Should Emollients Be Recommended for the Prevention of Atopic Dermatitis?—New Evidence and Current State of Knowledge
Abstract
1. Introduction
1.1. Pathogenesis of AD
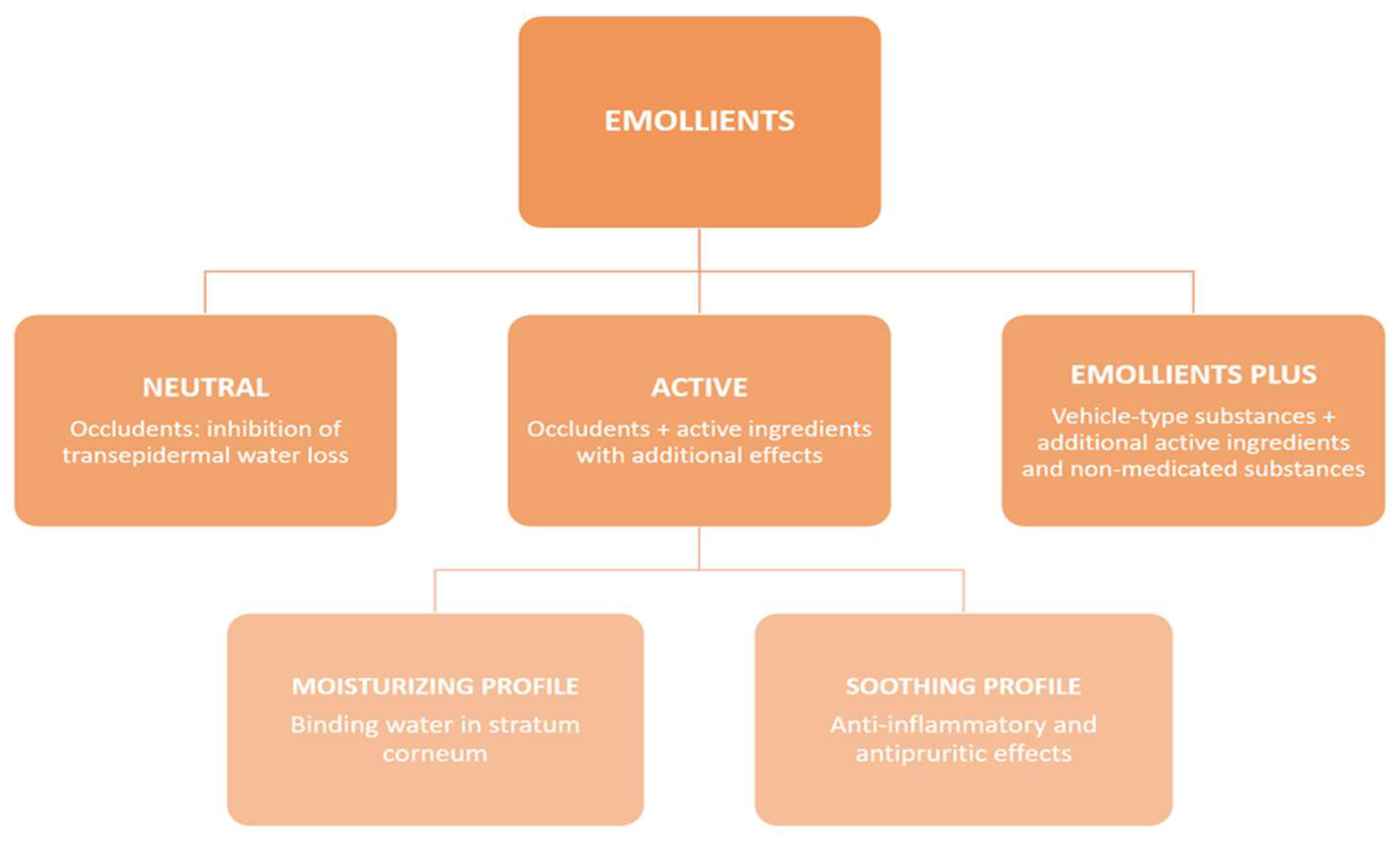
1.2. Skin Barrier in AD and Emollient Types
