Is the Elite Female Athlete’s Pelvic Floor Stronger?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instrumentation and Data Collection
2.2.1. Sociodemographic, Medical, and Sports Practice Data
2.2.2. Pelvic Floor Muscle Assessment: Modified Oxford Grading Scale (MOS)
2.2.3. Pelvic Floor Muscle Assessment: Intravaginal Dynamometry
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Areta, J.L.; Elliott-Sale, K.J. Nutrition for female athletes: What we know, what we don’t know, and why. Eur. J. Sport. Sci. 2022, 22, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Bø, K.; Nygaard, I.E. Is Physical Activity Good or Bad for the Female Pelvic Floor? A Narrative Review. Sports Med. 2020, 50, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, D.; Stania, M.; Kucab–Klich, K.; Błaszczak, E.; Kwaśna, K.; Smykla, A.; Hudziak, D.; Dolibog, P. Electromyographic characteristics of pelvic floor muscles in women with stress urinary incontinence following sEMG-assisted biofeedback training and Pilates exercises. PLoS ONE 2019, 14, e0225647. [Google Scholar] [CrossRef] [PubMed]
- Milsom, I.; Gyhagen, M. The prevalence of urinary incontinence. Climacteric 2019, 22, 217–222. [Google Scholar] [CrossRef]
- Jácome, C.; Oliveira, D.; Marques, A.; Sá-Couto, P. Prevalence and impact of urinary incontinence among female athletes. Int. J. Gynecol. Obs. 2011, 114, 60–63. [Google Scholar] [CrossRef]
- Carvalhais, A.; Natal Jorge, R.; Bø, K. Performing high-level sport is strongly associated with urinary incontinence in elite athletes: A comparative study of 372 elite female athletes and 372 controls. Br. J. Sports Med. 2018, 52, 1586–1590. [Google Scholar] [CrossRef]
- Rodríguez-López, E.S.; Calvo-Moreno, S.O.; Basas-García, Á.; Gutierrez-Ortega, F.; Guodemar-Pérez, J.; Acevedo-Gómez, M.B. Prevalence of urinary incontinence among elite athletes of both sexes. J. Sci. Med. Sport. 2021, 24, 338–344. [Google Scholar] [CrossRef]
- Reardon, C.L.; Hainline, B.; Aron, C.M.; Baron, D.; Baum, A.L.; Bindra, A.; Budgett, R.; Campriani, N.; Castaldelli-Maia, J.M.; Currie, A.; et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br. J. Sports Med. 2019, 53, 667–699. [Google Scholar] [CrossRef]
- Culleton-Quinn, E.; Bø, K.; Fleming, N.; Mockler, D.; Cusack, C.; Daly, D. Elite female athletes’ experiences of symptoms of pelvic floor dysfunction: A systematic review. Int. Urogynecol. J. 2022, 33, 2681–2711. [Google Scholar] [CrossRef]
- Bø, K.; Sundgot-Borgen, J. Are former female elite athletes more likely to experience urinary incontinence later in life than non-athletes? Scand. Med. Sci. Sports 2010, 20, 100–104. [Google Scholar] [CrossRef]
- Arbieto, E.R.M.; Dos Santos, K.M.; Da Luz, S.C.T.; Da Roza, T. Comparison of urinary incontinence, based on pelvic floor and abdominal muscle strength, between nulliparous female athletes and non-athletes: A secondary analysis. Neurourol. Urodyn. 2021, 40, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Dornowski, M.; Makar, P.; Sawicki, P.; Wilczyńska, D.; Vereshchaka, I.; Ossowski, Z. Effects of low- vs high-volume swimming training on pelvic floor muscle activity in women. Biol. Sport. 