Linear IgA Bullous Dermatosis in Korea Using the Nationwide Health Insurance Database
Abstract
1. Introduction
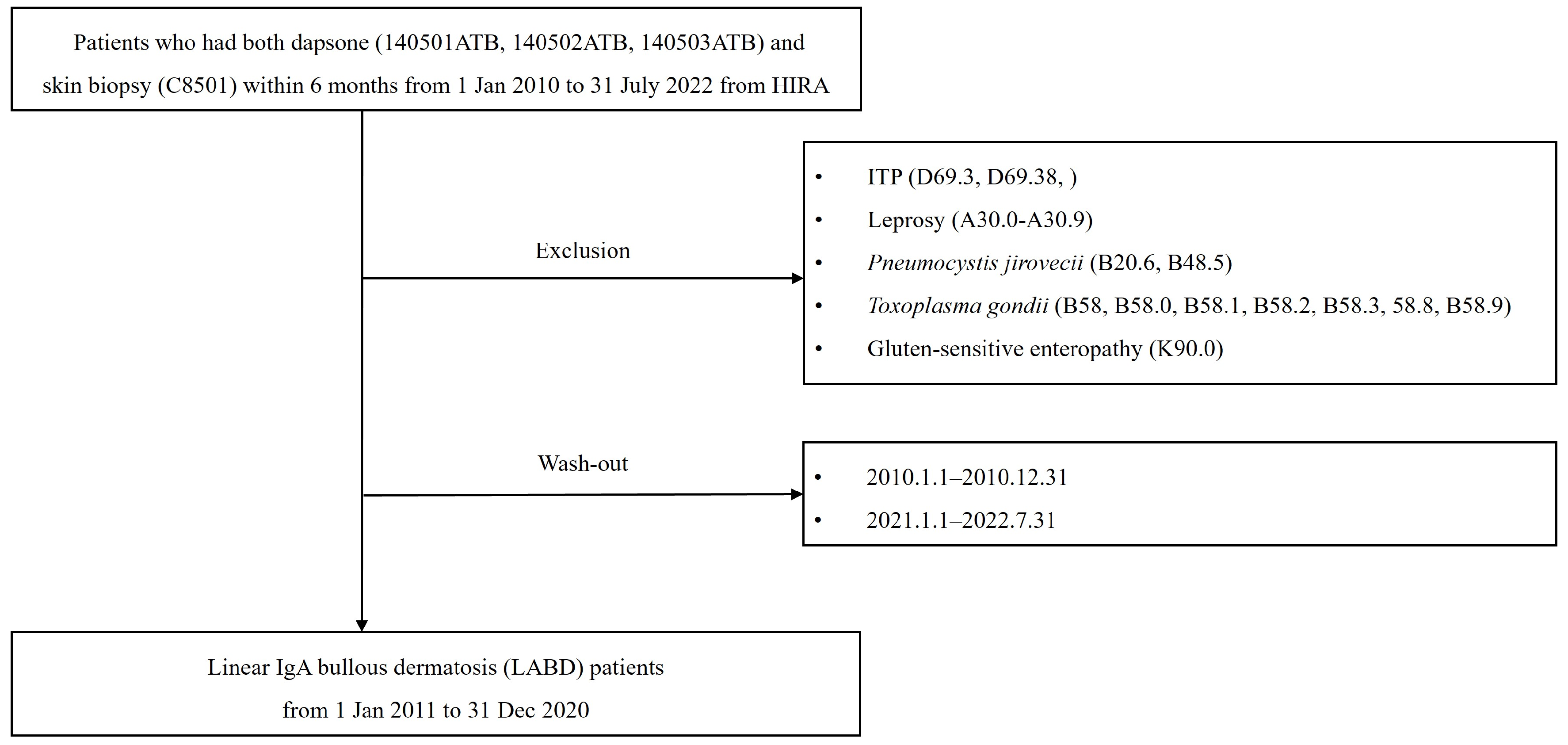
2. Materials and Methods
2.1. Data Sources
2.2. Case Definition
2.3. Statistical Analysis
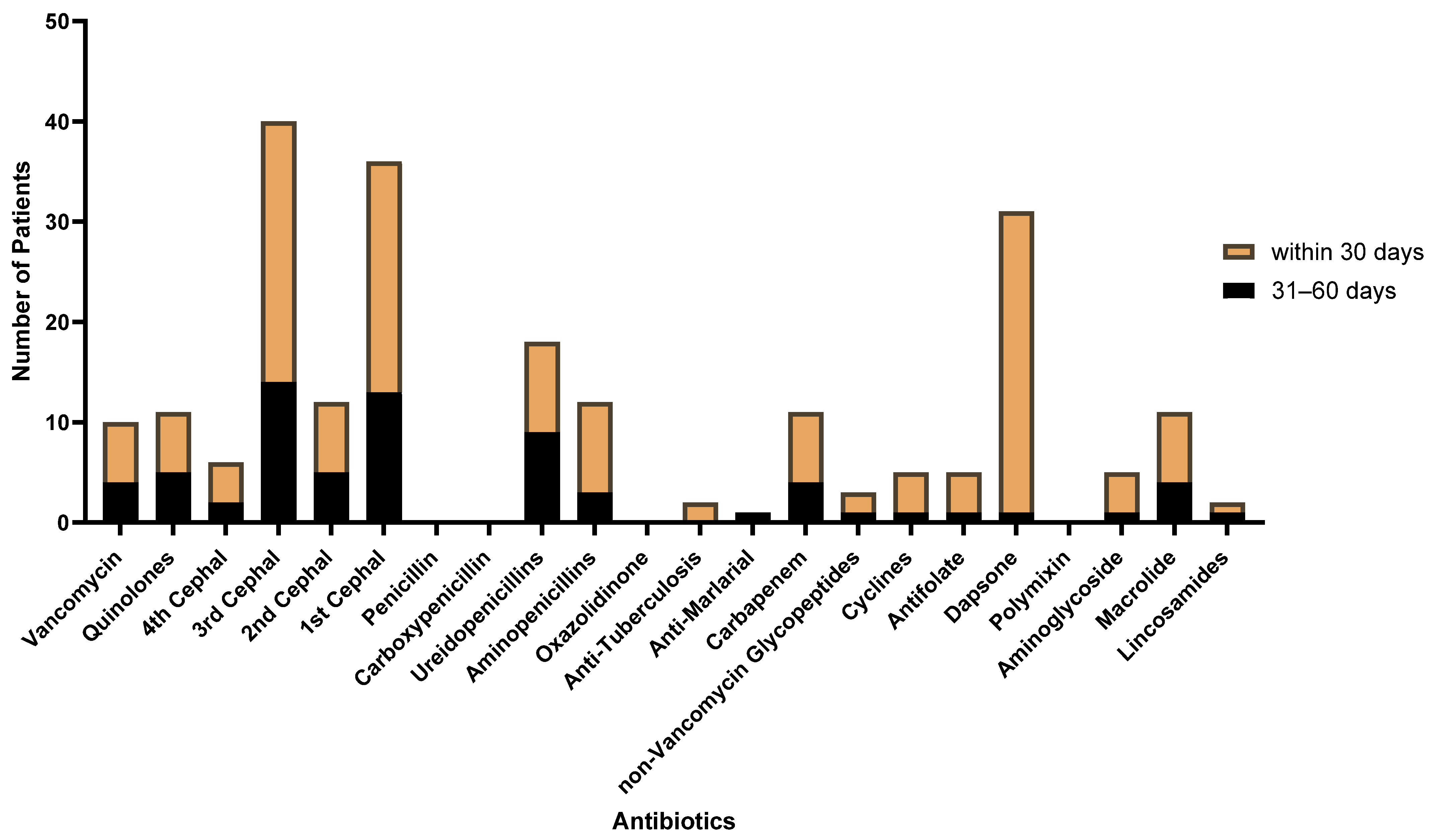
3. Results
3.1. Patient Characteristics
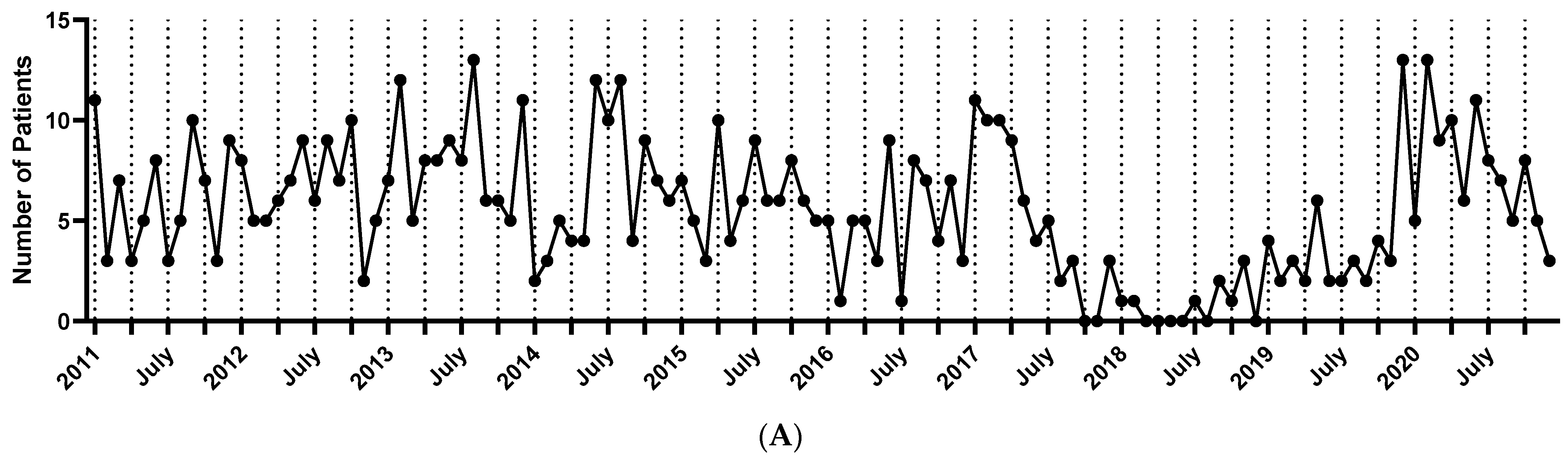
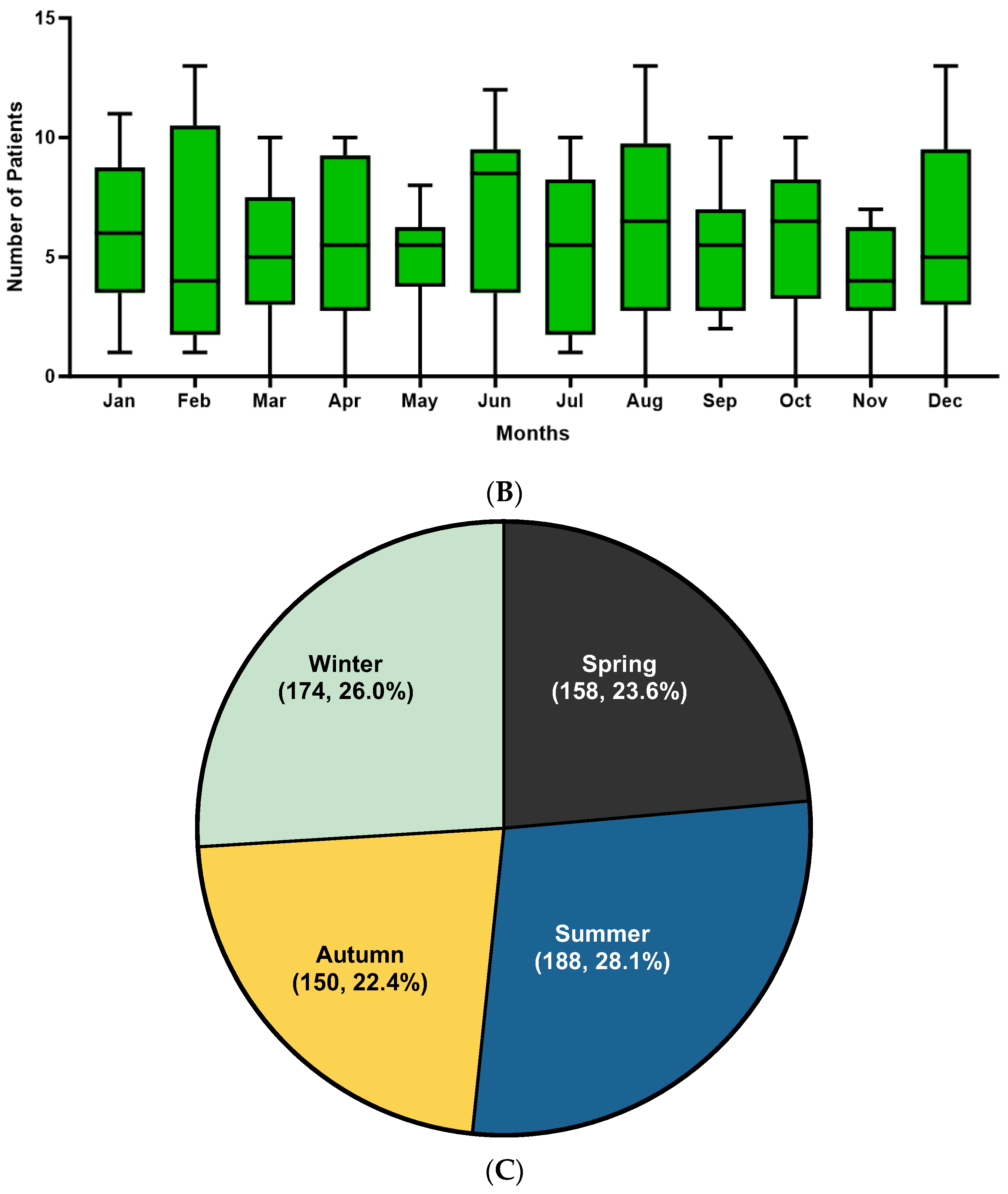
3.2. Trend Analysis of Linear IgA Bullous Dermatosis
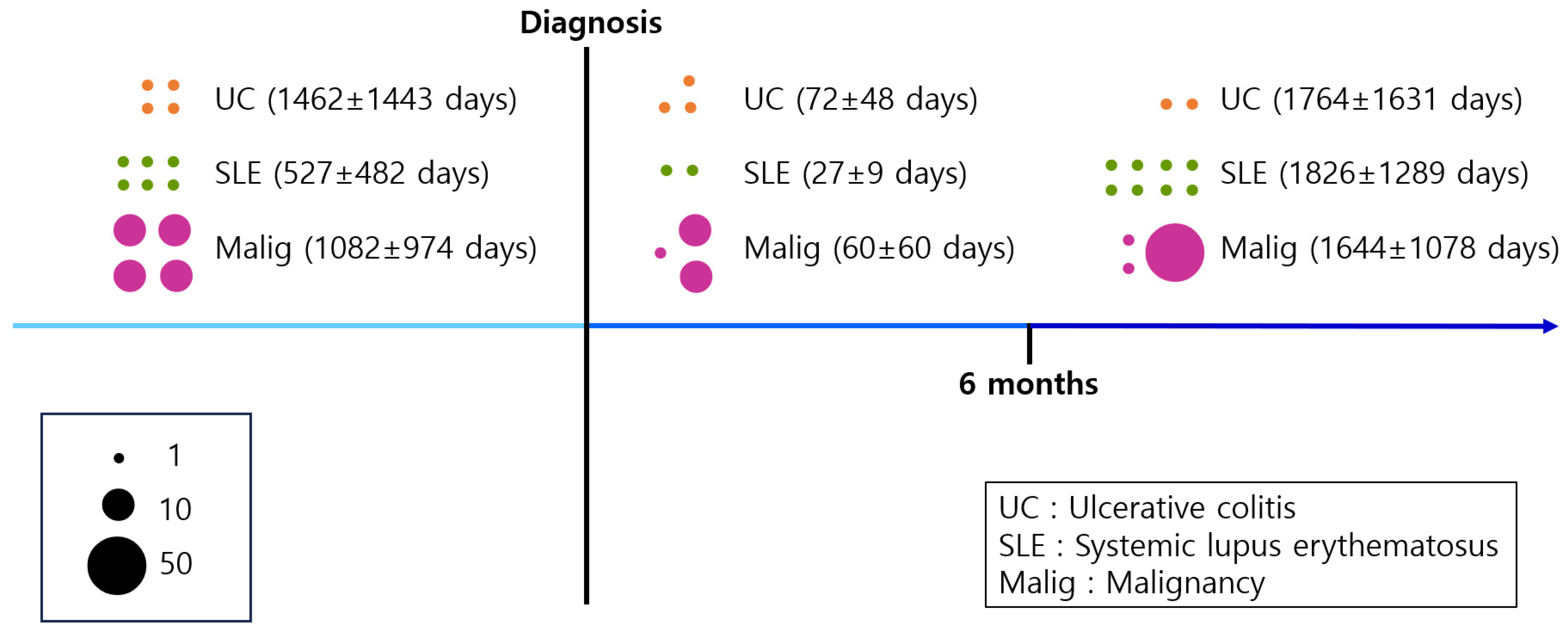
3.3. Associated Risk Factor of Linear IgA Bullous Dermatosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lings, K.; Bygum, A. Linear IgA bullous dermatosis: A retrospective study of 23 patients in Denmark. Acta Derm. Venereol. 2015, 95, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Chorzelski, T.P.; Jablonska, S. IgA linear dermatosis of childhood (chronic bullous disease of childhood). Br. J. Dermatol. 1979, 101, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bernat, B.A.; Laughlin, L.T.; Armstrong, R.N. Fosfomycin resistance protein (FosA) is a manganese metalloglutathione transferase related to glyoxalase I and the extradiol dioxygenases. Biochemistry 1997, 36, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, J.; Ingen-Housz-Oro, S.; Alexandre, M.; Grootenboer-Mignot, S.; Aucouturier, F.; Sbidian, E.; Tancrede, E.; Schneider, P.; Regnier, E.; Picard-Dahan, C.; et al. Idiopathic linear IgA bullous dermatosis: Prognostic factors based on a case series of 72 adults. Br. J. Dermatol. 