1.3. Study Justification and the Aim
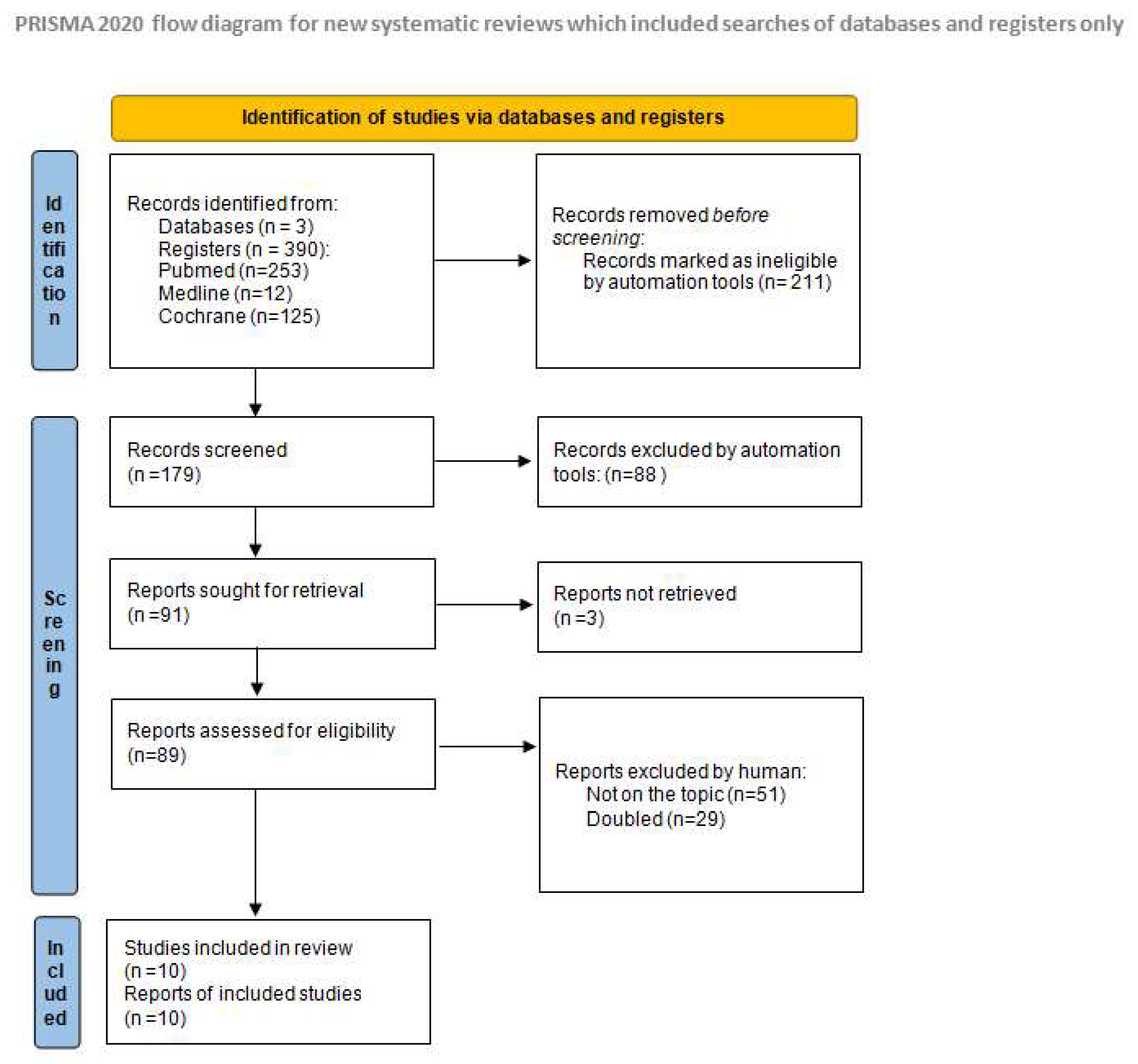
2. Materials and Methods
- Randomized clinical trials or metanalyses;
- Neonates from the group of AD risk (parents’ history of atopy);
- Studies published in the last 5 years.
