Exploring the Impact of Glycemic Control on Diabetic Retinopathy: Emerging Models and Prognostic Implications
Abstract
1. Introduction
1.1. Brief Overview of Type 1 Diabetes (T1D)
1.2. Importance of Understanding T1D and Its Microangiopathic Complications
1.3. Advancements in Treatment, Research Gaps, and Future Directions
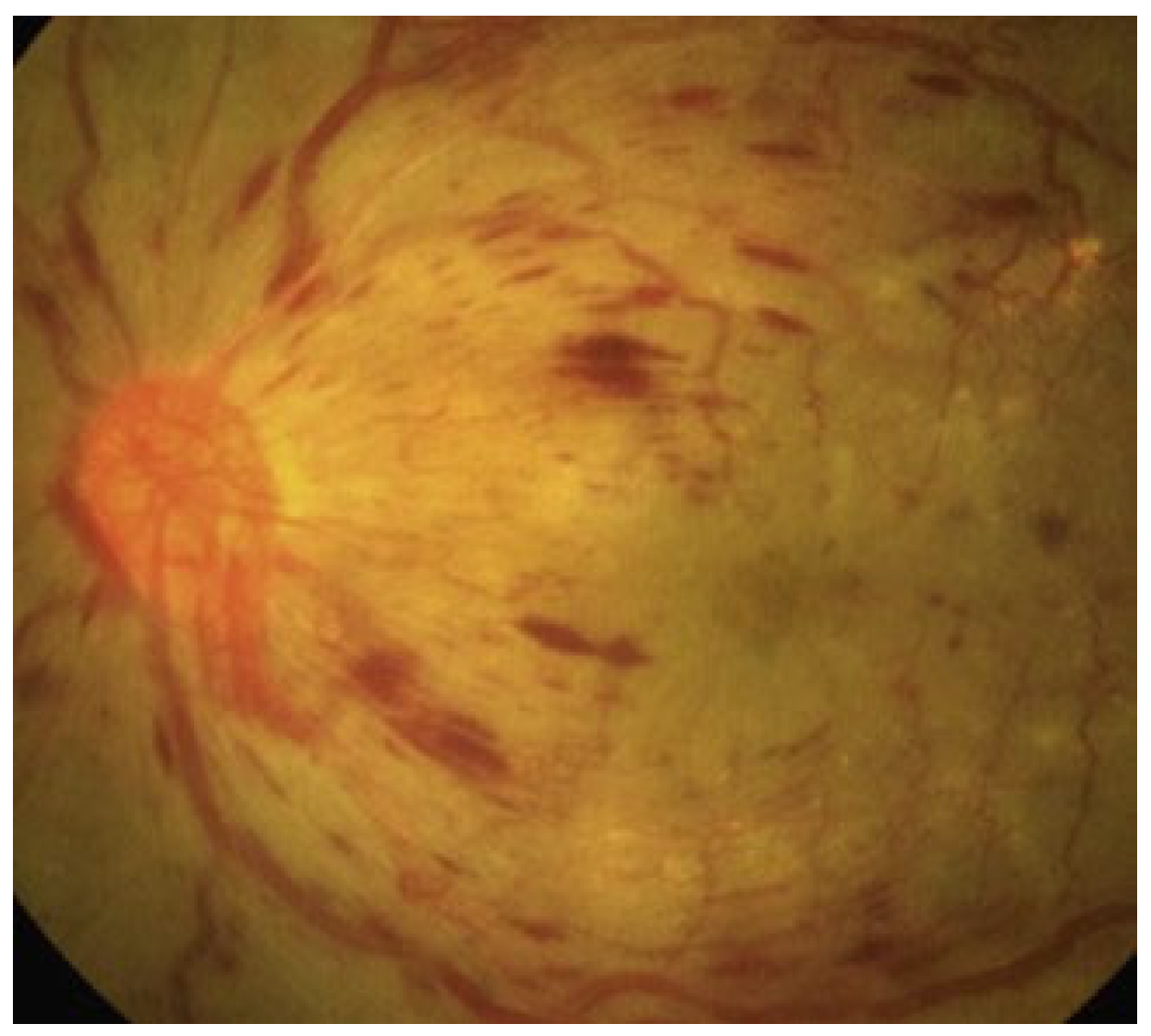
2. Diabetic Retinopathy: Clinical Features and Stages
2.1. Non-Proliferative Diabetic Retinopathy (NPDR) and Proliferative Diabetic Retinopathy (PDR)
2.2. Diabetic Macular Edema (DME)
2.3. Role of Dysglycemia in the Pathogenesis of Diabetic Retinopathy
2.4. Role of Inflammation and Retinal Degeneration in the Pathogenesis of Diabetic Retinopathy
3. Advancements in Diagnostic Techniques of Diabetic Retinopathy
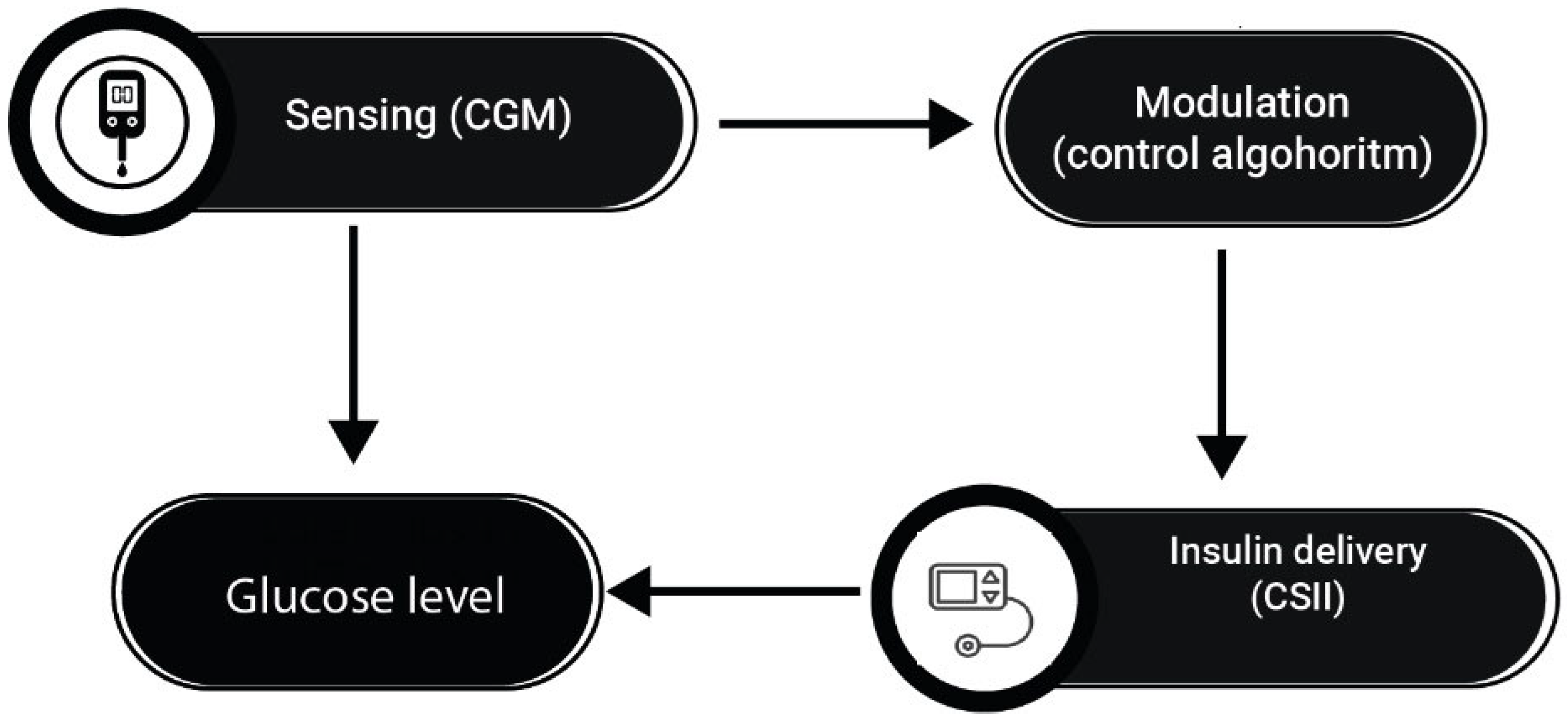
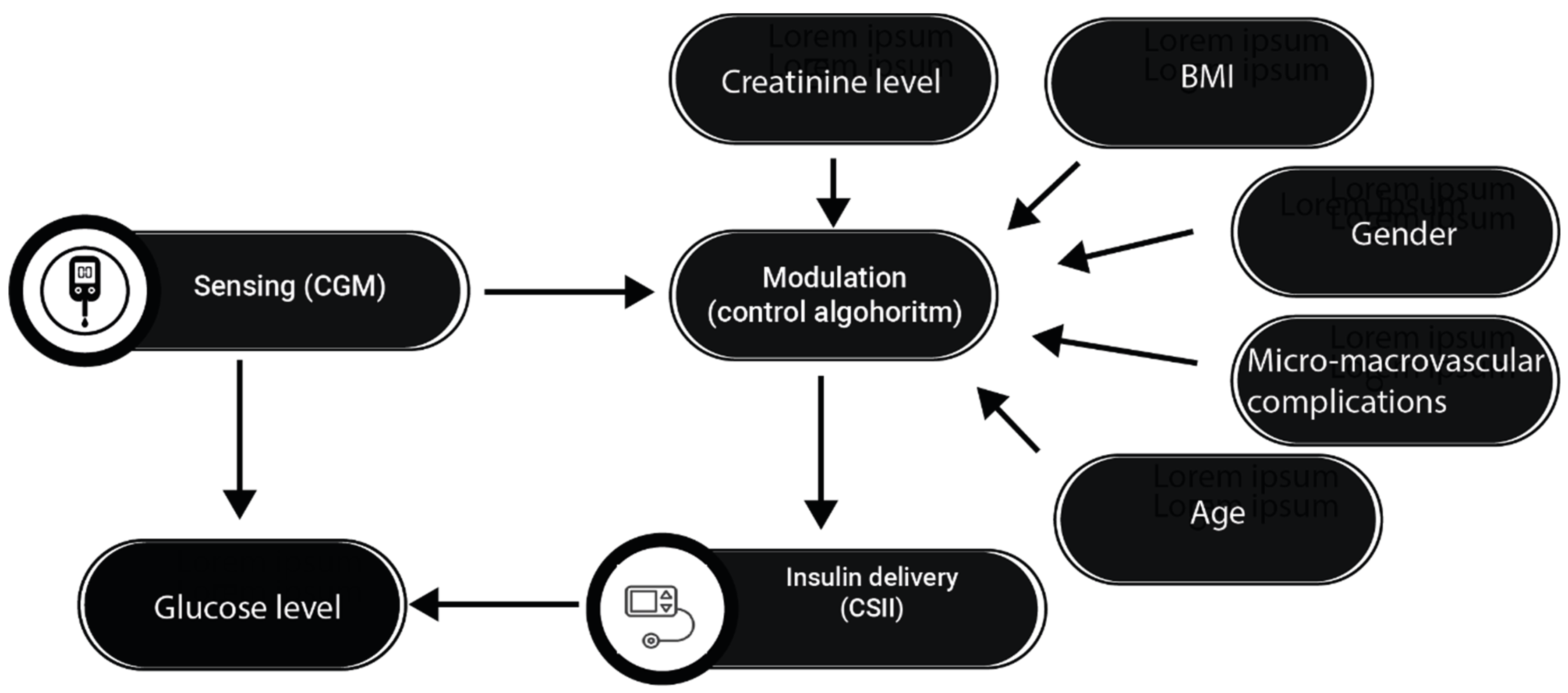
4. Advanced Hybrid Closed-Loop (AHCL) Systems and T1D
4.1. Intensive Insulin Therapy and Use of Continuous Glucose Monitors
4.2. Continuous Subcutaneous Insulin Infusion (CSII)
4.3. HCLSs and Glycemic Variability
