Contrast-Enhanced Mammography-Guided Biopsy: Preliminary Results of a Single-Center Retrospective Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and CEM Descriptors
2.2. CEM Protocol
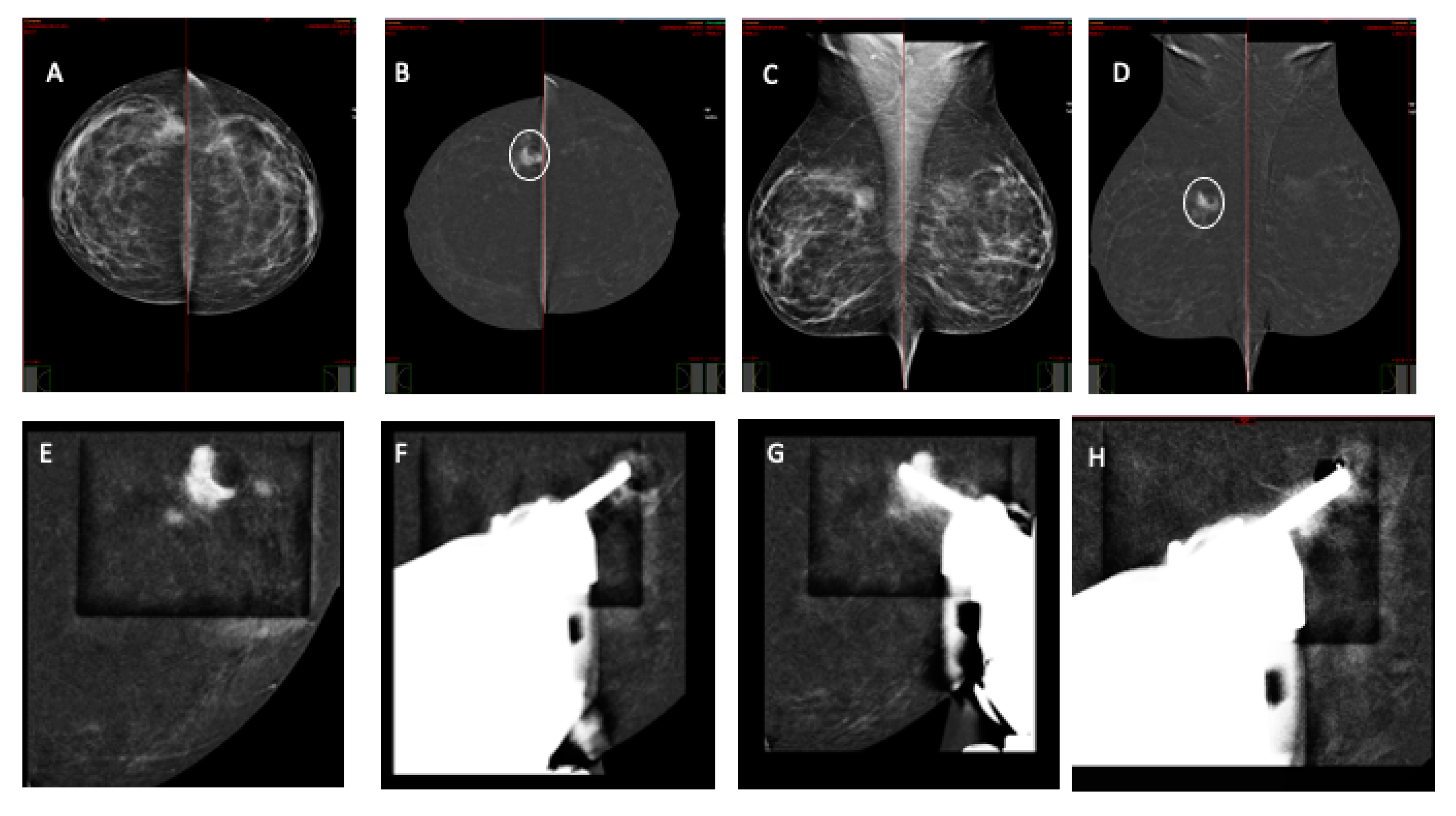
2.3. CEM-Guided Breast Biopsy Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef]
- Richter, V.; Hatterman, V.; Preibsch, H.; Bahrs, S.D.; Hahn, M.; Nikolaou, K.; Wiesinger, B. Contrast-enhanced spectral mammography in patients with MRI contraindications. Acta Radiol. 2017, 59, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.-C.; Lin, Y.-C.; Wan, Y.-L.; Yeow, K.-M.; Huang, P.-C.; Lo, Y.-F.; Tsai, H.-P.; Ueng, S.-H.; Chang, C.-J. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: Interobserver blind-reading analysis, Eur. Radiol. 2014, 24, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast- enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef]
- Iotti, V.; Ravaioli, S.; Vacondio, R.; Coriani, C.; Caffarri, S.; Sghedoni, R.; Nitrosi, A.; Ragazzi, M.; Gasparini, E.; Masini, C.; et al. Contrast- Enhanced spectral mammography in neoadjuvant chemotherapy monitoring: A comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017, 19, 106. [Google Scholar] [CrossRef]
- Lobbes, M.B.; Lalji, U.C.; Nelemans, P.J.; Houben, I.; Smidt, M.L.; Heuts, E.; de Vries, B.; Wildberger, J.E.; Beets-Tan, R.G. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J. Cancer 2015, 6, 144–150. [Google Scholar] [CrossRef]
- Lobbes, M.; Prevos, R.; Smidt, M. Response monitoring of breast cancer patients receiving neoadjuvant chemotherapy using breast MRI—A review of current knowledge. J. Cancer Ther. Res. 2012, 1, 34. [Google Scholar] [CrossRef][Green Version]
- Lobbes, M.B.I.; Prevos, R.; Smidt, M.; Tjan-Heijnen, V.C.G.; van Goethem, M.; Schipper, R.; Beets-Tan, R.G.; Wildberger, J.E. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: A systematic review. Insights Imaging 2013, 4, 163–175. [Google Scholar] [CrossRef]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Engelken, F.; Krohn, M.; Singh, J.M.; Ingold-Heppner, B.; Winzer, K.J.; Bick, U.; Renz, D.M. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur. Radiol. 2014, 24, 256–264. [Google Scholar] [CrossRef]
- van Nijnatten, T.J.; Jochelson, M.S.; Pinker, K.; Keating, D.M.; Sung, J.S.; Morrow, M.; Smidt, M.L.; Lobbes, M.B. Differences in degree of lesion enhancement on CEM between ILC and IDC. BJR Open 2019, 5, 20180046. [Google Scholar] [CrossRef]
- Houben, I.; Van de Voorde, P.; Jeukens, C.; Wildberger, J.; Kooreman, L.; Smidt, M.; Lobbes, M. Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur. J. Radiol. 2017, 94, 31–37. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Heerdt, A.S.; Thornton, C.; Moskowitz, C.S.; Ferrara, J.; Morris, E.A. Bilateral contrast-enhanced dual- energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013, 266, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Fallenberg, E.M.; Schmitzberger, F.F.; Amer, H.; Ingold-Heppner, B.; Balleyguier, C.; Diekmann, F.; Engelken, F.; Mann, R.M.; Renz, D.M.; Bick, U.; et al. Contrast-enhanced spectral mammography vs. mammography and MRI—Clinical performance in a multi-reader evaluation. Eur. Radiol. 2017, 27, 2752–2764. [Google Scholar] [CrossRef] [PubMed]