2019, 36, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lasak, A.M.; Jean-Michel, M.; Le, P.U.; Durgam, R.; Harroche, J. The Role of Pelvic Floor Muscle Training in the Conservative and Surgical Management of Female Stress Urinary Incontinence: Does the Strength of the Pelvic Floor Muscles Matter? PMR 2018, 10, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Bag Soytas, R.; Soytas, M.; Danacioglu, Y.O.; Citgez, S.; Yavuzer, H.; Can, G.; Onal, B.; Doventas, A. Relationship between the types of urinary incontinence, handgrip strength, and pelvic floor muscle strength in adult women. Neurourol. Urodyn. 2021, 40, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Deegan, E.G.; Stothers, L.; Kavanagh, A.; Macnab, A.J. Quantification of pelvic floor muscle strength in female urinary incontinence: A systematic review and comparison of contemporary methodologies. Neurourol. Urodyn. 2018, 37, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Yang, J.-M.; Chen, H.-F. Four-Dimensional Introital Ultrasound in Assessing Perioperative Pelvic Floor Muscle Functions of Women with Cystoceles. Ultraschall Der Med. 2021, 42, E31–E41. [Google Scholar] [CrossRef] [PubMed]
- Frawley, H.; Shelly, B.; Morin, M.; Bernard, S.; Kari, B.Ø.; Digesu, G.A.; Dickinson, T.; Goonewardene, S.; McClurg, D.; Rahnama’i, M.S.; et al. An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol. Urodyn. 2021, 40, 1217–1260. [Google Scholar] [CrossRef]
- Romero-Cullerés, G.; Peña-Pitarch, E.; Jané-Feixas, C.; Arnau, A.; Montesinos, J.; Abenoza-Guardiola, M. Intra-rater reliability and diagnostic accuracy of a new vaginal dynamometer to measure pelvic floor muscle strength in women with urinary incontinence. Neurourol. Urodyn. 2017, 36, 333–337. [Google Scholar] [CrossRef]
- Palmezoni, V.P.; Santos, M.D.; Pereira, J.M.; Bernardes, B.T.; Pereira-Baldon, V.S.; Resende, A.P.M. Pelvic floor muscle strength in primigravidae and non-pregnant nulliparous women: A comparative study. Int. Urogynecol. J. 2017, 28, 131–137. [Google Scholar] [CrossRef]
- Navarro Brazález, B.; Torres Lacomba, M.; de la Villa, P.; Sánchez Sánchez, B.; Prieto Gómez, V.; Asúnsolo del Barco, Á.; McLean, L. The evaluation of pelvic floor muscle strength in women with pelvic floor dysfunction: A reliability and correlation study. Neurourol. Urodyn. 2018, 37, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cullerés, G.; Peña-Pitarch, E.; Jané-Feixas, C.; Vilaseca-Grané, A.; Montesinos, J.; Arnau, A. Reliability and Diagnostic Accuracy of a New Vaginal Dynamometer to Measure Pelvic Floor Muscle Strength. Female Pelvic Med. Reconstr. Surg. 2020, 26, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol. Urodyn. 2017, 36, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Martinho, N.M.; Marques, J.; Silva, V.R.; Silva, S.L.A.; Carvalho, L.C.; Botelho, S. Intra and inter-rater reliability study of pelvic floor muscle dynamometric measurements. Braz. J. Phys. Ther. 2015, 19, 97–104. [Google Scholar] [CrossRef]
- Nygaard, I.; Barber, M.D.; Burgio, K.L.; Kenton, K.; Meikle, S.; Schaffer, J.; Spino, C.; Whitehead, W.E.; Wu, J.; Brody, D.J.; et al. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, X.; Yi, X.; Lai, P.; Lan, Y. Electromyographic Biofeedback for Stress Urinary Incontinence or Pelvic Floor Dysfunction in Women: A Systematic Review and Meta-Analysis. Adv. Ther. 2021, 38, 4163–4177. [Google Scholar] [CrossRef] [PubMed]