2017, 177, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sobjanek, M.; Sokolowska-Wojdylo, M.; Sztaba-Kania, M.; Baranska-Rybak, W.; Maciejewska, A.; Wlodarkiewicz, A. Clinical and immunopathological heterogeneity of 22 cases of linear IgA bullous dermatosis. J. Eur. Acad. Dermatol. Venereol. JEADV 2008, 22, 1131. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Ikoma, A.; Sakai, R.; Masatsugu, A.; Ohta, M.; Hashimoto, T. Linear IgA dermatosis: Report of an infantile case and analysis of 213 cases in Japan. J. Dermatol. 2008, 35, 737–743. [Google Scholar] [CrossRef]
- Chorzelski, T.; Jablonska, S.; Maciejowska, E.; Blaszczyk, M. Subepidermal bullous disease of childhood with linear IgA deposits. Arch. Immunol. Ther. Exp. 1978, 26, 783–789. [Google Scholar]
- Paige, D.G.; Leonard, J.N.; Wojnarowska, F.; Fry, L. Linear IgA disease and ulcerative colitis. Br. J. Dermatol. 1997, 136, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Tobon, G.J.; Toro, C.E.; Bravo, J.C.; Canas, C.A. Linear IgA bullous dermatosis associated with systemic lupus erythematosus: A case report. Clin. Rheumatol. 2008, 27, 391–393. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Stiller, M.J. Dapsone and sulfones in dermatology: Overview and update. J. Am. Acad. Dermatol. 2001, 45, 420–434. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Meier, M.; Zillikens, D.; Schmidt, E. Linear IgA disease: Successful application of immunoadsorption and review of the literature. Dermatology 2010, 220, 259–263. [Google Scholar] [CrossRef]
