Effect of Focal Laser Photocoagulation on the Ganglion Cell Complex Thickness in Acute Central Serous Chorioretinopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ophthalmic Examination
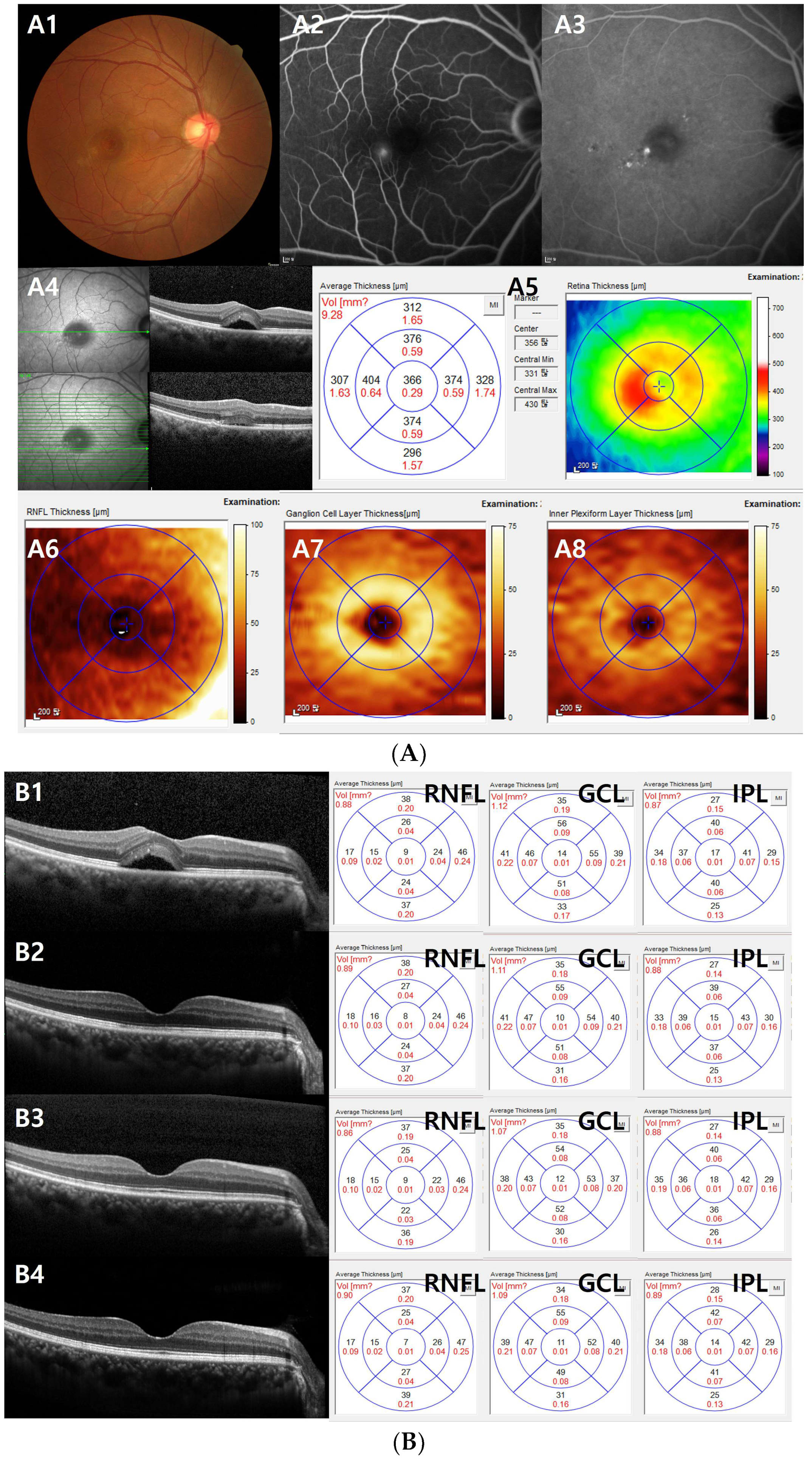
2.3. OCT
2.4. Statistical Analysis
3. Results
3.1. Changes in the Best-Corrected Visual Acuity, Intraocular Pressure (IOP), and CMT
3.2. Changes in the GCC Thickness (RNFL, GCL, and IPL)
3.3. Factors Related to Final Visual Acuity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yannuzzi, L.A.; Shakin, J.L.; Fisher, Y.L.; Altomonte, M.A. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology 1984, 91, 1554–1572. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Dirani, A.; Bousquet, E.; Zhao, M.; Farman, N.; Jaisser, F.; Behar-Cohen, F. Central serous chorioretinopathy: Recent findings and new physiopathology hypothesis. Prog. Retin. Eye Res. 2015, 48, 82–118. [Google Scholar] [CrossRef]
- Hamzah, F.; Shinojima, A.; Mori, R.; Yuzawa, M. Choroidal thickness measurement by enhanced depth imaging and swept-source optical coherence tomography in central serous chorioretinopathy. BMC Ophthalmol. 2014, 14, 145. [Google Scholar] [CrossRef]
- Jaisankar, D.; Kumar, M.; Rishi, P.; Singh, S.; Raman, R. Correlation of retinal changes with choroidal changes in acute and recurrent central serous chorioretinopathy assessed by swept-source optical coherence tomography. Ther. Adv. Ophthalmol. 2020, 12, 2515841419899823. [Google Scholar] [CrossRef]
- Piccolino, F.C.; de la Longrais, R.R.; Ravera, G.; Eandi, C.M.; Ventre, L.; Abdollahi, A.; Manea, M. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am. J. Ophthalmol. 2005, 139, 87–99. [Google Scholar] [CrossRef]
- Demirok, G.; Kocamaz, F.; Topalak, Y.; Altay, Y.; Sengun, A. Macular ganglion cell complex thickness in acute and chronic central serous chorioretinopathy. Int. Ophthalmol. 2017, 37, 409–416. [Google Scholar] [CrossRef]
- Han, K.J.; Kim, H.J.; Woo, J.M.; Min, J.K. Comparison of Retinal Layer Thickness and Capillary Vessel Density in the Patients with Spontaneously Resolved Acute Central Serous Chorioretinopathy. J. Clin. Med. 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Gawecki, M.; Grzybowski, A. Ganglion Cell Loss in the Course of Central Serous Chorioretinopathy. Ophthalmol. Ther. 2023, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Kim, J.Y. Serous Retinal Detachment Causes a Transient Reduction on Spectral Domain OCT Estimates of Ganglion Cell Layer Thickness. