozdemiroglu et al., 2017 [80] | All the pt. recruited were under mood stabilizers, antidepressants, or antipsychotics. |
| Perugi et al., 2002 [84] | In patients affected by BD-OCD, clomipramine and, to a lesser extent, SSRIs were associated with a higher rate of manic/hypomanic switches compared to those without BD-OCD (clomipramine: 39.1% vs. 4.1%; SSRIs: 13.9% vs. 0.0%). There was a more frequent occurrence of pharmacologic mania/hypomania in BD-OCD patients who did not concurrently receive mood stabilizers (38.7% vs. 8.8%). Polypharmacologic treatments were required in 31.6% (a combination of mood stabilizers, lithium, plus antiepilectics) and 10.5% (a combination of mood stabilizers with atypical antipsychotics, clozapine, olanzapine, and risperidone) of the BD-OCD patients. |
| Sahraian et al., 2017 [87] | Memantine, as an adjunctive agent, was more effective than placebo in decreasing the OC symptoms (with more than 34% decrease in the mean Y-BOCS score) in BD-I-OCD pt. in the manic phase. |
| Sahraian et al., 2018 [88] | Aripiprazole showed effectiveness in reducing OC symptoms (with more than 34% decline in the Y-BOCS score) in BD-OCD pt. compared to placebo (91.30% vs. 4.34%). |
| Sahraian et al., 2021 [89] | Quetiapine showed greater effectiveness than placebo in reducing OC symptoms (with more than 34% decline in the Y-BOCS score (p < 0.001)) in BD-OCD pt. (Y-BOCS score from 24.37 to 15.26 (p < 0.001)) compared to placebo (Y-BOCS from 24.21 to 23.94 (p = 1.97)). No serious adverse effects were reported in the two groups. |
| Saraf et al., 2016 [90] | OCD-BD pt. received more SSRI trials at baseline; 86% of OCD-BD pt. were treated with adjunctive mood stabiliser, 67% were treated with lithium, and 33% were treated with valproate. |
| Shashidhara et al., 2015 [93] | No significant difference in the pattern of use of various medications and their combinations was observed between BD-OCD pt. and BD pt. None of the OCD pt. received antidepressants during the index mood episodes for which they were hospitalized. |
| Tonna et al., 2021 [101] | All pt. received one or more mood stabilizers. Antipsychotics were added in 94.1% of hypomanic/manic pt., in 84.6% of mixed pt., in 23.2% of depressed pt. and in 51.6% of euthymic pt. Antidepressants were added in 88.3% of depressed pt., in 15.3% of mixed pt. and in 16.1% of euthymic pt. |
| References | Results |
|---|---|
| Age and type of onset | |
| Anholt et al., 2014 [33] | Early age of OCD onset pt. presented higher rates of lifetime BD co-morbidity. |
| Cederlöf et al., 2014 [24] | The risk of receiving a diagnosis of BD after an initial diagnosis of OCD was much greater (around 12 times, with a median of 2.7 years between diagnoses (IR = 3.5)) than the risk of receiving a diagnosis of OCD after an initial diagnosis of BD (around 1.2 times, with a median time between diagnoses of 1.1 years (IR = 2.0)). |
| Chen et al., 1995 [25] | The average onset age of OCD and BD was similar. |
| Jeon et al., 2017 [62] | BD-OCD pt. presented earlier age at onset compared to BD pt. In 46.2% of comorbid pt., OCD onset preceded BD, whereas in 34.6%, BD preceded OCD, and in 19.2%, OCD began during the first mood episode. |
| Kazhungil et al., 2017 [63] | No significant difference in age was observed at the onset of BD between BD-OCD pt. and BD pt. |
| Maina et al., 2007 [75] | In the majority of cases (52.4%), the onset of OCD in patients with comorbid BD occurred simultaneously with the first mood episode rather than preceding it (38.1%) or following it (9.5%). |
| Masi et al., 2018 [116] | Age at onset of BD and OCD were not different between BD and OCD pt. and BD-OCD pt. |