- Ingen-Housz-Oro, S.; Bernard, P.; Bedane, C.; Prost, C.; Joly, P.; Centres de reference des maladies bulleuses auto-immunes. Société Française de Dermatologie. Linear IgA dermatosis. Guidelines for the diagnosis and treatment. Centres de reference des maladies bulleuses auto-immunes. Societe Francaise de Dermatologie. Ann. Dermatol. Venereol. 2011, 138, 267–270. [Google Scholar] [CrossRef]
- Fortuna, G.; Marinkovich, M.P. Linear immunoglobulin A bullous dermatosis. Clin. Dermatol. 2012, 30, 38–50. [Google Scholar] [CrossRef]
- Kim, D.S. Special issue on the national health care system of South Korea. Soc. Work. Public Health 2010, 25, 125–126. [Google Scholar] [CrossRef]
- Kim, D.S. Introduction: Health of the health care system in Korea. Soc. Work. Public Health 2010, 25, 127–141. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Garel, B.; Ingen-Housz-Oro, S.; Afriat, D.; Prost-Squarcioni, C.; Tetart, F.; Bensaid, B.; Bara Passot, C.; Beylot-Barry, M.; Descamps, V.; Duvert-Lehembre, S.; et al. Drug-induced linear immunoglobulin A bullous dermatosis: A French retrospective pharmacovigilance study of 69 cases. Br. J. Clin. Pharmacol. 2019, 85, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Venegoni, L.; Fanoni, D.; Muratori, S.; Berti, E.; Marzano, A.V. Linear IgA bullous dermatosis in adults and children: A clinical and immunopathological study of 38 patients. Orphanet J. Rare Dis. 2019, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Nancy, A.L.; Yehuda, S. Prediction and prevention of autoimmune skin disorders. Arch. Dermatol. Res. 2009, 301, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Caricchio, R.; McPhie, L.; Cohen, P.L. Ultraviolet B radiation-induced cell death: Critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J. Immunol. 2003, 171, 5778–5786. [Google Scholar] [CrossRef] [PubMed]