- Fallenberg, E.M.; Schmitzberger, F.F.; Amer, H.; Ingold-Heppner, B.; Balleyguier, C.; Diekmann, F.; Engelken, F.; Mann, R.M.; Renz, D.M.; Bick, U.; et al. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123. [Google Scholar] [CrossRef]
- Kamal, R.M.; Helal, M.H.; Wessam, R.; Mansour, S.M.; Godda, I.; Alieldin, N. Contrastenhanced spectral mammography: Impact of the qualitative morphology descriptors on the diagnosis of breast lesions. Eur. J. Radiol. 2015, 84, 1049–1055. [Google Scholar] [CrossRef]
- Kamal, R.M.; Helal, M.H.; Mansour, S.M.; Haggag, M.A.; Nada, O.M.; Farahat, I.G.; Alieldin, N.H. Can we apply the MRI BI-RADS lexicon morphology descriptors on contrast-enhanced spectral mammography? Br. J. Radiol. 2016, 12, 20160157. [Google Scholar] [CrossRef] [PubMed]
- Tozaki, M.; Igarashi, T.; Fukuda, K. Breast MRI using the VIBE sequence: Clustered ring enhancement in the differential diagnosis of lesions showing nonmasslike enhancement. AJR Am. J. Roentgenol. 2006, 187, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rauch, G.M.; Dogan, B.E.; Smith, T.B.; Liu, P.; Yang, W.T. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am. J. Roentgenol. 2012, 198, 292–299. [Google Scholar] [CrossRef]
- Imschweiler, T.; Haueisen, H.; Kampmann, G.; Rageth, L.; Seifert, B.; Rageth, C.; Freiwald, B.; Kubik-Huch, A. MRI-guided vacuum-assisted breast biopsy: Comparison with stereotactically guided and ultrasound-guided techniques. Eur. Radiol. 2014, 24, 128–135. [Google Scholar] [CrossRef]
- Rauch, G.M.; Dogan, B.E.; Smith, T.B.; Liu, P.; Yang, W.T. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: Can underestimation be predicted? Eur. Radiol. 2014, 21, 582–589. [Google Scholar]
- McGrath, A.L.; Price, E.R.; Eby, P.R.; Rahbar, H. MRI-guided breast interventions. J. Magn. Reson. Imaging 2017, 46, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Hefler, L.; Casselman, J.; Amaya, B.; Heinig, A.; Alberich, T.; Koelbl, H.; Heywang-Köbrunner, S. Follow-up of breast lesions detected byMRI not biopsied due to absent enhancement of contrast medium. Eur. Radiol. 2003, 13, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.B.; Sung, J.S.; Dershaw, D.D.; Liberman, L.; Morris, E.A. Cancellation of MR imaging-guided breast biopsy due to lesion nonvisualization: Frequency and follow-up. Radiology 2011, 261, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Cheung, Y.C. Contrast-enhanced mammography-guided biopsy: Technique and initial outcomes. Quant. Imaging Med. Surg. 2023, 13, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, R.; Posso, M.; Pitarch, M.; Arenas, N.; Ejarque, B.; Iotti, V.; Besutti, G. Contrast-enhanced mammographyguided biopsy: Technical feasibility and first outcomes. Eur. Radiol. 2023, 33, 417–428. [Google Scholar] [CrossRef] [PubMed]
