The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, D.A.; Paradise, S.L.; Reeder, R.M. Amenorrhea: A Systematic Approach to Diagnosis and Management. Am. Fam. Physician 2019, 100, 39–48. [Google Scholar]
- Munster, K.; Helm, P.; Schmidt, L. Secondary amenorrhoea: Prevalence and medical contact--a cross-sectional study from a Danish county. Br. J. Obstet. Gynaecol. 1992, 99, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M. Clinical practice. Functional hypothalamic amenorrhea. N. Engl. J. Med. 2010, 363, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.; Dewailly, D.; Marculescu, R.; Ghobrial, S.; Parry, J.P.; Ott, J. Stress and polycystic ovarian morphology in functional hypothalamic amenorrhea: A retrospective cohort study. Reprod. Biol. Endocrinol. 2023, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Bonazza, F.; Politi, G.; Leone, D.; Vegni, E.; Borghi, L. Psychological factors in functional hypothalamic amenorrhea: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 981491. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Torbati, T.; Dutra, E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Blenck, C.L.; Harvey, P.A.; Reckelhoff, J.F.; Leinwand, L.A. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ. Res. 2016, 118, 1294–1312. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Pedrielli, G.; Bosoni, D.; Tiranini, L.; Cucinella, L.; Calogero, A.E.; Facchinetti, F.; Nappi, R.E. Sexual functioning in women with functional hypothalamic amenorrhea: Exploring the relevance of an underlying polycystic ovary syndrome (PCOS)-phenotype. J. Endocrinol. Investig. 2023, 46, 1623–1632. [Google Scholar] [CrossRef]
- Fontana, L.; Garzia, E.; Marfia, G.; Galiano, V.; Miozzo, M. Epigenetics of functional hypothalamic amenorrhea. Front. Endocrinol. 2022, 13, 953431. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Phylactou, M.; Clarke, S.A.; Patel, B.; Baggaley, C.; Jayasena, C.N.; Kelsey, T.W.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2021, 95, 239–252. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of the International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2023, 120, 767–793. [Google Scholar] [CrossRef]
- Makolle, S.; Catteau-Jonard, S.; Robin, G.; Dewailly, D. Revisiting the serum level of anti-Mullerian hormone in patients with functional hypothalamic anovulation. Hum. Reprod. 2021, 36, 1043–1051. [Google Scholar] [CrossRef]