- Ang, P.; Tay, Y.K. Treatment of linear IgA bullous dermatosis of childhood with colchicine. Pediatr. Dermatol. 1999, 16, 50–52. [Google Scholar] [CrossRef]
- Kaya Islamoglu, Z.G.; Akyurek, F.T. A case of recalcitrant linear IgA bullous dermatosis: Successfully treated with rituximab. Dermatol. Ther. 2019, 32, e12911. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Makino, T.; Jinnin, M.; Sakai, K.; Fukushima, S.; Inoue, Y.; Ihn, H. Association of linear IgA bullous disease with ulcerative colitis: A case of successful treatment with infliximab. Dermatology 2013, 227, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Barrios, M.; Velasco-Tamariz, V.; Tous-Romero, F.; Burillo-Martinez, S.; Zarco-Olivo, C.; Rodriguez-Peralto, J.L.; Ortiz-Romero, P.L. Linear immunoglobulin A dermatosis mimicking toxic epidermal necrolysis: A case report of etanercept treatment. Br. J. Dermatol. 2018, 178, 786–789. [Google Scholar] [CrossRef]
- Maalouf, N.S.; Hanna, D. Linear IgA bullous dermatosis successfully treated with omalizumab: A case report. JAAD Case Rep. 2019, 5, 966–969. [Google Scholar] [CrossRef]
- Fortuna, G.; Salas-Alanis, J.C.; Guidetti, E.; Marinkovich, M.P. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: A real and separate nosological entity? J. Am. Acad. Dermatol. 2012, 66, 988–994. [Google Scholar] [CrossRef]