3. Results
| Study Title and Type of Study | Participants | Method of Application | Emollient Type: | Results |
|---|---|---|---|---|
| Ní Chaoimh C et al. (2022) [26] Randomized controlled clinical trial | N = 321 infants 161 intervention and 160 control | Intervention group: Emollient use twice-daily, whole body except fingertip quantitation from scalp, implemented within days of life for the first 8 weeks Control group: standard routine skin-care advice | AVEENO® Dermexa Fast & Long Lasting Balm (Johnson & Johnson Santé Beauté France, JJSBF)—formulation with added ceramides, oat ingredients, fatty acids. | Daily emollient use until 2 months of age reduces the incidence of AD in the first year of life in high-risk infants. |
| Harder I et al. (2023) [36] Randomized controlled trial | N = 50 neonates | Intervention group: skin-care advice plus emollient 1 per day for 1 year Control group: general infant skin-care | Emollient containing a prebiotic Vitreos-cilla filiformis lysate | Daily emollient use did not significantly reduce the risk of developing AD or impact skin physiology development |
| Bradshaw LE et al. (2023) [32] Randomized controlled trial | N = 1394 term infants 693 emollient group; 701 controls | Intervention group: Emollient all over the body daily for the first year, for >3 days per week plus standard skin-care advice control—standard skin-care advice only. Emollients implemented 11 days of life. | Basic petroleum emollients | Daily emollient application during the first year of life does not prevent atopic dermatitis. |
| Skjerven HO et al. (2020) [33] Cluster randomised trial | N = 2397 newborn infants Assigned to different intervention groups | Intervention group: baths for 5–10 min with added emulsified oil and emollient applied to the entire face after the bath on at least 4 days per week, from age of 2 weeks to 8 months | Paraffin-based formulations | Skin emollients did not reduce development of atopic dermatitis by age 12 months. |
| McClanahan D et al. (2019) [15] Randomised controlled trial | N = 100 newborn infants | Intervention group: daily to all body surfaces excluding the scalp and diaper area | Emollient with shea, pseudoceramide-5 and two FLG breakdown products—arginine and sodium pyrrolidone carboxylic acid | No statistically significant effect in atopic dermatitis prevention of the ceramide and amino acid-containing emollient; |
| Techasatian L et al. (2021) [34] Randomised controlled study | N = 154 neonates 77 intervention group 77 control group | Intervention group: Once daily to the baby’s entire body surface (excluding the scalp), starting as soon as possible after birth (within a maximum of 3 weeks) till 6 months of age. | 5 types of emollients to choose: Four claimed to be therapeutic emollients, with a variety of anti-inflammatory ingredients. One is basic petrolatum-based emollient. | In tropical climate emollients put on skin in case of skin dryness protect infants against AD |
| Dissanayake E (2019) [35] Randomised Controlled study | N = 549 babies qualified to be randomized, 459 infants completed the intervention | Intervention group: 2–3 times/day, after a bath or on clean skin, particularly on the cheeks and the peri-oral area. | Cream containing ceramide, cholesterol, and free fatty acids | Emollient did not show any effect on reducing the development of AD and FA at 1 year of age |
| Lowe AJ et al. (2018) [37] Randomised trial | N = 80 children | Intervention group: Within the first three weeks 6 g of EpiCeram ™ to the full skin surface of their child twice per day | 6 g of EpiCeram complex ceramide-rich emollients | twice daily prophylactic use of a ceramide dominant emollient, reduced incidence of AD |
| Bellemere, G et al. (2019) [38,39] Randomised controlled trial | N= 120 infants | Intervention group: balm twice a day, cleansing cream and bath oil twice a week Control group: Standard skin-care | No information given | The beneficial effect of prevention maintained after 24 months of follow-up. |
| Kottner J et al. (2022) [40] Randomised trial | N = 160 infants | Intervention group: skin-care regimen including once daily leave-on product application Control group: standard skin-care | Lipd content 21% | No effect in prevention |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Napolitano, M.; Fabbrocini, G.; Neri, I.; Stingeni, L.; Boccaletti, V.; Piccolo, V.; Amoruso, G.F.; Malara, G.; De Pasquale, R.; Di Brizzi, E.V.; et al. Dupilumab Treatment in Children Aged 6–11 Years with Atopic Dermatitis: A Multicentre, Real-Life Study. Pediatr. Drugs 2022, 24, 671–678. [Google Scholar] [CrossRef]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Bylund, S.; Kobyletzki, L.; Svalstedt, M.; Svensson, Å. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm.-Venereol. 2020, 100, 320–329. [Google Scholar] [CrossRef] [PubMed]
- International League of Dermatological Societies; Arents, B.; van Zuuren, E.; Fedorowicz, Z.; Hughes, O. Global Report on Atopic Dermatitis 2022. Available online: https://www.atopicdermatitisatlas.org/en/explore-data/reports (accessed on 16 September 2023).