- Beitl, K.; Dewailly, D.; Seemann, R.; Hager, M.; Bunker, J.; Mayrhofer, D.; Holzer, I.; Ott, J. Polycystic Ovary Syndrome Phenotype D versus Functional Hypothalamic Amenorrhea with Polycystic Ovarian Morphology: A Retrospective Study about a Frequent Differential Diagnosis. Front. Endocrinol. 2022, 13, 904706. [Google Scholar] [CrossRef] [PubMed]
- Piltonen, T.T.; Komsi, E.; Morin-Papunen, L.C.; Korhonen, E.; Franks, S.; Järvelin, M.-R.; Arffman, R.K.; Ollila, M.-M. AMH as part of the diagnostic PCOS workup in large epidemiological studies. Eur. J. Endocrinol. 2023, 188, 547–554. [Google Scholar] [CrossRef]
- Berga, S.L.; Mortola, J.F.; Girton, L.; Suh, B.; Laughlin, G.; Pham, P.; Yen, S.S. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 1989, 68, 301–308. [Google Scholar] [CrossRef]
- Morrison, A.E.; Fleming, S.; Levy, M.J. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin. Endocrinol. 2021, 95, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Jonard, S.; Pigny, P.; Jacquesson, L.; Demerle-Roux, C.; Robert, Y.; Dewailly, D. The ovarian markers of the FSH insufficiency in functional hypothalamic amenorrhoea. Hum. Reprod. 2005, 20, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.D.; Meczekalski, B.; Podfigurna-Stopa, A.; Santagni, S.; Rattighieri, E.; Ricchieri, F.; Chierchia, E.; Simoncini, T. Estriol administration modulates luteinizing hormone secretion in women with functional hypothalamic amenorrhea. Fertil. Steril. 2012, 97, 483–488. [Google Scholar] [CrossRef]
- Marshall, J.C.; Dalkin, A.C.; Haisenleder, D.J.; Griffin, M.L.; Kelch, R.P. GnRH pulses—The regulators of human reproduction. Trans. Am. Clin. Climatol. Assoc. 1993, 104, 31–46. [Google Scholar]
- Tsutsumi, R.; Webster, N.J. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 2009, 56, 729–737. [Google Scholar] [CrossRef]
- Herpertz, S.; Hagenah, U.; Vocks, S.; von Wietersheim, J.; Cuntz, U.; Zeeck, A. The diagnosis and treatment of eating disorders. Dtsch. Arztebl. Int. 2011, 108, 678–685. [Google Scholar] [CrossRef]
- Schneider, L.F.; Warren, M.P. Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil. Steril. 2006, 86, 1744–1749. [Google Scholar] [CrossRef]
