Safety and Efficacy of PCSK9 Inhibitors in Patients with Acute Coronary Syndrome Who Underwent Coronary Artery Bypass Grafts: A Comparative Retrospective Analysis
Abstract
1. Introduction
2. Methods
2.1. Ethical Statement
2.2. Study Population
- (1)
- If without statins, they received a moderate-intensity statin (e.g., 40 mg) from the catheter laboratory (cath lab) table up to the 3-month follow-up;
- (2)
- On statins, they added Ezetimibe and a higher statin dosage (80 mg) from the cath lab table up to the 3-month follow-up.
- (1)
- If without statins, they received a moderate-intensity statin (e.g., 40 mg) + Ezetimibe + PCSK9i (Evolocumab 140 mg every 2 weeks) from the cath lab table up to the 3-month follow-up;
- (2)
- On statins, they added Ezetimibe and a higher statin dosage (80 mg) + PCSK9i (Evolocumab 140 mg every 2 weeks) from the cath lab table up to the 3-month follow-up.
2.3. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: Meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Koskinas, K.C.; Siontis, G.C.M.; Piccolo, R.; Mavridis, D.; Räber, L.; Mach, F.; Windecker, S. Effect of statins and nonstatin LDL-lowering medications on cardiovascular outcomes in secondary prevention: A meta-analysis of randomized trials. Eur. Heart J. 2018, 39, 1172–1180. [Google Scholar] [CrossRef]
- Ray, K.K.; Cannon, C.P.; McCabe, C.H.; Cairns, R.; Tonkin, A.M.; Sacks, F.M.; Jackson, G.; Braunwald, E. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: Results from the PROVE IT-TIMI 22 trial. J. Am. Coll. Cardiol. 2005, 46, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/ AphA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Desai, N.R.; Kohli, P.; Rogers, W.J.; Somaratne, R.; Huang, F.; Liu, T.; Mohanavelu, S.; Hoffman, E.B.; McDonald, S.T.; et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): A andomized, placebo-controlled, dose-ranging, phase 2 study. Lancet 2012, 380, 2007–2017. [Google Scholar] [CrossRef]
- Raal, F.J.; Stein, E.A.; Dufour, R.; Turner, T.; Civeira, F.; Burgess, L.; Langslet, G.; Scott, R.; Olsson, A.G.; Sullivan, D.; et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A andomized, double-blind, placebo-controlled trial. Lancet 2015, 385, 331–340. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; De Ferrari, G.M.; Giugliano, R.P.; Huber, K.; Lewis, B.S.; Ferreira, J.; Kuder, J.F.; Murphy, S.A.; Wiviott, S.D.; Kurtz, C.E.; et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation 2018, 138, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Koskinas, K.C.; Windecker, S.; Pedrazzini, G.; Mueller, C.; Cook, S.; Matter, C.M.; Muller, O.; Häner, J.; Gencer, B.; Crljenica, C.; et al. Evolocumab for Early Reduction of LDL Cholesterol Levels in Patients with Acute Coronary Syndromes (EVOPACS). J. Am. Coll. Cardiol. 