5. Technological Advancements in Diabetes Management: A Gateway to Preventing Diabetic Retinopathy and Other Microangiopatic Complications of T1D
5.1. Benefits of Using Continuous Glucose Monitors on Diabetic Retinopathy
5.2. Benefits of Using HCLSs and Insulin Pumps on Diabetic Retinopathy
6. Emerging Research and Future Perspectives
6.1. Open-Source HCLSs
6.2. Prospects for Future Research and Development
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Graham, D.; Ogle, F.W.; Gregory, G.A.; Maniam, J. Type 1 Diabetes Estimates in Children and Adults; International Diabetes Federation: Brussels, Belgium, 2023; Available online: https://diabetesatlas.org/atlas/t1d-index-2022/?dlmodal=active&dlsrc=https%3A%2F%2Fdiabetesatlas.org%2Fidfawp%2Fresource-files%2F2022%2F12%2FIDF-T1D-Index-Report.pdf (accessed on 4 January 2024).
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, S.; Timmins, P.; Hasan, S.S.; Babar, Z.-U. Cost analysis of type 2 diabetes mellitus treatment in economically developed countries. Expert Rev. Pharmacoeconom. Outcomes Res. 2019, 19, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.A.; Mohn, A.; Chiarelli, F. Technology and the issue of cost/benefit in diabetes. Diabetes/Metab. Res. Rev. 2009, 25, S34–S44. [Google Scholar] [CrossRef] [PubMed]