- Tinahones, F.J.; Martinez-Alfaro, B.; Gonzalo-Marin, M.; Garcia-Almeida, J.M.; Garrido-Sanchez, L.; Cardona, F. Recovery of menstrual cycle after therapy for anorexia nervosa. Eat. Weight. Disord. 2005, 10, e52–e55. [Google Scholar] [CrossRef]
- Hager, M.; Ott, J.; Marschalek, J.; Marschalek, M.L.; Kinsky, C.; Marculescu, R.; Dewailly, D. Basal and dynamic relationships between serum anti-Mullerian hormone and gonadotropins in patients with functional hypothalamic amenorrhea, with or without polycystic ovarian morphology. Reprod. Biol. Endocrinol. 2022, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, D.; Dewailly, D.; Hager, M.; Marculescu, R.; Beitl, K.; Ott, J. Functional hypothalamic amenorrhea with or without polycystic ovarian morphology: A retrospective cohort study about insulin resistance. Fertil. Steril. 2022, 118, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Update 2014, 20, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Robin, G.; Gallo, C.; Catteau-Jonard, S.; Lefebvre-Maunoury, C.; Pigny, P.; Duhamel, A.; Dewailly, D. Polycystic Ovary-Like Abnormalities (PCO-L) in women with functional hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 2012, 97, 4236–4243. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Fruzzetti, F.; Lobo, R.A. Features of polycystic ovary syndrome (PCOS) in women with functional hypothalamic amenorrhea (FHA) may be reversible with recovery of menstrual function. Gynecol. Endocrinol. 2018, 34, 301–304. [Google Scholar] [CrossRef]
- Alvero, R.; Kimzey, L.; Sebring, N.; Reynolds, J.; Loughran, M.; Nieman, L.; Olson, B.R. Effects of fasting on neuroendocrine function and follicle development in lean women. J. Clin. Endocrinol. Metab. 1998, 83, 76–80. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 77 (Suppl. S4), S3–S5. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Belda, X.; Fuentes, S.; Daviu, N.; Nadal, R.; Armario, A. Stress-induced sensitization: The hypothalamic-pituitary-adrenal axis and beyond. Stress 2015, 18, 269–279. [Google Scholar] [CrossRef]
- Selzer, C.; Ott, J.; Dewailly, D.; Marculescu, R.; Steininger, J.; Hager, M. Prolactin levels in Functional hypothalamic amenorrhea: A retrospective case-control study. Arch. Gynecol. Obstet. 2023, 309, 651–658. [Google Scholar] [CrossRef]