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar] [PubMed]
- Gomes, T.F.; Calado, R.; Gonçalo, M. Epidermal Barrier Dysfunction in Atopic Dermatitis. J. Port. Soc. Dermatol. Venereol. 2021, 79, 207–216. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Camelo, A.; Rosignoli, G.; Ohne, Y.; Stewart, R.A.; Overed-Sayer, C.; Sleeman, M.A.; May, R.D. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 2017, 1, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Klonowska, J.; Gleń, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.-S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.-I.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon Monoxide and Nitric Oxide as Examples of the Youngest Class of Transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, D.; Wong, A.; Kezic, S.; Samrao, A.; Hajar, T.; Hill, E.; Simpson, E. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2087–2094. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Pincelli, C.; Sparavigna, A.; Luger, T. The Role of a Novel Generation of Emollients, “Emollients Plus” in Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2705–2719. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Wang, X.; Zhang, L.; Hu, M.; Le, Y.; Chen, L.; Zheng, J. Topical emollient prevents the development of atopic dermatitis and atopic march in mice. Exp. Dermatol. 2023, 32, 1007–1015. [Google Scholar] [CrossRef]
- Shindo, S.; Murota, H.; Seki, T.; Mori, K.; Kaizu, K.; Nishizaka, T.; Takagi, Y.; Katayama, I. Effects of a moisturizer containing pseudo-ceramide and a eucalyptus extract on sweating function in adult atopic dermatitis: A double-blind, randomized, controlled left-right comparison clinical trial. J. Cosmet. Dermatol. 2022, 21, 4503–4509. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682, Erratum in: J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1436. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Valle, G.A.; Barbarot, S.; Bieber, T.; Brough, H.; Pinton, P.C.; Christen-Zäch, S.; et al. European guideline (EuroGuiDerm) on atopic eczema—part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1904–1926. [Google Scholar] [CrossRef]
- Nowicki, R.; Trzeciak, M.; Kaczmarski, M.; Wilkowska, A.; Czarnecka-Operacz, M.; Kowalewski, C.; Rudnicka, L.; Kulus, M.; Mastalerz-Migas, A.; Peregud-Pogorzelski, J.; et al. Atopic dermatitis. Interdisciplinary diagnostic and therapeutic recommendations of the PTD, PTA, PTP, and PTMR. Part I. Prophylaxis, topical treatment, and phototherapy. Lek. POZ 2019, 5, 335–348. [Google Scholar]
- Katoh, N.; Ohya, Y.; Ikeda, M.; Ebihara, T.; Katayama, I.; Saeki, H.; Shimojo, N.; Tanaka, A.; Nakahara, T.; Nagao, M.; et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J. Dermatol. 2019, 46, 1053–1101. [Google Scholar] [CrossRef] [PubMed]
- Rubel, D.; Thirumoorthy, T.; Soebaryo, R.W.; Weng, S.C.K.; Gabriel, T.M.; Villafuerte, L.L.; Chu, C.; Dhar, S.; Parikh, D.; Wong, L.; et al. Consensus guidelines for the management of atopic dermatitis: An Asia–Pacific perspective. J. Dermatol. 2013, 40, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Ridd, M.J.; Santer, M.; MacNeill, S.J.; Sanderson, E.; Wells, S.; Webb, D.; Banks, J.; Sutton, E.; Roberts, A.; Liddiard, L.; et al. Effectiveness and safety of lotion, cream, gel, and ointment emollients for childhood eczema: A pragmatic, randomised, phase 4, superiority trial. Lancet Child Adolesc. Health 2022, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Neri, I.; Ricci, G.; Baldo, E.; Barone, M.; Fortina, A.B.; Bernardini, R.; Berti, I.; Caffarelli, C.; Calamelli, E.; et al. Consensus Conference on Clinical Management of pediatric Atopic Dermatitis. Ital. J. Pediatr. 2016, 42, 26. [Google Scholar] [CrossRef]