| Ortiz et al., 2011 [79] | Comorbid OCD was associated with an early onset of BD and an early onset of depressive episodes in patients with BD. |
| Ozdemiroglu et al., 2017 [80] | Age at onset of BD was found to be earlier in BD-OCD pt. compared to BD pt. (23.9 vs. 29.2); age of onset of BD, compared to the age of onset of OCD, was found to be earlier in BD-OCD pt. (23.9 vs. 30). The first affective episode was major depression in half of BD-OCD pt. |
| Paul et al., 2015 [117] | OCD symptoms pre-dated the onset of mood disorder in all BD-OCD pt. compared to BD pt. |
| Perugi et al., 1997 [81] | OCD-BD showed a greater tendency toward a gradual onset compared to those without OCD-BD (68.5% vs. 49.0%). |
| Perugi et al., 2002 [84] | In the majority of cases (52.6%), the onset of OCD in patients with BD-OCD occurred simultaneously with the first mood episode, rather than preceding it (31.6%) or following it (15.8%). |
| Saraf et al., 2016 [90] | No difference between OCD-BD and OCD pt. with respect to age of onset of OCD was found. |
| Shashidhara et al., 2015 [93] | No significant difference was found in age at the onset of BD between BD-OCD pt. and BD pt. |
| Zutshi et al., 2007 [105] | In the majority of cases (54%), patients with OCD-BD experienced the onset of OCD before the onset of BD. |
| Course of illness | |
| Bener et al., 2016 [36] | BD pt. presented a longer duration of illness compared to BD-OCD pt. (10.96 ± 3.81 vs. 9.06 ± 3.32). |
| de Filippis et al., 2018 [49] | No differences in illness duration, number of depressive and manic episodes among BD, OCD and BD-OCD pt. were found. |
| Fineberg et al., 2013 [27] | OCD-BD exhibited a longer total illness duration (the time gap from the first manifestation until the last manifestation) compared to patients with only OCD (OR = 2.53; 95%; CI: 0.92–6.96). |
| Goes et al., 2012 [56] | Patients suffering from BD-OCD experienced a greater number of depressive episodes compared to those without BD-OCD (19.7 vs. 10.3). |
| Hofer et al., 2017 [107] | Prior OCD was associated with an increased risk of BD (OR = 6.9; 95% CI: 2.8–17.3). |
| Issler et al., 2005 [59] | A greater percentage of BD-OCD patients experienced a chronic course of OCD compared to those without BD-OCD (86.7% vs. 13.3%). (b) |
| Issler et al., 2010 [60] | BD-OCD had a greater number of depressive episodes compared to those without BD-OCD (8.9 ± 4.2 vs. 4.1 ± 2.7). Additionally, there was a higher rate of a chronic course of bipolar disorder in patients with BD-OCD compared to those without BD-OCD (66.7% vs. 20%). |
| Jeon et al., 2017 [62] | No statistically significant differences between the BD-OCD pt. and BD pt. in terms of duration of illness, number of previous episodes, type of the first epsiode, and the most recent episode were found. |
| Kazhungil et al., 2017 [63] | BD-OCD pt. presented higher rates of manic and depressive episodes compared to BD pt. |
| Khan et al., 2019 [64] | BD-I-OCD pt. presented a longer duration of illness compared to BD pt. |
| Kim et al., 2014 [67] | Comorbid OCD was associated with lower remission rates in BD-I pt. both in the first and in the second year of the study evaluation compared to BD-I pt. |
| Koyuncu et al., 2010 [68] | BD-OCD exhibited a higher rate of a chronic course of BD compared to those without BD-OCD (17.1% vs. 0.0%). (c) |
| Mahasuar et al., 2011 [74] | An episodic course was more prevalent in patients affected by OCD-BD compared to those without comorbidity (53% vs. 12%). On the other hand, higher rates of a continuous course (40% vs. 35%) or a subclinical course (32% vs. 6%) were observed in patients without OCD-BD compared to those with OCD-BD. (a),(b) |