2019, 74, 2452–2462. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E.; Vrablik, M.; Bunc, M.; Dyrbus, K.; Fedacko, J.; Gaita, D.; Gierlotka, M.; Jarai, Z.; Magda, S.L.; et al. Optimal use of lipid-lowering therapy after acute coronary syndromes: A Position Paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2021, 166, 105499. [Google Scholar] [CrossRef]
- Gummert, J.F.; Funkat, A.; Beckmann, A.; Schiller, W.; Hekmat, K.; Ernst, M.; Haverich, A. Cardiac Surgery in Germany during 2008. A report on behalf of the German society for thoracic and cardiovascular surgery. Thorac. Cardiovasc. Surg. 2009, 57, 315–323. [Google Scholar] [CrossRef]
- Kim, L.K.; Looser, P.; Swaminathan, R.V.; Minutello, R.M.; Wong, S.C.; Girardi, L.; Feldman, D.N. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J. Thorac. Cardiovasc. Surg. 2016, 151, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Morice, M.-C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Van Den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- LaPar, D.J.; Crosby, I.K.; Rich, J.B.; Fonner, E., Jr.; Kron, I.L.; Ailawadi, G.; Speir, A.M. Investigators for Virginia Cardiac Surgery Quality Initiative. A contemporary cost analysis of postoperative morbidity after coronary artery bypass grafting with and without concomitant aortic valve replacement to improve patient quality and cost-effective care. Ann. Thorac. Surg. 2013, 96, 1621–1627. [Google Scholar]
- Verma, S.; Fedak, P.W.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.K.; Dhillon, B.; Yau, T.M. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kapoor, A.; Agarwal, S.K.; Pande, S.; Tewari, P.; Majumdar, G.; Sinha, A.; Kashyap, S.; Khanna, R.; Kumar, S.; et al. Statin reload before off-pump coronary artery bypass graft: Effect on biomarker release kinetics. Ann. Card. Anaesth. 2020, 23, 27–33. [Google Scholar]
- Mannacio, V.A.; Iorio, D.; De Amicis, V.; Di Lello, F.; Musumeci, F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2008, 136, 1541–1548. [Google Scholar] [CrossRef]
- Pan, W.; Pintar, T.; Anton, J.; Lee, V.V.; Vaughn, W.K.; Collard, C.D. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004, 110 (Suppl. S1), II-45–II-49. [Google Scholar] [CrossRef]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M.; Investigators, P. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef]