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2019, 96, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Moghimi, S.; Proudfoot, J.A.; Ghahari, E.; Penteado, R.C.; Bowd, C.; Yang, D.; Weinreb, R.N. Ganglion Cell Complex Thickness and Macular Vessel Density Loss in Primary Open-Angle Glaucoma. Ophthalmology 2020, 127, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Rebolleda, G.; Sanchez-Sanchez, C.; Gonzalez-Lopez, J.J.; Contreras, I.; Munoz-Negrete, F.J. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 682–692. [Google Scholar] [CrossRef]
- Sun, J.K.; Lin, M.M.; Lammer, J.; Prager, S.; Sarangi, R.; Silva, P.S.; Aiello, L.P. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014, 132, 1309–1316. [Google Scholar] [CrossRef]
- Jackson, G.R.; Scott, I.U.; Quillen, D.A.; Walter, L.E.; Gardner, T.W. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012, 96, 699–703. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, Y.; Huang, S.; Wu, Q.; Chen, Q.; Shen, M.; Lu, F. Macular Inner Retinal Layer Thickening and Outer Retinal Layer Damage Correlate With Visual Acuity During Remission in Behcet’s Disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5470–5478. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Senatore, A.; Di Antonio, L.; Di Nicola, M.; Carpineto, P.; Mastropasqua, L. Macular Features in Retinitis Pigmentosa: Correlations Among Ganglion Cell Complex Thickness, Capillary Density, and Macular Function. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6360–6366. [Google Scholar] [CrossRef]
- Zheng, Z.; Yan, M.; Li, L.; Zhang, D.; Zhang, L. Analysis of ganglion cell-inner plexiform layer thickness in retinal vein occlusion with resolved macular edema. Int. Ophthalmol. 2023, 43, 655–664. [Google Scholar] [CrossRef]
- Kaye, R.; Chandra, S.; Sheth, J.; Boon, C.J.F.; Sivaprasad, S.; Lotery, A. Central serous chorioretinopathy: An update on risk factors, pathophysiology and imaging modalities. Prog. Retin. Eye Res. 2020, 79, 100865. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kim, K.; Kang, M.S.; Kim, E.S.; Yu, S.Y. Central serous chorioretinopathy: Treatment. Taiwan. J. Ophthalmol. 2022, 12, 394–408. [Google Scholar] [CrossRef]
- van Rijssen, T.J.; van Dijk, E.H.C.; Yzer, S.; Ohno-Matsui, K.; Keunen, J.E.E.; Schlingemann, R.O.; Sivaprasad, S.; Querques, G.; Downes, S.M.; Fauser, S.; et al. Central serous chorioretinopathy: Towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019, 73, 100770. [Google Scholar] [CrossRef]