| Ozdemiroglu et al., 2017 [80] | A more episodic course of OCD (34.4% vs. 14.8%) was higher in BD-OCD pt. compared to OCD pt.; rates of rapid cycling (21.9% vs. 6.3%) and seasonality (50% vs. 20.8%) were found to be higher in BD-OCD pt. compared to BD pt. |
| Perugi et al., 1997 [81] | OCD-BD showed a greater inclination toward an episodic course of OCD symptoms compared to those without OCD-BD (42.6% vs. 26.9%). (a) |
| Perugi et al., 1998 [82] | There were statistically non-significant trends indicating a higher rate of an episodic course of OCD in patients with comorbid BD-II compared to those without comorbidity. (a) |
| Perugi et al., 2002 [84] | BD-OCD exhibited a higher rate of an episodic course of OCD compared to those without BD-OCD (52.6% vs. 16.7%). (a) |
| Saraf et al., 2016 [90] | Episodic OCD was significantly overrepresented in OCD-BD pt. compared to OCD pt. (29% vs. 2%). All OCD-BD pt. presented a deterioration of OCD during depression; 86% reported improvement during mania/hypomania, whereas 14% reported complete remission during this phase. |
| Shashidhara et al., 2015 [93] | No significant difference in the total illness duration between BD-OCD pt. and BD pt. was found. |
| Strakowski et al., 1998 [96] | In 44% of patients with BD-OCD observed over a 12-month period following their initial hospitalization, BD and OCD exhibited cyclical patterns concurrently. |
| Tukel et al., 2006 [102] | A greater percentage of OCD-BD pt. experienced an episodic course of OCD compared to those without OCD-BD (42.3% vs. 10.9%). (a) |
| Tukel et al., 2007 [103] | Patients with episodic OCD exhibited a higher frequency of BD compared to those with chronic OCD (20.8% vs. 3.8%). (a) |
| Zutshi et al., 2007 [105] | There was a higher rate of an episodic course of OCD in patients with OCD-BD compared to patients without the comorbidity (75% vs. 3%). Conversely, there was a higher rate of a chronic course of OCD in patients without OCD-BD compared to those with comorbidity (97% vs. 14%). Among OCD-BD patients, the majority (78%) experienced OCD either exclusively during depressive episodes or reported worsening of OCD symptoms during depression. Improvement in obsessive–compulsive symptoms was noted in 64% of patients during manic/hypomanic episodes. (a),(b) |
| Global functioning and quality of life | |
| Angst et al., 2005 [16] | There was a statistically significant difference in global impairment (100.0% vs. 87.5%) and work impairment (100.0% vs. 78.1%) between OCD-BD pt. and those with “pure” OCD. |
| Bener et al., 2016 [36] | Higher CGI (4.69 ± 0.66 vs. 4.12 ± 0.76) and GAF (39.67 ± 5.32 vs. 37.11 ± 6.86) scores were found in BD pt. compared to BD-OCD pt. |
| Carta et al., 2020 [23] | OCD-BDS pt. presented significantly poorer QoL compared to OCD pt. (33.6 ± 6.7 vs. 36.1 ± 7.1). |
| Centorrino et al., 2006 [45] | There were no statistically significant differences in terms of GAF and CGI-S scores between patients with “pure” OCD and BD and those with comorbidity. |
| Fineberg et al., 2013 [27] | Relationship issues were more frequently reported in OCD-BD pt. compared to OCD pt. (separation/divorce, OR = 4.11; 95% CI: 1.13–14.89; problems with partner, OR = 5.04; 95% CI: 1.27–20.10). |
| Joshi, Wozniak et al., 2010 [109] | Patients with BD-OCD and those with OCD-BD exhibited lower levels of lifetime and current functioning on the GAF scale compared to patients without a comorbidity. |
| Kazhungil et al., 2017 [63] | BD-OCD pt. showed lower GAF scores and more dysfunctional impairment compared to BD pt. |
| Khan et al., 2017 [64] | BD-I-OCD pt. presented a lower level of education and higher divorce rates compared to BD-I pt. |
| Magalhaes et al., 2010 [73] | Patients with BD-OCD scored lower on all domains of the WHOQOL questionnaire, including physical, psychological, social, and environmental domains, compared to those without OCD-BD |