- Brull, D.J.; Sanders, J.; Rumley, A.; Lowe, G.D.; Humphries, S.E.; Montgomery, H.E. Statin therapy and the acute inflammatory response after coronary artery bypass grafting. Am. J. Cardiol. 2001, 88, 431–433. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Mellis, S.; Yancopoulos, G.D.; Stahl, N.; Logan, D.; Smith, W.B.; Lisbon, E.; Gutierrez, M.; Webb, C.; Wu, R.; et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 2012, 366, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Kolodziejczak, M.; Kereiakes, D.J.; Tantry, U.S.; O’Connor, C.; Gurbel, P.A. Proprotein convertase subtilisin/kexin type 9 monoclonal antibodies for acute coronary syndrome: A narrative review. Ann. Intern. Med. 2016, 164, 600–607. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Palee, S.; McSweeney, C.M.; Maneechote, C.; Moisescu, D.M.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, S.C.; Chattipakorn, N. PCSK9 inhibitor improves cardiac function and reduces infarct size in rats with ischaemia/reperfusion injury: Benefits beyond lipid-lowering effects. J. Cell. Mol. Med. 2019, 23, 7310–7319. [Google Scholar] [CrossRef]
- Na, H.R.; Kwon, O.S.; Kang, J.K.; Kim, Y.H.; Lim, J.Y. Evolocumab administration prior to Coronary Artery Bypass Grafting in patients with multivessel coronary artery disease (EVOCABG): Study protocol for a randomized controlled clinical trial. Trials 2022, 23, 430. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Musumeci, G.; Annibali, G.; Delnevo, F. Acute coronary syndromes: Hospital management of dyslipidaemia with proprotein convertase subtilisin/kexin 9 inhibitors: Time to act. Eur. Heart J. Suppl. 2023, 25, B114–B118. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Riccio, C.; Navazio, A.; Valente, S.; Cipriani, M.; Corda, M.; De Nardo, A.; Francese, G.M.; Napoletano, C.; Tizzani, E.; et al. Position paper ANMCO: Gestione dell’ipercolesterolemia nei pazienti con sindrome coronarica acuta. G. Ital. Cardiol. 2023, 24, 229–240. [Google Scholar]
- Fogacci, F.; Yerlitaş, S.I.; Giovannini, M.; Zararsız, G.; Lido, P.; Borghi, C.; Cicero, A.F.C. Sex X Time Interactions in Lp(a) and LDL-C Response to Evolocumab. Biomedicines 2023, 11, 3271. [Google Scholar] [CrossRef] [PubMed]
| GROUP STEVO | GROUP STEZE | p | |
|---|---|---|---|
| pts = 31 | pts = 43 | ||
| Age, yrs | 68.5 ± 6.8 | 70 ± 7.4 | NS |
| Male | 26 (83.9%) | 34 (79.1%) | NS |
| Body mass index, kg/m2 | 26.9 ± 3 | 26.4 ± 2.9 | NS |
| Diabetes mellitus | 12 (38.7%) | 18 (41.8%) | NS |
| Insulin-treated | 7 (22.6%) | 10 (23.2%) | NS |
| Arterial hypertension | 21 (67.7%) | 30 (69.8%) | NS |
| Active smoking | 19 (61.3%) | 25 (58.1%) | NS |
| Previous myocardial infarction | 7 (22.6%) | 10 (23.2%) | NS |
| Previous PCI | 15 (48.4%) | 20 (46.5%) | NS |
| Previous CABG | 0 | 0 | |
| Peripheral arterial disease | 2 (6.4%) | 4 (9.3%) | NS |
| History of stroke | 0 | 2 (4.6%) | NS |
| History of TIA | 1 (3.2%) | 2 (4.6%) | NS |