- Chanal, J.; Ingen-Housz-Oro, S.; Ortonne, N.; Duong, T.A.; Thomas, M.; Valeyrie-Allanore, L.; Lebrun-Vignes, B.; André, C.; Roujeau, J.C.; Chosidow, O.; et al. Linear IgA bullous dermatosis: Comparison between the drug-induced and spontaneous forms. Br. J. Dermatol. 2013, 169, 1041–1048. [Google Scholar] [CrossRef]
- Shipman, A.R.; Reddy, H.; Wojnarowska, F. Association between the subepidermal autoimmune blistering diseases linear IgA disease and the pemphigoid group and inflammatory bowel disease: Two case reports and literature review. Clin. Exp. Dermatol. 2012, 37, 461–468. [Google Scholar] [CrossRef]
- Kanda, N.; Nakadaira, N.; Otsuka, Y.; Ishii, N.; Hoashi, T.; Saeki, H. Linear IgA bullous dermatosis associated with ulcerative colitis: A case report and literature review. Australas. J. Dermatol. 2020, 61, e82–e86. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.; Patel, S.; Motaparthi, K. Successful Treatment of Linear IgA Disease and Ulcerative Colitis with Sulfasalazine. Cureus 2023, 15, e37210. [Google Scholar] [CrossRef]
- Malipatel, R.; Gnanapriya, V.; Manocha, A.; Inchara, Y.K. Systemic Lupus Erythematosus with Linear IgA Bullous Dermatosis and Renal Vascular Lesions: An Extremely Rare Association. Indian J. Nephrol. 2018, 28, 465–467. [Google Scholar] [CrossRef]
- Andriano, T.M.; Tannenbaum, R.; Xu, H.; Magro, C.M. Linear IgA bullous dermatosis in the setting of angioimmunoblastic T-cell lymphoma. J. Cutan. Pathol. 