- Kim, D.Y.; Joe, S.G.; Yang, S.J.; Lee, J.Y.; Kim, J.G.; Yoon, Y.H. The association between choroidal thickness variations and response to intravitreal bevacizumab in central serous chorioretinopathy. Korean J. Ophthalmol. KJO 2015, 29, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Agharokh, S.; Akhlaghi, M.R.; Kianersi, F.; Dehghani, A.; Jahanbani-Ardakani, H.; Abtahi, S.H. Short-Term Effects of Photodynamic Therapy on Segmentation of Retinal Layers in Central Serous Chorioretinopathy. Adv. Biomed. Res. 2021, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Cidad, P.; Gonzalez, E.; Asencio, M.; Garcia, J. Structural and Functional Outcomes in Chronic Central Serous Chorioretinopathy Treated with Photodynamic Therapy. Korean J. Ophthalmol. KJO 2015, 29, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Yoon, C.K.; Kim, B.H.; Yu, H.G. Evaluation of the Safety and Efficacy of Selective Retina Therapy Laser Treatment in Patients with Central Serous Chorioretinopathy. Korean J. Ophthalmol. KJO 2021, 35, 51–63. [Google Scholar] [CrossRef]
- Nicholson, B.; Noble, J.; Forooghian, F.; Meyerle, C. Central serous chorioretinopathy: Update on pathophysiology and treatment. Surv. Ophthalmol. 2013, 58, 103–126. [Google Scholar] [CrossRef] [PubMed]
| CSC Group | Controls | p-Value | |
|---|---|---|---|
| Patients (n) | 30 | 45 | |
| Age (years) | 54.21 ± 9.72 | 54.51 ± 7.96 | 0.883 a |
| Sex | 0.377 b | ||
| Male | 21 | 27 | |
| Female | 9 | 18 | |
| BCVA (logMAR) | 0.20 ± 0.14 | 0.08 ± 0.15 | 0.005 |
| IOP (mmHg) | 14.60 ± 2.57 | 15.83 ± 2.85 | 0.101 |
| CMT (µm) | 423.90 ± 118.62 | 270.38 ± 24.28 | <0.001 |
| CSC Eye | Fellow Eye | Control Eye | p a | p b | p c | |
|---|---|---|---|---|---|---|
| GCC | 104.83 ± 10.82 | 105.18 ± 8.35 | 106.20 ± 6.41 | 0.155 | 0.494 | 0.566 |
| RNFL | 33.01 ± 4.85 | 32.95 ± 4.06 | 33.90 ± 3.61 | 0.159 | 0.366 | 0.310 |
| GCL | 39.56 ± 4.40 | 39.83 ± 3.39 | 39.88 ± 2.33 | 0.594 | 0.722 | 0.948 |
| IPL | 32.26 ± 3.04 | 32.40 ± 2.68 | 32.42 ± 2.20 | 0.863 | 0.799 | 0.975 |
| Baseline | 1 Month | 3 Months | 6 Months | p-Value a | |
|---|---|---|---|---|---|
| GCC | 104.83 ± 10.82 | 105.20 ± 11.16 | 103.66 ± 12.71 | 105.16 ± 8.79 | 0.962 |
| p-value b | 0.304 | 0.208 | 0.310 | ||
| RNFL | 33.01 ± 4.85 (0.93 ± 0.14) | 32.92 ± 4.81 (0.93 ± 0.14) | 31.91 ± 5.44 (0.90 ± 0.15) | 32.56 ± 3.95 (0.92 ± 0.11) | 0.854 |
| p-value b | 0.750 | 0.090 | 0.285 | ||
| GCL | 39.56 ± 4.40 (1.12 ± 0.12) | 39.59 ± 4.41 (1.12 ± 0.12) | 39.32 ± 4.83 (1.11 ± 0.14) | 39.71 ± 3.66 (1.12 ± 0.10) | 0.993 |
| p-value b | 0.889 | 0.331 | 0.064 | ||
| IPL | 32.26 ± 3.04 (0.91 ± 0.09) | 32.69 ± 3.21 (0.92 ± 0.09) | 32.43 ± 3.57 (0.92 ± 0.10) | 32.89 ± 2.58 (0.93 ± 0.07) | 0.902 |
| p-value b | 0.084 | 0.204 | 0.354 |
| Baseline | 1 Month | 3 Months | 6 Months | p-Value a | |
|---|---|---|---|---|---|