| Mahasuar et al., 2011 [74] | There were no statistically significant differences in GAF, CGI-S, and CGI-I scores between patients with OCD with or without comorbid BD. |
| Masi et al., 2004 [111] | There was a higher mean global severity score, measured during the first visit using the CGI-S, in patients with BD-OCD compared to those with only OCD (4.80 ± 0.7 vs. 4.4 ± 0.5). The scores were similar in patients with BD and those with BD-OCD (5.0 ± 0.8 and 4.80 ± 0.7, respectively). Furthermore, there were higher rates of severity at the end of the follow-up in patients with BD and BD-OCD compared to those with obsessive–compulsive disorder alone (3.1 ± 1.0 and 2.7 ± 1.0, respectively, vs. 2.0 ± 0.7). |
| Masi et al., 2007 [113] | OCD-BD exhibited greater functional impairment (C-GAS) at the baseline (43.1 ± 8.7 vs. 46.4 ± 9.0) and greater severity (CGI-S) both at the baseline and at the end of the follow-up compared to those without OCD-BD. |
| Shashidhara et al., 2015 [93] | BD-OCD pt. presented significantly lower GAF scores (41.9 vs. 35.8) and higher rates of unemployment (10% vs. 5%) compared to BD pt. |
| Simon et al., 2004 [94] | Having a current anxiety disorder (excluding OCD) in patients with BD was linked to lower functioning (measured by LIFE-RIFT) and a lower quality of life (Q-LES-Q). |
| Timpano et al., 2012 [100] | There were no statistically significant differences in the GAF total score between patients with OCD with or without comorbid BD. |
| Suicide ideations and attempts | |
| Chen et al., 1995 [25] | Patients with BD-OCD had higher lifetime rates of “thoughts of suicide,” “suicide attempts,” “thoughts of death,” and “wanting to die” compared to those without BD-OCD. |
| Di Salvo et al., 2020 [50] | No significant differences were found comparing lifetime suicide attempts rates between BD pts with or without comorbid OCD. In the BD-OCD group, more pt. performed suicide attempts with violent methods (48.3% vs. 28.7%), with a correlation between comorbid OCD and male gender and violent suicide attempts. |
| Dilsaver et al., 2006 [52] | BD-OCD was associated with a 2.4-fold increase in the odds of suicidal ideation compared to patients without BD-OCD (95% CI = 1.0–5.8). |
| Domingues-Castro et al., 2019 [54] | OCD-BD pt. presented a higher suicide risk compared to OCD pt., with more suicide ideations (OR 2.08, 95% CI = 1.26–3.43), plans (OR 2.80, 95% CI = 1.66–4.72), and attempts (OR 2.64, 95% CI = 1.41–4.91). |
| Fineberg et al., 2013 [27] | Suicidal acts were more commonly reported in patients with OCD-BD compared to those with only OCD (OR = 10.38; 95%; CI: 3.14–34.35). |
| Goes et al., 2012 [56] | BD-OCD had higher rates of a history of suicide attempts compared to those without BD-OCD (48.3% vs. 29.6%). |
| Jeon et al., 2017 [62] | No statistically significant differences were observed between BD-OCD pt. and BD pt. in terms of a history of suicide attempts. |
| Kazhungil et al., 2017 [63] | BD-OCD pt. presented higher rates of suicidal attempts compared to BD pt. |
| Koyuncu et al., 2010 [68] | There were no statistically significant differences between pt. with BD-OCD and pt. without BD-OCD in terms of lifetime suicide attempts. |
| Kruger et al., 2000 [70] | There was a higher incidence of prior suicide attempts in patients with BD-OCD compared to those without BD-OCD (90% vs. 38%). |
| Magalhaes et al., 2010 [73] | Patients with BD-OCD had higher rates of a history of suicide attempts compared to those without BD-OCD (70% vs. 35%). |
| Ozdemiroglu et al., 2017 [80] | A previous history of suicidal attempts was more likely to be higher in BD-OCD pt. (46.9%) compared to BD pt. (20.8%) and OCD pt. (11.5%). |