2023, 50, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Colmant, C.; Camboni, A.; Dekeuleneer, V.; Marot, L.; Dachelet, C.; Baeck, M. Linear IgA dermatosis in association with angioimmunoblastic T-cell lymphoma infiltrating the skin: A case report with literature review. J. Cutan. Pathol. 2020, 47, 251–256. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, R.I.; van de Scheur, M.R.; Pas, H.H.; Jonkman, M.F.; Van Groeningen, C.J.; Nieboer, C.; Starink, T.M. Linear IgA bullous dermatosis in a patient with renal cell carcinoma. Br. J. Dermatol. 2001, 144, 870–873. [Google Scholar] [CrossRef]
- Yang, C.S.; Robinson-Bostom, L.; Landow, S. Linear IgA bullous dermatosis associated with metastatic renal cell carcinoma. JAAD Case Rep. 2015, 1, 91–92. [Google Scholar] [CrossRef][Green Version]
- Wojnarowska, F.; Marsden, R.A.; Bhogal, B.; Black, M.M. Chronic bullous disease of childhood, childhood cicatricial pemphigoid, and linear IgA disease of adults. A comparative study demonstrating clinical and immunopathologic overlap. J. Am. Acad. Dermatol. 1988, 19, 792–805. [Google Scholar] [CrossRef]
- Ho, J.C.; Ng, P.L.; Tan, S.H.; Giam, Y.C. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr. Dermatol. 2007, 24, E40–E43. [Google Scholar] [CrossRef]
- Nantel-Battista, M.; Al Dhaybi, R.; Hatami, A.; Marcoux, D.; Desroches, A.; Kokta, V. Childhood linear IgA bullous disease induced by trimethoprim-sulfamethoxazole. J. Dermatol. Case Rep. 2010, 4, 33–35. [Google Scholar] [CrossRef]
- Baden, L.A.; Apovian, C.; Imber, M.J.; Dover, J.S. Vancomycin-induced linear IgA bullous dermatosis. Arch. Dermatol. 1988, 124, 1186–1188. [Google Scholar] [CrossRef]