- Da Roza, T.; de Araujo, M.P.; Viana, R.; Viana, S.; Jorge, R.N.; Bø, K.; Mascarenhas, T. Pelvic floor muscle training to improve urinary incontinence in young, nulliparous sport students: A pilot study. Int. Urogynecol. J. 2012, 23, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Marcellino, C.; Henn, R.L.; Anselmo Olinto, M.T.; Bressan, A.W.; Vieira Paniz, V.M.; Pattussi, M.P. Physical Inactivity and Associated Factors Among Women From a Municipality in Southern Brazil. J. Phys. Act. Health 2014, 11, 777–783. [Google Scholar] [CrossRef]
- Limongelli, G.; Nunziato, M.; D’Argenio, V.; Esposito, M.V.; Monda, E.; Mazzaccara, C.; Caiazza, M.; D’Aponte, A.; D’Andrea, A.; Bossone, E.; et al. Yield and clinical significance of genetic screening in elite and amateur athletes. Eur. J. Prev. Cardiol. 2021, 28, 1081–1090. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Busquets, C.M.; Serra, T.R. Validación del cuestionario International Consultation on Incontinence Questionnaire Short-Form (ICIQ-SF) en una población chilena usuaria del Fondo Nacional de Salud (FONASA). Rev. Méd. Chile 2012, 140, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Espuña Pons, M.; Rebollo Álvarez, P.; Puig Clota, M. Validación de la versión española del International Consultation on Incontinence Questionnaire-Short Form. Un cuestionario para evaluar la incontinencia urinaria. Med. Clínica 2004, 122, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cullerés, G.; Jané-Feixas, C.; Vilaseca-Grané, A.; Arnau, A.; Montesinos, J.; Abenoza-Guardiola, M. Inter-rater reliability of the digital palpation of pelvic floor muscle by the modified Oxford grading scale in continent and incontinent women. Arch. Esp. Urol. 2019, 72, 602–607. [Google Scholar]
- Chen, J.; Ren, Y.; Zhu, L. Correlation between modified Oxford grading scale and pelvic floor surface electromyography in assessment of pelvic floor muscle function in female patients with stress urinary incontinence. Zhonghua Yi Xue Za Zhi 2020, 100, 2908–2912. [Google Scholar] [PubMed]
- Ferreira, C.H.J.; Barbosa, P.B.; de Oliveira Souza, F.; Antônio, F.I.; Franco, M.M.; Bø, K. Inter-rater reliability study of the modified Oxford Grading Scale and the Peritron manometer. Physiotherapy 2011, 97, 132–138. [Google Scholar] [CrossRef]
- Ben Ami, N.; Dar, G. What is the most effective verbal instruction for correctly contracting the pelvic floor muscles? Neurourol. Urodyn. 2018, 37, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Nagano, R.C.; Biasotto-Gonzalez, D.A.; Da Costa, G.L.; Amorim, K.M.; Fumagalli, M.A.; Amorim, C.F.; Fumagalli, M.A.; Amorim, C.F.; Politti, F. Test-retest reliability of the different dynamometric variables used to evaluate pelvic floor musculature during the menstrual cycle. Neurourol. Urodyn. 2018, 37, 2606–2613. [Google Scholar] [CrossRef]
- Miller, J.M.; Ashton-Miller, J.A.; Perruchicni, D.; DeLancey, J.O.L. TestRetest reliability of an instrumented speculum for measuring vaginal closure force. Neurourol. Urodyn. 2007, 26, 858–863. [Google Scholar] [CrossRef]
- Richardson, J.T. Eta squared and partial eta squared as measurements of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Statistics National Institute. Sports Habits Survey 2022. Available online: https://www.cultura.gob.es/dam/jcr:f4310d9d-6322-43a7-b29c-714348931faf/encuesta-de-habitos-deportivos-2022-cuadro-resumen.pdf (accessed on 11 January 2024).