| History of malignancy | 6 (19.3%) | 8 (18.6%) | NS |
| Euroscore II | 2.14 ± 0.75 | 2.05 ± 0.6 | NS |
| Time of symptom onset, h | 98 ± 30 | 91 ± 27 | NS |
| Culprit primary PCI | 4 (12.9%) | 5 (11.6%) | NS |
| NSTE-ACS | 21 (67.7%) | 30 (69.8%) | NS |
| STE-ACS | 4 (12.9%) | 5 (11.6%) | NS |
| Instable angina | 6 (19.3%) | 8 (18.6%) | NS |
| GROUP STEZE | GROUP STEVO | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| January 2022– January 2023 | No Statin | Low- or Moderate- Intensity Statin | High- Intensity Statin | Ezetimibe Treatment | February 2023–June 2023 | No Statin | Low- or Moderate- Intensity Statin | High- Intensity Statin | Ezetimibe Treatment |
| Pt. 1 | x | Pt. 1 | x | ||||||
| Pt. 2 | x | Pt. 2 | x | ||||||
| Pt. 3 | x | Pt. 3 | x | ||||||
| Pt. 4 | x | Pt. 4 | x | ||||||
| Pt. 5 | x | Pt. 5 | x | ||||||
| Pt. 6 | x | Pt. 6 | x | ||||||
| Pt. 7 | x | Pt. 7 | x | ||||||
| Pt. 8 | x | Pt. 8 | x | ||||||
| Pt. 9 | X | Pt. 9 | x | ||||||
| Pt. 10 | x | Pt. 10 | x | ||||||
| Pt. 11 | x | Pt. 11 | x | ||||||
| Pt. 12 | x | Pt. 12 | x | ||||||
| Pt. 13 | x | Pt. 13 | x | ||||||
| Pt. 14 | x | Pt. 14 | x | ||||||
| Pt. 15 | x | Pt. 15 | x | ||||||
| Pt. 16 | x | Pt. 16 | x | ||||||
| Pt. 17 | x | Pt. 17 | x | ||||||
| Pt. 18 | x | Pt. 18 | x | ||||||
| Pt. 19 | x | Pt. 19 | x | ||||||
| Pt. 20 | X | Pt. 20 | x | ||||||
| Pt. 21 | x | Pt. 21 | x | ||||||
| Pt. 22 | x | Pt. 22 | x | ||||||
| Pt. 23 | x | Pt. 23 | x | ||||||
| Pt. 24 | x | Pt. 24 | x | ||||||
| Pt. 25 | X | Pt. 25 | x | ||||||
| Pt. 26 | x | Pt. 26 | x | ||||||
| Pt. 27 | x | Pt. 27 | x | ||||||
| Pt. 28 | x | Pt. 28 | x | ||||||
| Pt. 29 | x | Pt. 29 | x | ||||||
| Pt. 30 | x | Pt. 30 | x | ||||||
| Pt. 31 | x | Pt. 31 | x | ||||||
| Pt. 32 | x | 71% | 16.1% | 9.7% | 3.2% | ||||
| Pt. 33 | x | ||||||||
| Pt. 34 | x | ||||||||
| Pt. 35 | x | ||||||||
| Pt. 36 | x | ||||||||
| Pt. 37 | x | ||||||||
| Pt. 38 | x | ||||||||
| Pt. 39 | x | ||||||||
| Pt. 40 | X | ||||||||
| Pt. 41 | x | ||||||||
| Pt. 42 | x | ||||||||
| Pt. 43 | x | ||||||||
| 74.4% | 14% | 9% | 2.6% | ||||||
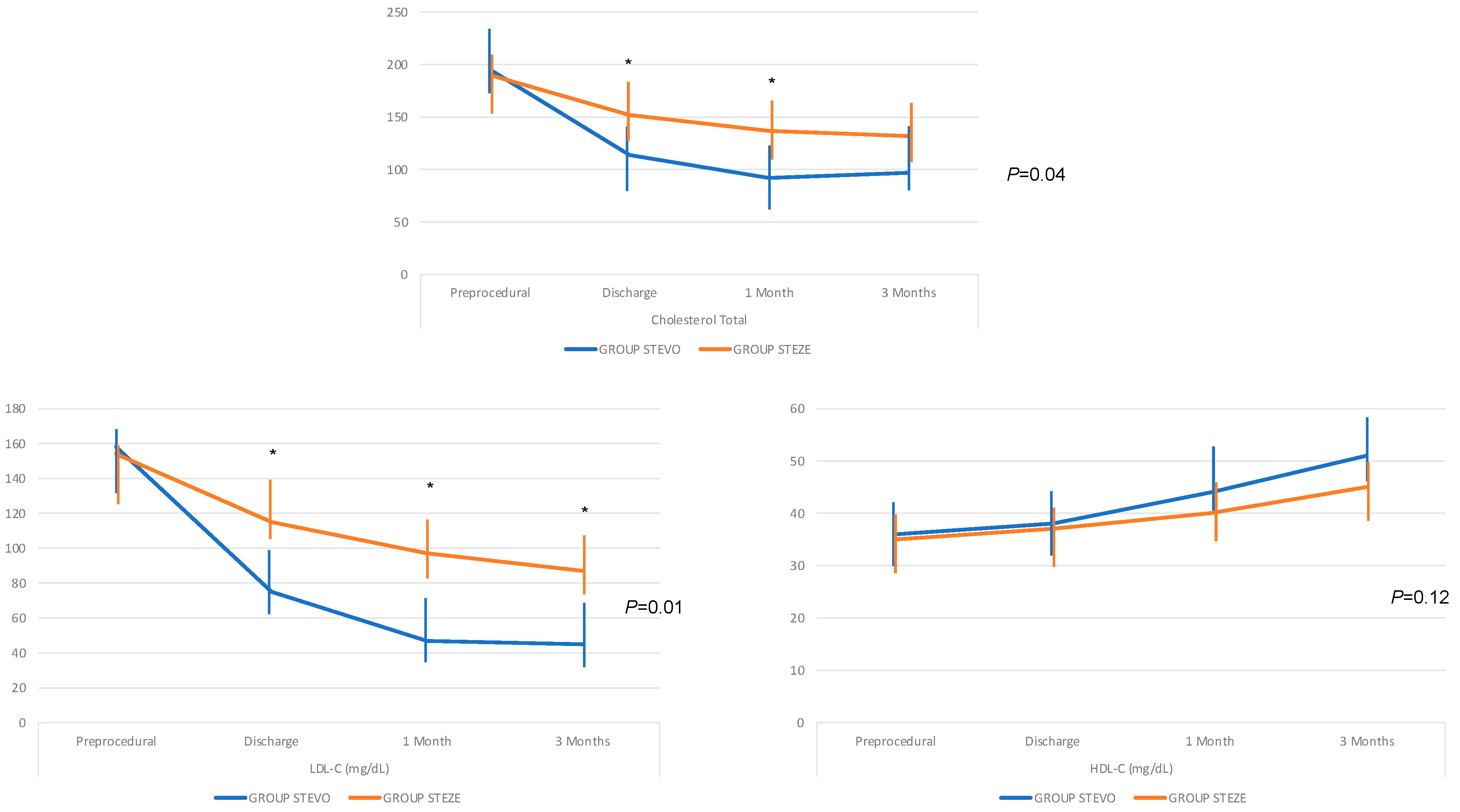
| Total Cholesterol (mg/dL) | |||||
|---|---|---|---|---|---|
| Preprocedural | Discharge | 1 Month | 3 Months | p | |
| GROUP STEVO | 195 ± 13 | 114 ± 16 | 92 ± 12 | 97 ± 9 | 0.04 |
| GROUP STEZE | 190 ± 17 | 152 ± 12 | 137 ± 16 | 132 ± 17 | |
| LDL-C (mg/dL) | |||||
| Preprocedural | Discharge | 1 Month | 3 Months | p | |
| GROUP STEVO | 158 ± 14 | 75 ± 16 | 47 ± 10 | 45 ± 10 | 0.01 |
| GROUP STEZE | 154 ± 18 | 115 ± 11 | 97 ± 19 | 87 ± 22 | |
| HDL-C (mg/dL) | |||||
| Preprocedural | Discharge | 1 Month | 3 Months | p | |
| GROUP STEVO | 36 ± 4 | 38 ± 3 | 44 ± 5 | 51 ± 6 | 0.12 |