- Arimone, Y.; Bidault, I.; Dutertre, J.P.; Gerardin, M.; Guy, C.; Haramburu, F.; Hillaire-Buys, D.; Meglio, C.; Penfornis, C.; Theophile, H.; et al. Updating the French method for the causality assessment of adverse drug reactions. Therapie 2013, 68, 69–76. [Google Scholar] [CrossRef] [PubMed]

| Variables | N (%) | Annual Incidence * | |
|---|---|---|---|
| Total number of patients | 670 (100.0) | 1.3 | |
| Mean age (years) | 55.9 ± 21.2 | ||
| Age group | |||
| 0–19 years | 46 (6.9) | 0.5 | |
| 20–39 years | 105 (15.7) | 0.7 | |
| 40–59 years | 199 (29.7) | 0.5 | |
| ≥60 years | 320 (47.8) | 3.2 | |
| Sex | |||
| Male | 394 (46.2) | 1.2 | |
| Female | 458 (53.8) | 1.4 | |
| Location | |||
| Seoul | 269 (23.1) | 2.7 | |
| Busan | 14 (6.4) | 0.4 | |
| Incheon | 53 (5.1) | 2.1 | |
| Daegu | 22 (5.6) | 0.7 | |
| Gwangju | 82 (4.4) | 5.6 | |
| Daejeon | 27 (4.4) | 1.8 | |
| Ulsan | 29 (1.5) | 2.5 | |
| Gyeonggi | 71 (22.8) | 0.6 | |
| Gangwon | 36 (3.6) | 2.3 | |
| Chungbuk | - | 0.0 | |
| Chungnam | - | 0.0 | |
| Jeonbuk | 47 (3.5) | 2.5 | |
| Jeonnam | 2 (2.3) | 0.1 | |
| Gyeongbuk | 15 (3.1) | 0.6 | |
| Gyeongnam | - | 0.0 | |
| Jeju | 3 (1.8) | 0.5 | |
| Sejong | 0.0 | ||
| Insurance type | |||
| Medical insurance | 610 (91.0) | 1.2 | |
| Medical aid | 56 (8.4) | 0.1 | |
| Free | 4 (0.6) | 0 | |
| Clinical course | |||
| Dapsone medication (days) | 30.7 ± 56.7 | ||
| Hospital visit (days) | 1.3 ± 0.7 | ||
| Hospitalization (days) | 19.8 ± 19.7 |
| Disease | Total N (%) | Before Diagnosis of LABD | After Diagnosis of LABD | |||
|---|---|---|---|---|---|---|