- Chaoimh, C.N.; Lad, D.; Nico, C.; Puppels, G.J.; Wong, X.F.C.C.; Common, J.E.; Murray, D.M.; Irvine, A.D.; Hourihane, J.O. Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants—The STOP-AD randomised controlled trial. Allergy 2023, 78, 984–994. [Google Scholar] [CrossRef]
- Fostini, A.C.; Georgescu, V.; Decoster, C.J.; Girolomoni, G. A cream based on Aquaphilus dolomiae extracts alleviates non-histaminergic pruritus in humans. Eur. J. Dermatol. 2017, 27, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Aries, M.-F.; Hernandez-Pigeon, H.; Vaissière, C.; Delga, H.; Caruana, A.; Lévêque, M.; Bourrain, M.; Helffer, K.R.; Chol, B.; Nguyen, T.; et al. Anti-inflammatory and immunomodulatory effects of Aquaphilus dolomiae extract on in vitro models. Clin. Cosmet. Investig. Dermatol. 2016, 9, 421–434. [Google Scholar] [CrossRef]
- Bianchi, P.; Theunis, J.; Casas, C.; Villeneuve, C.; Patrizi, A.; Phulpin, C.; Bacquey, A.; Redoulès, D.; Mengeaud, V.; Schmitt, A. Effects of a New Emollient-Based Treatment on Skin Microflora Balance and Barrier Function in Children with Mild Atopic Dermatitis. Pediatr. Dermatol. 2016, 33, 165–171. [Google Scholar] [CrossRef]
- Martin, H.; Laborel-Préneron, E.; Fraysse, F.; Nguyen, T.; Schmitt, A.-M.; Redoulès, D.; Davrinche, C. Aquaphilus dolomiae extract counteracts the effects of cutaneous S. aureus secretome isolated from atopic children on CD4+ T cell activation. Pharm. Biol. 2016, 54, 2782–2785. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bradshaw, L.E.; Wyatt, L.A.; Brown, S.J.; Haines, R.H.; Montgomery, A.A.; Perkin, M.R.; Lawton, S.; Sach, T.H.; Chalmers, J.R.; Ridd, M.J.; et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy 2023, 78, 995–1006. [Google Scholar] [CrossRef]
- Skjerven, H.O.; Rehbinder, E.M.; Vettukattil, R.; LeBlanc, M.; Granum, B.; Haugen, G.; Hedlin, G.; Landrø, L.; Marsland, B.J.; Rudi, K.; et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet 2020, 395, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Techasatian, L.; Kiatchoosakun, P. Effects of an emollient application on newborn skin from birth for prevention of atopic dermatitis: A randomized controlled study in Thai neonates. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, E.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Suzuki, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; et al. Skin Care and Synbiotics for Prevention of Atopic Dermatitis or Food Allergy in Newborn Infants: A 2 × 2 Factorial, Randomized, Non-Treatment Controlled Trial. Int. Arch. Allergy Immunol. 2019, 180, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Harder, I.; Stölzl, D.; Sander, N.; Hartmann, J.; Rodriguez, E.; Mazur, C.; Kerzel, S.; Kabesch, M.; Küster, D.; Schmitt, J.; et al. Effects of Early Emollient Use in Children at High Risk of Atopic Dermatitis: A German Pilot Study. Acta Derm.-Venereol. 2023, 103, adv5671. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.; Su, J.; Allen, K.; Abramson, M.; Cranswick, N.; Robertson, C.; Forster, D.; Varigos, G.; Hamilton, S.; Kennedy, R.; et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: The PEBBLES pilot study. Br. J. Dermatol. 2018, 178, e19–e21. [Google Scholar] [CrossRef] [PubMed]
- Bellemere, G.; Boyer, G.; De Belilovsky, C.; Baudouin, C. 563 Prevention of atopic dermatitis using emollients for 6 months—Follow-up for 24 months. J. Investig. Dermatol. 2019, 139, S97. [Google Scholar] [CrossRef]
- Bellemere, G.; Boyer, G.; De Belilovsky, C.; Moga, A.; Fontanie, M.; Baudouin, C. Early atopic dermatitis: Prevention study. Pediatr. Dermatol. 2018, 35, S4–S5. [Google Scholar] [CrossRef]