| GROUP STEZE | 35 ± 5 | 37 ± 4 | 40 ± 5 | 45 ± 9 | |
| Triglyceride (mg/dL) | |||||
| Preprocedural | Discharge | 1 Month | 3 Months | p | |
| GROUP STEVO | 176 ± 17 | 166 ± 15 | 141 ± 27 | 133 ± 22 | 0.18 |
| GROUP STEZE | 179 ± 20 | 173 ± 17 | 170 ± 16 | 173 ± 16 | |
| Alanine Aminotransferease (ALT) (U/L) | |||||
| Preprocedural | Discharge | 1 Month | 3 Months | p | |
| GROUP STEVO | 37 ± 5 | 45 ± 9 | 62 ± 22 | 58 ± 24 | 0.44 |
| GROUP STEZE | 35 ± 2 | 39 ± 6 | 59 ± 22 | 57 ± 20 | |
| Troponin I (microgr/L) | |||||
| Preprocedural | 24 h | 72 h | Discharge | p | |
| GROUP STEVO | 613 ± 257 | 829 ± 323 | 364 ± 109 | 138 ± 66 | 0.06 |
| GROUP STEZE | 613 ± 273 | 821 ± 316 | 507 ± 218 | 298 ± 184 | |
| CRP (mg/dL) | |||||
| Preprocedural | Discharge | 1 Month | 3 Months | p | |
| GROUP STEVO | 52 ± 31 | 24 ± 15 | 3.5 ± 2 | 3.1 ± 2 | 0.10 |
| GROUP STEZE | 64 ± 58 | 64 ± 58 | 6.9 ± 3 | 5.8 ± 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasso, G.; Larosa, C.; Bartolomucci, F.; Brigiani, M.S.; Contegiacomo, G.; Demola, M.A.; Vignaroli, W.; Tripoli, A.; Girasoli, C.; Lisco, R.; et al. Safety and Efficacy of PCSK9 Inhibitors in Patients with Acute Coronary Syndrome Who Underwent Coronary Artery Bypass Grafts: A Comparative Retrospective Analysis. J. Clin. Med. 2024, 13, 907. https://doi.org/10.3390/jcm13030907
Nasso G, Larosa C, Bartolomucci F, Brigiani MS, Contegiacomo G, Demola MA, Vignaroli W, Tripoli A, Girasoli C, Lisco R, et al. Safety and Efficacy of PCSK9 Inhibitors in Patients with Acute Coronary Syndrome Who Underwent Coronary Artery Bypass Grafts: A Comparative Retrospective Analysis. Journal of Clinical Medicine. 2024; 13(3):907. https://doi.org/10.3390/jcm13030907
Chicago/Turabian StyleNasso, Giuseppe, Claudio Larosa, Francesco Bartolomucci, Mario Siro Brigiani, Gaetano Contegiacomo, Maria Antonietta Demola, Walter Vignaroli, Alessandra Tripoli, Cataldo Girasoli, Rosanna Lisco, and et al. 2024. "Safety and Efficacy of PCSK9 Inhibitors in Patients with Acute Coronary Syndrome Who Underwent Coronary Artery Bypass Grafts: A Comparative Retrospective Analysis" Journal of Clinical Medicine 13, no. 3: 907. https://doi.org/10.3390/jcm13030907
APA StyleNasso, G., Larosa, C., Bartolomucci, F., Brigiani, M. S., Contegiacomo, G., Demola, M. A., Vignaroli, W., Tripoli, A., Girasoli, C., Lisco, R., Trivigno, M., Tunzi, R. M., Loizzo, T., Hila, D., Franchino, R., Amodeo, V., Ventra, S., Diaferia, G., Schinco, G., ... Speziale, G. (2024). Safety and Efficacy of PCSK9 Inhibitors in Patients with Acute Coronary Syndrome Who Underwent Coronary Artery Bypass Grafts: A Comparative Retrospective Analysis. Journal of Clinical Medicine, 13(3), 907. https://doi.org/10.3390/jcm13030907