| Saraf et al., 2016 [90] | OCD-BD pt. presented a higher rate of lifetime suicide attempts compared to OCD pt. (57% vs. 14%). |
| Simon et al., 2004 [95] | Patients with BD-OCD experienced higher rates of a history of suicide attempts compared to those without the comorbidity. |
| Hospitalization | |
| Bener et al., 2016 [36] | No statistically significant difference was observed in the number of hospitalizations between BD-OCD pt. and BD pt. |
| Centorrino et al., 2006 [45] | Re-hospitalization rates were comparable between patients affected by BD-OCD and those with BD alone, but they were 2.9 times more frequent in BD-OCD patients compared to those without comorbidity. |
| Joshi, Wozniak et al., 2010 [109] | There were higher rates of hospitalization in BD-OCD and OCD-BD compared to patients without comorbidity (BD-OCD: 41.2% vs. 10.4%; OCD-BD: 31.6% vs. 10.4%). |
| Kazhungil et al., 2017 [63] | BD-OCD pt. presented higher rates of hospitalizations compared to BD pt. |
| Kim et al., 2014 [67] | BD-I-OCD pt. were more likely to be hospitalized during the 24-month study period compared to BD-I pt. (54.2% vs. 35.3%), (difference statistically significant). |
| Mahasuar et al., 2011 [74] | There was a higher total number of hospitalizations in patients with OCD-BD compared to those without OCD-BD (1.55 ± 1.70 vs. 0.16 ± 0.41). |
| Masi et al., 2007 [113] | There was a higher frequency of the need for hospitalization in patients with OCD-BD compared to those without OCD-BD (62.8% vs. 28.6%). |
| Shashidhara et al., 2015 [93] | No statistically significant difference was found in the number of hospitalizations between BD-OCD pt. and BD pt. |
| Timpano et al., 2012 [100] | There was a more frequent requirement for hospitalization in patients with the BD-OCD comorbidity compared to those without OCD-BD (58.2% vs. 13.8%). |
| Substance and alcohol abuse | |
| Angst et al., 2005 [16] | Patients with obsessive–compulsive symptoms and comorbid BD (OCS-BD) exhibited higher rates of substance abuse/dependence (50% vs. 31.3%) and alcohol abuse/dependence (37.5% vs. 18.8%) compared to those with “pure” obsessive–compulsive symptoms (OCS pt.). |
| Boylan et al., 2004 [39] | BD-OCD had higher rates of past substance abuse or dependence compared to pt. without BD-OCD (50% vs. 20%). |
| Chen et al., 1995 [25] | Pt. with OCD-BD comorbidity exhibited rates of alcohol and drug abuse of 31.4% and 37.1%, respectively. Non-comorbid patients had rates of alcohol and drug abuse of 36.4% and 28.2%, respectively. |
| Fineberg et al., 2013 [27] | The occurrence of substance abuse disorders was more commonly reported in patients with OCD-BD compared to those with only OCD (OR = 3.65; 95%; CI 1.34–9.94). |
| Magalhaes et al., 2010 [73] | BD-OCD pt. had higher rates of lifetime alcohol dependence compared to pt. without BD-OCD (31% vs. 10%). |
| Maina et al., 2007 [75] | There was a significant association between OCD-BD and substance use disorders (28.6% vs. 4.9%) compared to pt. without OCD-BD. |
| Perugi et al., 2002 [84] | There was a stronger association between BD-OCD and the use of sedatives, nicotine, alcohol, and coffee (p = 0.03) compared to patients without this comorbidity. |
| Timpano et al., 2012 [100] | There was a robust association between OCD-BD and substance use disorders (OR 3.18, 95%; CI = 1.81–5.58), as well as alcohol use disorders (odds ratio 2.21, 95% CI = 1.34–3.65), compared to pt. without OCD-BD. |
| Other psychiatric comorbidities | |
| Domingues-Castro et al., 2019 [54] | BD-OCD pt. presented higher panic disorder with agoraphobia (OR 2.54, 95% CI = 1.53–4.20) and impulse control disorders (OR 2.41, 95% CI = 1.49–3.92) compared to OCD pt. |