- Macotela, Y.; Triebel, J.; Clapp, C. Time for a New Perspective on Prolactin in Metabolism. Trends Endocrinol. Metab. 2020, 31, 276–286. [Google Scholar] [CrossRef]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.; Endocrine, S. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, J.; Blizzard, L.; Oddy, W.H.; Dwyer, T.; Bazzano, L.A.; Hickey, M.; Harville, E.W.; Venn, A.J. Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: The Childhood Determinants of Adult Health Study and the Bogalusa Heart Study. Hum. Reprod. 2020, 35, 1185–1198. [Google Scholar] [CrossRef]
- Ezeh, U.; Pisarska, M.D.; Azziz, R. Association of severity of menstrual dysfunction with hyperinsulinemia and dysglycemia in polycystic ovary syndrome. Hum. Reprod. 2022, 37, 553–564. [Google Scholar] [CrossRef]
- La Marca, A.; Pati, M.; Orvieto, R.; Stabile, G.; Carducci Artenisio, A.; Volpe, A. Serum anti-mullerian hormone levels in women with secondary amenorrhea. Fertil. Steril. 2006, 85, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lie Fong, S.; Schipper, I.; Valkenburg, O.; de Jong, F.H.; Visser, J.A.; Laven, J.S. The role of anti-Mullerian hormone in the classification of anovulatory infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 186, 75–79. [Google Scholar] [CrossRef]
- Luisi, S.; Ciani, V.; Podfigurna-Stopa, A.; Lazzeri, L.; De Pascalis, F.; Meczekalski, B.; Petraglia, F. Serum anti-Mullerian hormone, inhibin B, and total inhibin levels in women with hypothalamic amenorrhea and anorexia nervosa. Gynecol. Endocrinol. 2012, 28, 34–38. [Google Scholar] [CrossRef]
- Qu, X.; Donnelly, R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2020, 21, 8191. [Google Scholar] [CrossRef]
| Age (years), median (IQR) 1 | 26 (22;29) |
| BMI (kg/m2), median (IQR) 1 | 20.3 (18.6;22.0) |
| Gravidity: n (%) 2 | |
| 0 | 134 (99.3) |
| 1 | 1 (0.7) |
| Parity: n (%) 2 | |
| 0 | 134 (99.3) |
| 1 | 1 (0.7) |
| Causes for FHA: n (%) 2,3 | |
| Stress | 44 (32.6) |
| Excessive exercise | 55 (40.7) |
| Anorexia nervosa | 30 (22.2) |
| Acute weight loss | 33 (24.4) |
| Underweight | 24 (17.8) |
| Duration since last menstrual bleeding (months), median (IQR) 1 | 14 (10;24) |
| Hormones, median (IQR) 1 | |
| TSH (IU/mL) | 1.57 (1.12;2.03) |
| Prolactin (ng/mL) | 8.9 (6.6;12.9) |
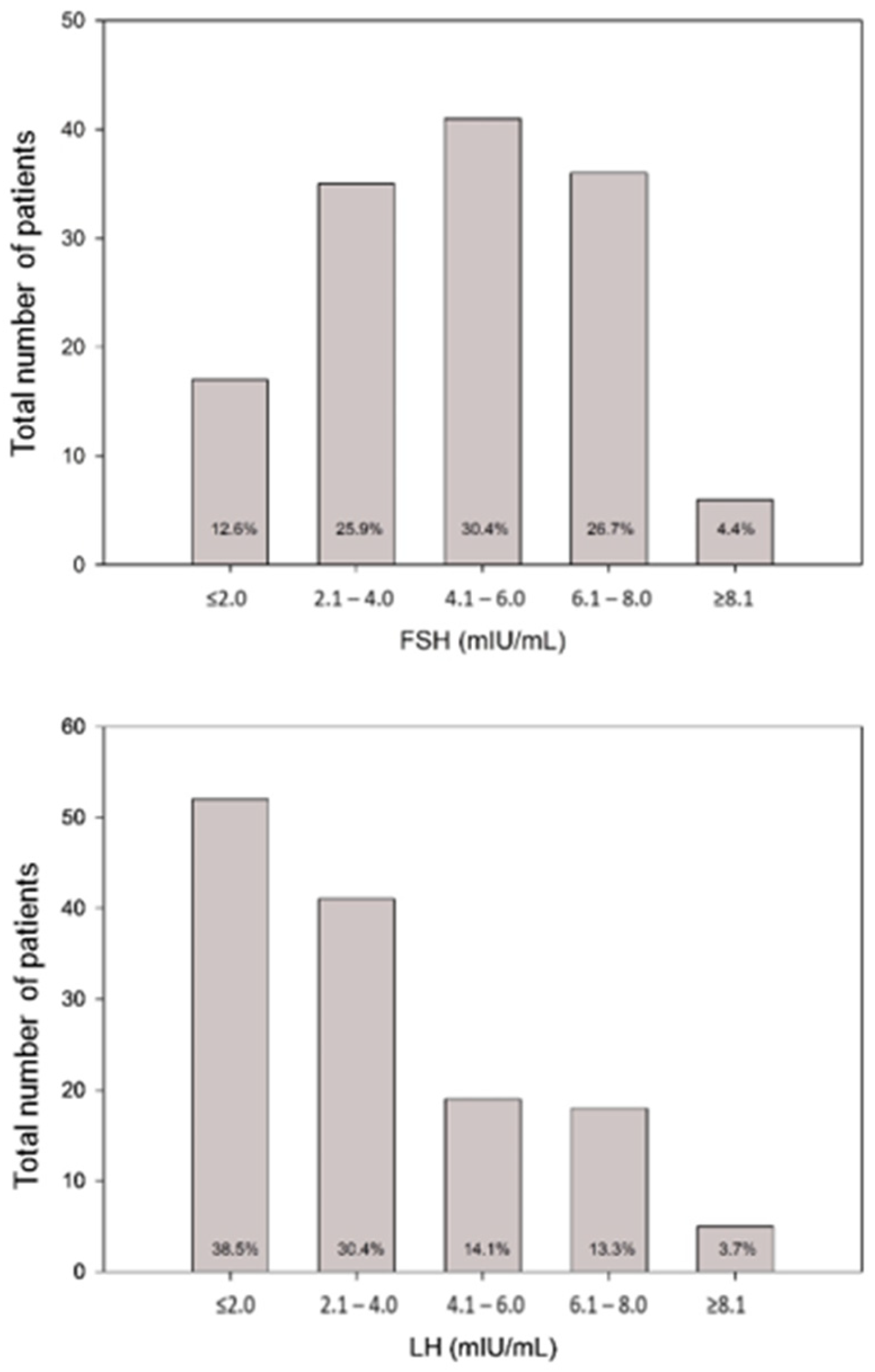
| FSH (mIU/mL) | 4.7 (3.3;6.5) |