- Kottner, J.; Hillmann, K.; Fastner, A.; Conzade, R.; Heidingsfelder, S.; Neumann, K.; Blume-Peytavi, U. ADAPI Study Group Effectiveness of a standardized skin care regimen to prevent atopic dermatitis in infants at risk for atopy: A randomized, pragmatic, parallel-group study. J. Eur. Acad. Dermatol. Venereol. 2022, 37, 540–548. [Google Scholar] [CrossRef]
- Simpson, E.L.; Keck, L.E.; Chalmers, J.R.; Williams, H.C. How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies. J. Allergy Clin. Immunol. 2012, 130, 137–144. [Google Scholar] [CrossRef]
- Xu, D.; Stengel, R.; Sun, P. Effectiveness of Emollients in the Prevention of Atopic Dermatitis in Infants: A Meta-Analysis. Dermatology 2021, 238, 711–716. [Google Scholar] [CrossRef]
- Priyadarshi, M.; Balachander, B.; Gupta, S.; Sankar, M.J. Topical emollient application in term healthy newborns: A systematic review. J. Glob. Health 2022, 12, 12002. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.M.; Phillips, R.; Brown, S.J.; Cro, S.; Cornelius, V.; Carlsen, K.C.L.; O Skjerven, H.; Rehbinder, E.M.; Lowe, A.J.; Dissanayake, E.; et al. Skin care interventions in infants for preventing eczema and food allergy. Cochrane Database Syst. Rev. 2022, 11, CD013534. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Hu, F.; Tang, H.; Jiang, F.; Sang, Y.; Hong, Y.; Wang, Q.; Nuer, K.; Kang, X. Systematic review and network meta-analysis of different types of emollient for the prevention of atopic dermatitis in infants. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 501–510. [Google Scholar] [CrossRef]
- Zhong, Y.; Samuel, M.; van Bever, H.; Tham, E.H. Emollients in infancy to prevent atopic dermatitis: A systematic review and meta-analysis. Allergy 2022, 77, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.M.; Cro, S.; Van Vogt, E.; Cornelius, V.; Carlsen, K.C.L.; Skjerven, H.O.; Rehbinder, E.M.; Lowe, A.; Dissanayake, E.; Shimojo, N.; et al. Skincare interventions in infants for preventing eczema and food allergy: A cochrane systematic review and individual participant data meta-analysis. Clin. Exp. Allergy 2021, 51, 402–418. [Google Scholar] [CrossRef]
- Chalmers, J.R.; Haines, R.H.; E Bradshaw, L.; A Montgomery, A.; Thomas, K.S.; Brown, S.J.; Ridd, M.J.; Lawton, S.; Simpson, E.L.; Cork, M.J.; et al. Daily emollient during infancy for prevention of eczema: The BEEP randomised controlled trial. Lancet 2020, 395, 962–972. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]
- Serup, J.; Winther, A.; Blichmann, C.W. Effects of repeated application of a moisturizer. Acta Derm Venereol. 1989, 69, 457–459. [Google Scholar]
- Kunkiel, K.; Sojewska, M.; Feleszko, W. Contact haptens in emollients marketed in two European countries (Poland and Spain). Allergol. Immunopathol. 2020, 48, 814–818. [Google Scholar] [CrossRef]
- Sach, T.H.; Lartey, S.T.; Davies, C.; Chalmers, J.R.; Haines, R.H.; Bradshaw, L.E.; Montgomery, A.A.; Thomas, K.S.; Brown, S.J.; Ridd, M.J.; et al. Emollients for preventing atopic eczema: Cost-effectiveness analysis of the BEEP trial. Clin. Exp. Allergy 2023, 53, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Cabout, E.; Eymere, S.; Launois, R.; Séité, S.; Delvigne, V.; Taïeb, C.; Reguai, Z. Cost-effectiveness of Emollients in the Prevention of Relapse among French Patients with Atopic Dermatitis. Acta Derm.-Venereol. 2021, 101, adv00509. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.C.; Visitsunthorn, K.; Ong, P.Y. Genetic/Environmental Contributions and Immune Dysregulation in Children with Atopic Dermatitis. J. Asthma Allergy 2022, 15, 1681–1700. [Google Scholar] [CrossRef] [PubMed]
| Article | Number of Articles and Participants | Population | Intervention | Relative Effect | Conclusion |
|---|---|---|---|---|---|