| GCC | 109.70 ± 18.93 | 110.97 ± 19.13 | 108.95 ± 21.10 | 109.50 ± 17.14 | 0.984 |
| p-Value b | 0.053 | 0.903 | 0.417 |
| Final VA | ||
|---|---|---|
| Standardized Coefficients Beta | p-Value | |
| Age | −0.303 | 0.320 |
| Sex | 0.443 | 0.053 |
| Baseline VA | 0.697 | 0.017 |
| Baseline RNFL | −0.961 | 0.131 |
| Baseline GCL | 1.866 | 0.168 |
| Baseline IPL | 1.120 | 0.168 |
| RNFL at 6 months | 1.578 | 0.072 |
| GCL at 6 months | −2.671 | 0.106 |
| IPL at 6 months | −0.331 | 0.561 |
| Authors | Subjects (Number) | OCT Machine | Study Focus | Key Findings |
|---|---|---|---|---|
| Demirok et al. [6] | 16 | Cirrus SD-OCT | GCL-IPL thickness in acute CSC vs. normal group | Lesser GCL-IPL thickness in acute CSC |
| Nam et al. [9] | 30 | Cirrus SD-OCT | GCL-IPL thickness change with/without SRF in acute CSC | GCL-IPL thickness less with SRF, normalized after SRF reabsorption |
| Jaisankar et al. [4] | 7 | Topcon SS-OCT | RNFL and GCL thickness change with SRF in acute CSC | Decrease in RNFL and GCL thickness as SRF diminished |
| Gawecki et al. [8] | 13 | REVO NX SD-OCT | GCL-IPL thickness in acute and chronic CSC vs. controls | No significant difference in acute CSC but reduced GCL-IPL thickness in chronic CSC |
| Han et al. [7] | 34 | Topcon SS-OCT | Outer retinal layer and GCC layer thickness in resolved acute CSC | Thinning of outer retinal layer but unaffected GCC in resolved acute CSC |
| Our study | 30 | Spectralis SD-OCT | GCC, RNFL, GCL, IPL thickness | No significant change |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.Y.; Choi, J.; Lee, S.U.; Kim, C.W.; Hwang, D.D.-J. Effect of Focal Laser Photocoagulation on the Ganglion Cell Complex Thickness in Acute Central Serous Chorioretinopathy. J. Clin. Med. 2024, 13, 1064. https://doi.org/10.3390/jcm13041064
Lee AY, Choi J, Lee SU, Kim CW, Hwang DD-J. Effect of Focal Laser Photocoagulation on the Ganglion Cell Complex Thickness in Acute Central Serous Chorioretinopathy. Journal of Clinical Medicine. 2024; 13(4):1064. https://doi.org/10.3390/jcm13041064
Chicago/Turabian StyleLee, A Young, Jinyoung Choi, Sang Un Lee, Chul Woo Kim, and Daniel Duck-Jin Hwang. 2024. "Effect of Focal Laser Photocoagulation on the Ganglion Cell Complex Thickness in Acute Central Serous Chorioretinopathy" Journal of Clinical Medicine 13, no. 4: 1064. https://doi.org/10.3390/jcm13041064
APA StyleLee, A. Y., Choi, J., Lee, S. U., Kim, C. W., & Hwang, D. D.-J. (2024). Effect of Focal Laser Photocoagulation on the Ganglion Cell Complex Thickness in Acute Central Serous Chorioretinopathy. Journal of Clinical Medicine, 13(4), 1064. https://doi.org/10.3390/jcm13041064