| Within 6 Months | After 6 Months | Total | ||||
| Ulcerative colitis | ||||||
| Number of patients | 9 (1.3) | 4 | 3 | 2 | 5 | |
| Time interval to diagnosis (days) | 1462 ± 1443 | 72 ± 48 | 1764 ± 1631 | 749 ± 1235 | ||
| Systemic lupus erythematosus | ||||||
| Number of patients | 16 (2.3) | 6 | 2 | 8 | 10 | |
| Time interval to diagnosis (days) | 527 ± 482 | 27 ± 9 | 1826 ± 1289 | 1466 ± 1367 | ||
| Malignancy | ||||||
| Number of patients | 113 (16.9) | 40 | 21 | 52 | 73 | |
| Time interval to diagnosis (days) | 1082 ± 974 | 60 ± 60 | 1644 ± 1078 | 1188 ± 1160 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.R.; Kim, J.H.; Kim, S.W.; Lee, J.M.; Bae, J.S. Linear IgA Bullous Dermatosis in Korea Using the Nationwide Health Insurance Database. J. Clin. Med. 2024, 13, 1159. https://doi.org/10.3390/jcm13041159
Kim YR, Kim JH, Kim SW, Lee JM, Bae JS. Linear IgA Bullous Dermatosis in Korea Using the Nationwide Health Insurance Database. Journal of Clinical Medicine. 2024; 13(4):1159. https://doi.org/10.3390/jcm13041159
Chicago/Turabian StyleKim, Yu Rim, Ji Hyeon Kim, Sang Won Kim, Jae Min Lee, and Jacob S. Bae. 2024. "Linear IgA Bullous Dermatosis in Korea Using the Nationwide Health Insurance Database" Journal of Clinical Medicine 13, no. 4: 1159. https://doi.org/10.3390/jcm13041159
APA StyleKim, Y. R., Kim, J. H., Kim, S. W., Lee, J. M., & Bae, J. S. (2024). Linear IgA Bullous Dermatosis in Korea Using the Nationwide Health Insurance Database. Journal of Clinical Medicine, 13(4), 1159. https://doi.org/10.3390/jcm13041159