| Xu D et al. (2022) [42] | 9 RCT Intervention = 1483 Control = 1509 | 0–12 months | Daily use of emollient vs. no regular administration | RR = 0.7 CI = 0.48–1.01 | No statistically significant difference in incidence rate of AD |
| Priyadarshi M et al. (2022) [43] | 2 RCT N = 1408 Intervention = 695 Control = 713 | 0–28 days on-term babies AD diagnosis up to 1 year No risk factors of AD | Emollient application vs. no emollient application | RR = 1.29 CI = 0.96–1.72 | No difference in the incidence of AD at 12 months of age |
| Priyadarshi M et al. (2022) [43] | 11 RCT N = 1988 Intervention = 1015 Control = 1022 | 0–28 days on-term babies AD diagnosis up to 1 year Risk factors of AD | Emollient appliance after bathing, at least four days a week vs. | RR = 0.74 CI = 0.55–1.00 | Intervention probably lowers the risk of atopic dermatitis among ‘at risk’ newborns |
| Kelleher MM et al. (2022) [44] | 33 RCT N = 25,827 | 0–14 days almost all participants | Skin barrier intervention versus standard care or no skin-care intervention | RR = 1.03 CI = 0.81–1.31 | Skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema, and probably increase risk of skin infection. |
| Liang J et al. (2023) [45] | 11 RCT N = 3483 Intervention = 1740 Control = 1743 | 0–12 months | Early application of emollients vs. no treatment in high-risk infants | RR = 0.64 CI = 0.47–0.88 | Early application of emollients is an effective strategy for preventing AD development in high-risk infants |
| Zhong Y et al. (2022) [46] | 2 RCT N = 1349 Intervention = 713 Control = 716 | 0–6 weeks general population | prophylactic emollient treatment vs. placebo or no treatment | RR = 0.84 CI = 0.64–1.01 | No significant reduction in the development of AD |
| Zhong Y et al. (2022) [46] | 8 RCT N = 2158 Intervention = 1033 Control = 955 | 0–6 weeks high risk for AD, based on strong family history | prophylactic emollient treatment vs. placebo or no treatment | RR = 0.75 CI = 0.43–0.81 | significant benefit of prophylactic emollients in the high-risk population |
| Kelleher MM et al. (2021) [47] | 7 RCT N= 3075 Intervention = 1489 Control = 1586 | 0–12 months | Skin care intervention compared to standard skin-care or no skin-care intervention | RR = 1.03 CI= 0.81–1.31 | Skincare interventions probably do not change risk of eczema but they probably increase risk of local skin infections, and may increase risk of infant slippage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grześk-Kaczyńska, M.; Petrus-Halicka, J.; Kaczyński, S.; Bartuzi, Z.; Ukleja-Sokołowska, N. Should Emollients Be Recommended for the Prevention of Atopic Dermatitis?—New Evidence and Current State of Knowledge. J. Clin. Med. 2024, 13, 863. https://doi.org/10.3390/jcm13030863
Grześk-Kaczyńska M, Petrus-Halicka J, Kaczyński S, Bartuzi Z, Ukleja-Sokołowska N. Should Emollients Be Recommended for the Prevention of Atopic Dermatitis?—New Evidence and Current State of Knowledge. Journal of Clinical Medicine. 2024; 13(3):863. https://doi.org/10.3390/jcm13030863
Chicago/Turabian StyleGrześk-Kaczyńska, Magdalena, Justyna Petrus-Halicka, Szymon Kaczyński, Zbigniew Bartuzi, and Natalia Ukleja-Sokołowska. 2024. "Should Emollients Be Recommended for the Prevention of Atopic Dermatitis?—New Evidence and Current State of Knowledge" Journal of Clinical Medicine 13, no. 3: 863. https://doi.org/10.3390/jcm13030863
APA StyleGrześk-Kaczyńska, M., Petrus-Halicka, J., Kaczyński, S., Bartuzi, Z., & Ukleja-Sokołowska, N. (2024). Should Emollients Be Recommended for the Prevention of Atopic Dermatitis?—New Evidence and Current State of Knowledge. Journal of Clinical Medicine, 13(3), 863. https://doi.org/10.3390/jcm13030863