- James, J. Contrast-enhanced spectral mammography (CESM)-guided breast biopsy as an alternative to MRI-guided biopsy. Br. J. Radiol. 2022, 95, 20211287. [Google Scholar] [CrossRef]
- Perlet, C.; Heywang-Kobrunner, S.H.; Heinig, A.; Sittek, H.; Casselman, J.; Anderson, I.; Taourel, P. Magnetic resonance-guided, vacuum-assisted breast biopsy: Results from a European multicenter study of 538 lesions. Cancer 2006, 106, 982–990. [Google Scholar] [CrossRef]
- Eby, P.R.; Lehman, C.D. Magnetic resonance imaging-guidedbreast interventions. Top. Magn. Reson. Imaging 2008, 19, 151–162. [Google Scholar] [CrossRef]

| Shape | Margins | Internal Enhancement Pattern | Contrast Enhancement |
|---|---|---|---|
| Irregular | Non-circumscribed | Heterogeneous | Moderate |
| Ring-enhancement | Intense |
| Spatial Distribution | Internal Enhancement Pattern | Symmetric/Not Symmetric |
|---|---|---|
| Linear | Heterogeneous | Not symmetric |
| Segmental | Clumped | |
| Clustered-ring |
| Variables | Value (n = 69) |
|---|---|
| Age (mean, range), yrs | 52 (range: 45–77) |
| Breast density,n % | |
| Dense | 57 (82%) |
| Non-dense | 12 (18%) |
| Imaging findings, % | |
| Mass | 24 (35%) |
| Non-mass | 45 (65%) |
| Procedural time (mean ± SD), min | 10 ± 4 min |
| Needle approach, n (%) | |
| Vertical | 12 (21%) |
| Horizontal | 57 (79%) |
| AGD (mean ± SD) | 14.8 ± 10.2 |
| Biopsy results | |
| B2 n (%) | 24 (33.8%) |
| B3 n (%) | 24 (33.8%) |
| Tumors, n (%) | 20 (28%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammarra, M.; Piccolo, C.L.; Sarli, M.; Stefanucci, R.; Tommasiello, M.; Orsaria, P.; Altomare, V.; Beomonte Zobel, B. Contrast-Enhanced Mammography-Guided Biopsy: Preliminary Results of a Single-Center Retrospective Experience. J. Clin. Med. 2024, 13, 933. https://doi.org/10.3390/jcm13040933
Sammarra M, Piccolo CL, Sarli M, Stefanucci R, Tommasiello M, Orsaria P, Altomare V, Beomonte Zobel B. Contrast-Enhanced Mammography-Guided Biopsy: Preliminary Results of a Single-Center Retrospective Experience. Journal of Clinical Medicine. 2024; 13(4):933. https://doi.org/10.3390/jcm13040933
Chicago/Turabian StyleSammarra, Matteo, Claudia Lucia Piccolo, Marina Sarli, Rita Stefanucci, Manuela Tommasiello, Paolo Orsaria, Vittorio Altomare, and Bruno Beomonte Zobel. 2024. "Contrast-Enhanced Mammography-Guided Biopsy: Preliminary Results of a Single-Center Retrospective Experience" Journal of Clinical Medicine 13, no. 4: 933. https://doi.org/10.3390/jcm13040933
APA StyleSammarra, M., Piccolo, C. L., Sarli, M., Stefanucci, R., Tommasiello, M., Orsaria, P., Altomare, V., & Beomonte Zobel, B. (2024). Contrast-Enhanced Mammography-Guided Biopsy: Preliminary Results of a Single-Center Retrospective Experience. Journal of Clinical Medicine, 13(4), 933. https://doi.org/10.3390/jcm13040933