| LH (mIU/mL) | 2.6 (1.3;4.7) |
| Estradiol (pg/mL) | 23 (12;31) |
| Testosterone (ng/mL) | 0.20 (0.13;0.29) |
| DHEAS (µg/mL) | 2.03 (1.40;2.73) |
| SHBG (nmol/L) | 73.0 (55.1;101.8) |
| AMH (ng/mL) | 3.1 (1.6;6.2) |
| Polycystic ovarian morphology on ultrasound, n (%) 2 | 58 (43.0) |
| FSH | LH | LH:FSH Ratio | Estradiol | Testosterone | AMH | Prolactin | ||
|---|---|---|---|---|---|---|---|---|
| FSH | r2 | - | 0.556 | −0.141 | 0.291 | 0.045 | 0.221 | 0.323 |
| p | <0.001 | 0.103 | <0.001 | 0.604 | 0.010 | 0.134 | ||
| LH | r2 | 0.556 | - | 0.633 | 0.387 | 0.218 | 0.060 | 0.315 |
| p | <0.001 | <0.001 | <0.001 | 0.011 | 0.134 | <0.001 | ||
| LH:FSH ratio | r2 | −0.141 | 0.633 | - | 0.271 | 0.159 | −0.076 | 0.261 |
| p | 0.103 | <0.001 | 0.002 | 0.066 | 0.385 | 0.002 | ||
| Estradiol | r2 | 0.291 | 0.387 | 0.271 | - | 0.243 | 0.076 | 0.182 |
| p | <0.001 | <0.001 | 0.002 | 0.005 | 0.386 | 0.037 | ||
| Testosterone | r2 | 0.045 | 0.218 | 0.159 | 0.243 | - | 0.126 | 0.268 |
| p | 0.604 | 0.011 | 0.066 | 0.005 | 0.134 | 0.002 | ||
| AMH | r2 | 0.221 | 0.060 | −0.076 | 0.076 | 0.126 | - | −0.051 |
| p | 0.010 | 0.134 | 0.385 | 0.386 | 0.134 | 0.555 | ||
| Prolactin | r2 | 0.323 | 0.315 | 0.261 | 0.182 | 0.268 | −0.051 | - |
| p | 0.134 | <0.001 | 0.002 | 0.037 | 0.002 | 0.555 |
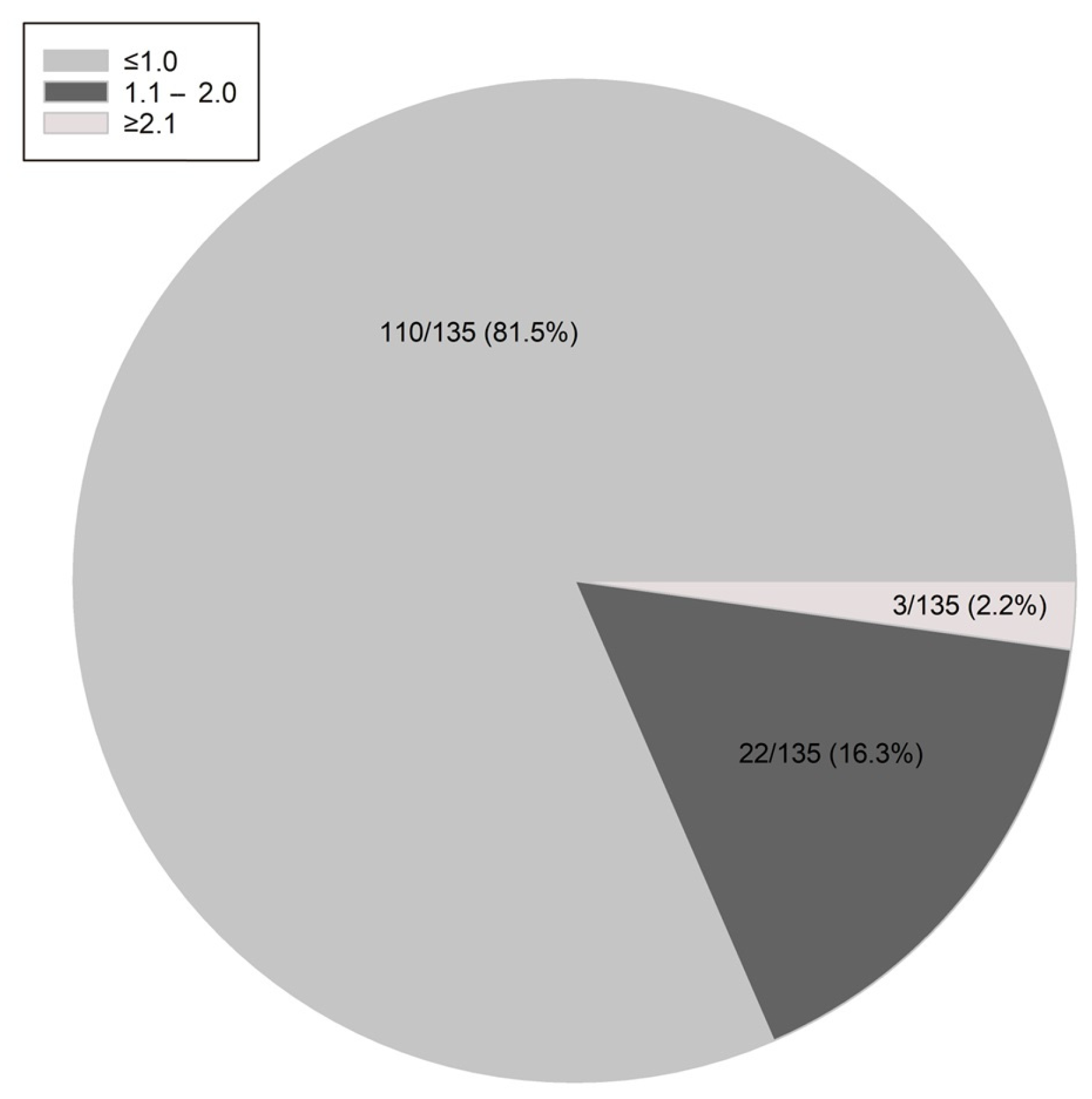
| LH:FSH Ratio ≤ 1 (n = 110) | LH:FSH Ratio > 1 (n = 25) | OR (95%CI) | p | OR (95%CI) | p | |
|---|---|---|---|---|---|---|
| Age (years) 1 | 26 (22;29) | 24 (22;28) | 1.047 (0.956;1.147) | 0.321 | - | - |
| BMI (kg/m2) 1 | 19.9 (18.6;21.7) | 21.2 (18.6;22.3) | 0.880 (0.740;1.047) | 0.149 | - | - |
| Causes for FHA: | ||||||
| Stress 2,3 | 33 (30.0) | 11 (44.0) | 0.545 (0.224;1.327) | 0.181 | - | - |
| Excessive exercise 2,3 | 45 (40.9) | 10 (40.0) | 1.038 (0.428;2.518) | 0.933 | - | - |
| Anorexia nervosa 2,3 | 22 (20.0) | 8 (32.0) | 0.531 (0.203;1.389) | 0.197 | - | - |
| Acute weight loss 2,3 | 27 (24.5) | 6 (24.0) | 1.030 (0.373;2.844) | 0.954 | - | - |
| Underweight 2,3 | 19 (17.3) | 5 (20.0) | 0.835 (0.279;2.503) | 0.748 | - | - |
| Duration since last bleeding (months) 1 | 14 (12;24) | 12 (8;16) | 1.039 (0.998;1.082) | 0.065 | - | - |
| FSH (mIU/mL) 1 | 4.7 (3.4;6.3) | 4.7 (2.6;6.5) | 1.056 (0.868;1.284) | 0.588 | - | - |