| Issler et al., 2010 [60] | There were higher prevalence rates of any anxiety disorder other than OCD (93.3% vs. 53.3%), impulse control disorders (60% vs. 13.3%), EDs (33.3% vs. 0%), and tic disorders (33.3% vs. 0%) in patients with BD-OCD compared to those without BD-OCD. |
| Jeon et al., 2017 [62] | BD-OCD pt. presented a higher rate of comorbid panic disorder and eating disorders compared to BD pt. |
| Maina et al., 2007 [75] | There were higher rates of at least one Cluster A personality disorder (p = 0.027), at least one Cluster B personality disorder, and narcissistic and antisocial personality disorders in patients with OCD-BD compared to those without OCD-BD. |
| Masi et al., 2004 [111] | There were higher rates of ADHD-CD (51.3% vs. 20.0%) and CD (29.7% vs. 13.3%) in patients with BD compared to those with BD-OCD. Conversely, there were lower rates of ADHD (2.8% vs. 16.7%) and higher rates of GAD (77.1% vs. 16.7%) in patients with OCD compared to those with comorbid BD-OCD. |
| Masi et al., 2007 [113] | Patients with OCD-BD had higher comorbidity rates with ADHD and ODD and lower comorbidity rates with GAD. |
| Perugi et al., 2002 [84] | There were higher rates of current comorbidity with panic disorder/agoraphobia in patients with BD-OCD compared to those without BD-OCD (52.6% vs. 16.7%). |
| Shashidhara et al., 2015 [93] | BD-OCD pt. presented higher rates of comorbid social anxiety (10% vs. 1.4%) and anxious avoidant personality disorder (26.7% vs. 2.7%). |
| Tasdemir et al., 2015 [99] | Although not statistically significant, BD-OCD comorbidity was more common in BD pt. with adult separation anxiety disorder. |
| Timpano et al., 2012 [100] | Patients with OCD-BD were more than twice as likely to also be diagnosed with panic disorder (OR 2.26, 95% CI = 1.38–3.71), agoraphobia (OR 2.30, 95% CI = 1.35–3.91), PTSD (OR 2.85, 95% CI = 1.49–5.45), and an ED (OR 2.03, p < 0.001, 95% CI = 1.14–3.60) compared to patients without the comorbidity. |
| Medical comorbidities | |
| Kemp et al., 2014 [65] | BD-OCD pt. presented a higher rate of medical comorbidity compared to BD pt. without OCD (14.4% vs. 5.6%). |
| Khan et al., 2019 [64] | BD-I-OCD pt. presented fewer medical comorbidities compared to BD-I pt. but a higher frequency of cardiovascular conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Filippis, R.; Aguglia, A.; Costanza, A.; Benatti, B.; Placenti, V.; Vai, E.; Bruno, E.; De Berardis, D.; Dell’Osso, B.; Albert, U.; et al. Obsessive–Compulsive Disorder as an Epiphenomenon of Comorbid Bipolar Disorder? An Updated Systematic Review. J. Clin. Med. 2024, 13, 1230. https://doi.org/10.3390/jcm13051230
de Filippis R, Aguglia A, Costanza A, Benatti B, Placenti V, Vai E, Bruno E, De Berardis D, Dell’Osso B, Albert U, et al. Obsessive–Compulsive Disorder as an Epiphenomenon of Comorbid Bipolar Disorder? An Updated Systematic Review. Journal of Clinical Medicine. 2024; 13(5):1230. https://doi.org/10.3390/jcm13051230
Chicago/Turabian Stylede Filippis, Renato, Andrea Aguglia, Alessandra Costanza, Beatrice Benatti, Valeria Placenti, Eleonora Vai, Edoardo Bruno, Domenico De Berardis, Bernardo Dell’Osso, Umberto Albert, and et al. 2024. "Obsessive–Compulsive Disorder as an Epiphenomenon of Comorbid Bipolar Disorder? An Updated Systematic Review" Journal of Clinical Medicine 13, no. 5: 1230. https://doi.org/10.3390/jcm13051230
APA Stylede Filippis, R., Aguglia, A., Costanza, A., Benatti, B., Placenti, V., Vai, E., Bruno, E., De Berardis, D., Dell’Osso, B., Albert, U., De Fazio, P., Amore, M., Serafini, G., Ghaemi, N. S., & Amerio, A. (2024). Obsessive–Compulsive Disorder as an Epiphenomenon of Comorbid Bipolar Disorder? An Updated Systematic Review. Journal of Clinical Medicine, 13(5), 1230. https://doi.org/10.3390/jcm13051230