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic Press: New York, NY, USA, 1969; pp. 278–280. [Google Scholar]
- Morin, M.; Dumoulin, C.; Gravel, D.; Bourbonnais, D.; Lemieux, M.C. Reliability of speed of contraction and endurance dynamometric measurements of the pelvic floor musculature in stress incontinent parous women. Neurourol. Urodyn. 2007, 26, 397–403. [Google Scholar] [CrossRef]
- Morin, M.; Dumoulin, C.; Bourbonnais, D.; Gravel, D.; Lemieux, M.C. Pelvic floor maximal strength using vaginal digital assessment compared to dynamometric measurements. Neurourol. Urodyn. 2004, 23, 336–341. [Google Scholar] [CrossRef] [PubMed]
- El-Sayegh, B.; Cacciari, L.P.; Primeau, F.L.; Sawan, M.; Dumoulin, C. The state of pelvic floor muscle dynamometry: A scoping review. Neurourol. Urodyn. 2023, 42, 478–499. [Google Scholar] [CrossRef] [PubMed]
- Piernicka, M.; Błudnicka, M.; Kortas, J.; Duda-Biernacka, B.; Szumilewicz, A. High-impact aerobics programme supplemented by pelvic floor muscle training does not impair the function of pelvic floor muscles in active nulliparous women: A randomized control trial. Medicine 2021, 100, e26989. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.P.D.; Parmigiano, T.R.; Negra, L.G.D.; Torelli, L.; Carvalho, C.G.D.; Wo, L.; Manito, A.C.A.; Castello Girao, M.J. çB.; Sartori, M.G.F. Avaliação do assoalho pélvico de atletas: Existe relação com a incontinência urinária? Rev. Bras Med. Esporte 2015, 21, 442–446. [Google Scholar] [CrossRef]
- Rodríguez-López, E.S.; Acevedo-Gómez, M.B.; Romero-Franco, N.; Basas-García, Á.; Ramírez-Parenteau, C.; Calvo-Moreno, S.O.; Fernández-Domínguez, J.C. Urinary Incontinence Among Elite Track and Field Athletes According to Their Event Specialization: A Cross-Sectional Study. Sports Med.-Open 2022, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Simons, G.D.; Mense, S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain 1998, 75, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Binik, Y.M.; Bourbonnais, D.; Khalifé, S.; Ouellet, S.; Bergeron, S. Heightened Pelvic Floor Muscle Tone and Altered Contractility in Women with Provoked Vestibulodynia. J. Sex. Med. 2017, 14, 592–600. [Google Scholar] [CrossRef]