| LH (mIU/mL) 1 | 2.2 (1.2;3.5) | 6.3 (4.0;7.7) | 0.499 (0.385;0.647) | <0.001 | 0.520 (0.400;0.675) | <0.001 |
| Prolactin (ng/mL) 1 | 8.1 (6.4;12.4) | 12.0 (8.8;14.2) | 0.960 (0.894;1.031) | 0.260 | - | - |
| Estradiol (pg/mL) 1 | 21 (11;28) | 25 (22;39) | 0.956 (0.928;0.986) | 0.004 | 0.975 (0.939;1.013) | 0.196 |
| Testosterone (ng/mL) 1 | 0.19 (0.13;0.29) | 0.25 (0.14;0.33) | 0.015 (0.000;1.030) | 0.052 | - | - |
| SHBG (nmol/L) 1 | 74.0 (57.4;99.1) | 70.0 (48.6;113.0) | 0.998 (0.986;1.010) | 0.720 | - | - |
| AMH (ng/mL) 1 | 2.8 (1.6;6.1) | 4.5 (2.2;6.2) | 0.955 (0.863;1.087) | 0.486 | - | - |
| PCOM 2 | 42 (38.2) | 13 (52.0) | 0.570 (0.238;1.366) | 0.208 | - | - |
| PCOM (n = 58) | Non-PCOM (n = 77) | p | |
|---|---|---|---|
| Age (years) | 26 (22;28) | 26 (22;30) | 0.299 |
| BMI (kg/m2) | 20.4 (18.8;22.5) | 20.0 (18.4;21.4) | 0.190 |
| Prolactin (ng/mL) | 9.6 (6.6;13.0) | 8.8 (6.7;12.8) | 0.964 |
| FSH (mIU/mL) | 4.8 (3.5;6.5) | 4.7 (3.2;6.2) | 0.426 |
| LH (mIU/mL) | 2.8 (1.5;5.5) | 2.6 (1.3;4.6) | 0.290 |
| LH:FSH ratio | 0.8 (0.3;1.0) | 0.7 (0.4;1.0) | 0.728 |
| Estradiol (pg/mL) | 24 (11;34) | 22 (12;29) | 0.719 |
| Testosterone (ng/mL) | 0.20 (0.14;0.29) | 0.19 (0.13;0.29) | 0.881 |
| SHBG (nmol/L) | 67.0 (39.7;94.1) | 79.4 (63.3;104.0) | 0.008 |
| AMH (ng/mL) | 6.3 (4.9;7.6) | 2.0 (1.1;2.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boegl, M.; Dewailly, D.; Marculescu, R.; Steininger, J.; Ott, J.; Hager, M. The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study. J. Clin. Med. 2024, 13, 1201. https://doi.org/10.3390/jcm13051201
Boegl M, Dewailly D, Marculescu R, Steininger J, Ott J, Hager M. The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study. Journal of Clinical Medicine. 2024; 13(5):1201. https://doi.org/10.3390/jcm13051201
Chicago/Turabian StyleBoegl, Magdalena, Didier Dewailly, Rodrig Marculescu, Johanna Steininger, Johannes Ott, and Marlene Hager. 2024. "The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study" Journal of Clinical Medicine 13, no. 5: 1201. https://doi.org/10.3390/jcm13051201
APA StyleBoegl, M., Dewailly, D., Marculescu, R., Steininger, J., Ott, J., & Hager, M. (2024). The LH:FSH Ratio in Functional Hypothalamic Amenorrhea: An Observational Study. Journal of Clinical Medicine, 13(5), 1201. https://doi.org/10.3390/jcm13051201