- Czyrnyj, C.S.; Bérubé, M.È.; Varette, K.; McLean, L. The impact of a familiarization session on the magnitude and stability of active and passive pelvic floor muscle forces measured through intravaginal dynamometry. Neurourol. Urodyn. 2019, 38, 902–911. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; Da Roza, T.; Mochizuki, L.; Arbieto, E.R.M.; Tonon Da Luz, S.C. Assessment of abdominal and pelvic floor muscle function among continent and incontinent athletes. Int. Urogynecol. J. 2019, 30, 693–699. [Google Scholar] [CrossRef]
- Bø, K.; Finckenhagen, H.B. Vaginal palpation of pelvic floor muscle strength: Inter-test reproducibility and comparison between palpation and vaginal squeeze pressure. Acta Obstet. Gynecol. Scand. 2001, 80, 883–887. [Google Scholar]
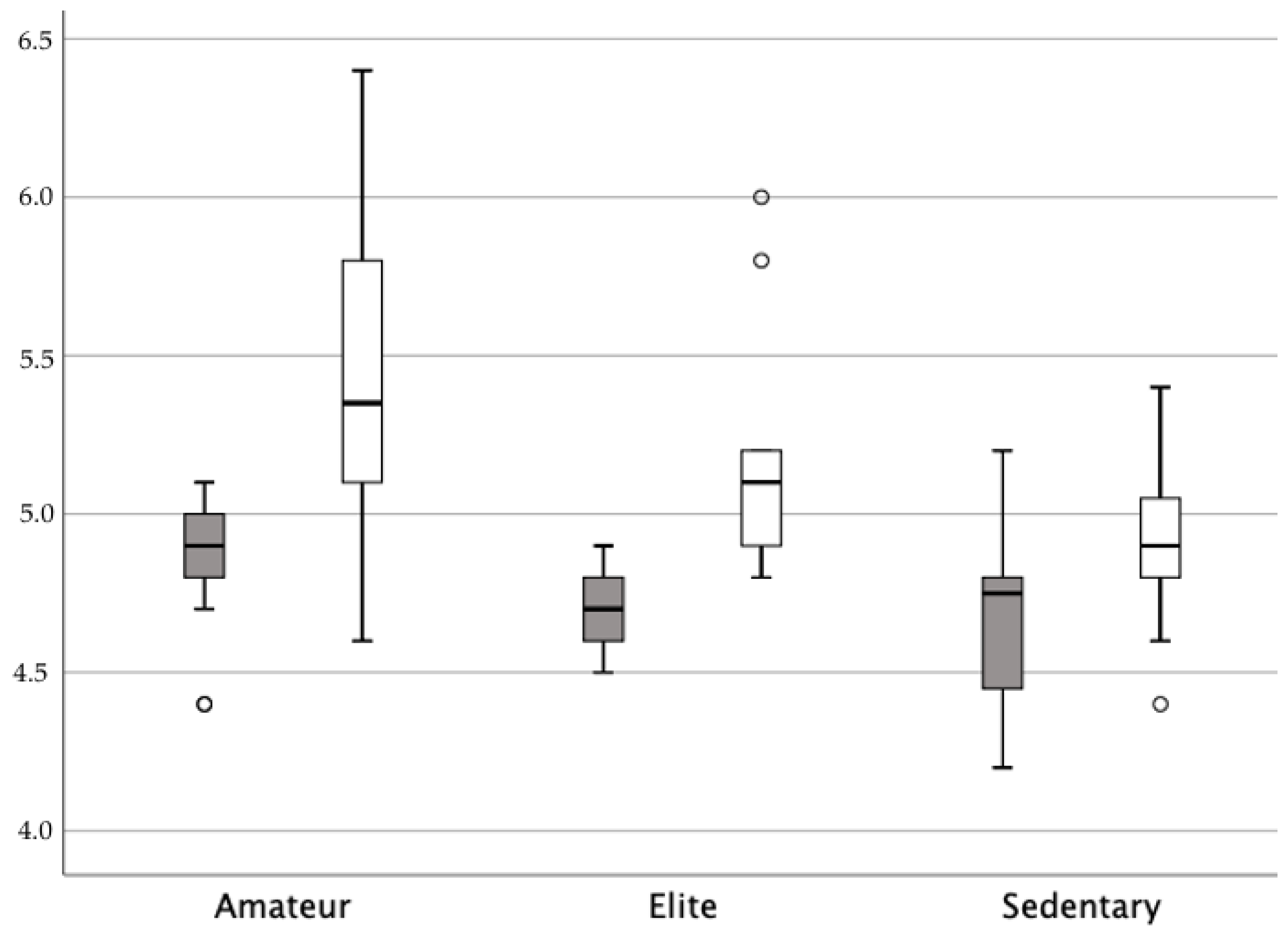
| Physically Active (n = 38) | Sedentary (n = 16) | Between Groups p Value * | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Strength (Modified Oxford Grading Scale) | 3.68 | 0.57 | 3.43 | 0.51 | 0.175 |
| Baseline strength (N) 1st measurement | 4.79 | 0.11 | 4.63 | 0.26 | <0.001 |
| Baseline strength (N) 2nd measurement | 4.82 | 0.18 | 4.68 | 0.29 | 0.048 |
| Contraction strength (N) 1st measurement | 5.32 | 0.56 | 4.83 | 0.21 | <0.001 |
| Contraction strength (N) 2nd measurement | 5.38 | 0.50 | 4.89 | 0.25 | <0.001 |
| Strength (N) 1st measurement | 0.53 | 0.55 | 0.20 | 0.14 | 0.047 |
| Strength (N) 2nd measurement | 0.56 | 0.50 | 0.21 | 0.15 | 0.021 |
| Physically Active (n = 38) | Sedentary (n = 16) | Between Groups p Value * | η2 | |||||
|---|---|---|---|---|---|---|---|---|
| Amateur (n = 25) | Elite (n = 13) | |||||||
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | Mean (SD) | 95% CI | |||
| Age (years) | 26.08 (5.31) | (23.89–28.27) | 25.08 (5.72) | (21.62–28.53) | 25.19 (5.23) | (22.40–27.97) | 0.865 | ----- |
| BMI (kg/m2) | 20.93 (2.44) | (19.92–21.94) | 20.12 (1.36) | (19.3–20.95) | 23.03 (3.91) | (20.95–25.12) | 0.076 | ----- |
| Strength (Modified Oxford Grading Scale) | 3.68 (0.55) | (3.45–3.9) | 3.69 (0.63) | (3.11–4.07) | 3.43 (0.51) | (3.16–3.71) | 0.398 | ----- |
| Baseline strength (N) 1st measurement | 4.83 (0.09) | (4.79–4.87) | 4.70 (0.10) | (4.63–4.77) | 4.63 (0.26) | (4.49–4.76) | 0.001 | 0.240 |
| Baseline strength (N) 2nd measurement | 4.87 (0.18) | (4.8–4.95) | 4.72 (0.12) | (4.64–4.79) | 4.68 (0.29) | (4.52–4.83) | 0.004 | 0.158 |
| Contraction strength (N) 1st measurement | 5.45 (0.61) | (5.20–5.70) | 5.05 (0.28) | (4.88–5.22) | 4.83 (0.21) | (4.72–4.95) | <0.001 | 0.260 |
| Contraction strength (N) 2nd measurement | 5.50 (0.52) | (5.29–5.72) | 5.14 (0.35) | (4.92–5.36) | 4.89 (0.25) | (4.75–5.02) | <0.001 | 0.290 |
| Strength (N) 1st measurement | 0.62 (0.63) | (0.35–0.88) | 0.34 (0.28) | (0.17–0.51) | 0.20 (0.14) | (0.12–0.28) | 0.039 | 0.142 |
| Strength (N) 2nd measurement | 0.63 (0.55) | (0.40–0.86) | 0.42 (0.33) | (0.22–0.62) | 0.21 (0.15) | (0.13–0.29) | 0.019 | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Gómez, M.B.; Rodríguez-López, E.S.; Oliva-Pascual-Vaca, Á.; Fernández-Rodríguez, T.; Basas-García, Á.; Ojedo-Martín, C. Is the Elite Female Athlete’s Pelvic Floor Stronger? J. Clin. Med. 2024, 13, 908. https://doi.org/10.3390/jcm13030908
Acevedo-Gómez MB, Rodríguez-López ES, Oliva-Pascual-Vaca Á, Fernández-Rodríguez T, Basas-García Á, Ojedo-Martín C. Is the Elite Female Athlete’s Pelvic Floor Stronger? Journal of Clinical Medicine. 2024; 13(3):908. https://doi.org/10.3390/jcm13030908
Chicago/Turabian StyleAcevedo-Gómez, María Barbaño, Elena Sonsoles Rodríguez-López, Ángel Oliva-Pascual-Vaca, Tomás Fernández-Rodríguez, Ángel Basas-García, and Cristina Ojedo-Martín. 2024. "Is the Elite Female Athlete’s Pelvic Floor Stronger?" Journal of Clinical Medicine 13, no. 3: 908. https://doi.org/10.3390/jcm13030908
APA StyleAcevedo-Gómez, M. B., Rodríguez-López, E. S., Oliva-Pascual-Vaca, Á., Fernández-Rodríguez, T., Basas-García, Á., & Ojedo-Martín, C. (2024). Is the Elite Female Athlete’s Pelvic Floor Stronger? Journal of Clinical Medicine, 13(3), 908. https://doi.org/10.3390/jcm13030908