- Ogle, G.D.; von Oettingen, J.E.; Middlehurst, A.C.; Hanas, R.; Orchard, T.J. Levels of type 1 diabetes care in children and adolescents for countries at varying resource levels. Pediatr. Diabetes 2019, 20, 93–98. [Google Scholar] [CrossRef]
- Petrie, D.; Lung, T.W.C.; Rawshani, A.; Palmer, A.J.; Svensson, A.-M.; Eliasson, B.; Clarke, P. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia 2016, 59, 1167–1176. [Google Scholar] [CrossRef]
- Rawshani, A.; Franzen, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjornsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J.; Hanson, K.; Jain, A.B.; Kichler, J.C.; Mehta, G.; Melamed, O.C.; Vallis, M.; Bajaj, H.S.; Barnes, T.; Gilbert, J.; et al. Diabetes and Mental Health. Can. J. Diabetes 2023, 47, 308–344. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 2016, 39, 686–693. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care 2016, 39, 1378–1383. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Han, K.; Kim, B.; Lee, S.-E.; Jun, J.E.; Ahn, J.; Kim, G.; Jin, S.-M.; Kim, J.H. Risk of early mortality and cardiovascular disease in type 1 diabetes: A comparison with type 2 diabetes, a nationwide study. Cardiovasc. Diabetol. 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [PubMed]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in diabetes. Diabetes Care 2004, 27, S84–S87. [Google Scholar] [CrossRef] [PubMed]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; De Boer, I.H. Kidney Disease and Increased Mortality Risk in Type 2 Diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Zhang, P.; Wirth, K.E.; Lee, P.P.; Hoerger, T.J.; McCall, N.; Klein, R.; Tielsch, J.M.; Vijan, S.; Saaddine, J. The Economic Burden of Major Adult Visual Disorders in the United States. Arch. Ophthalmol. 2006, 124, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Kochar, A.; Saini, D.; Poonia, R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J. Ophthalmol. 2021, 69, 3364–3368. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z. Mechanistic Pathogenesis of Endothelial Dysfunction in Diabetic Nephropathy and Retinopathy. Front. Endocrinol. 2022, 13, 816400. [Google Scholar] [CrossRef]
- Ng, S.M.; Wright, N.P.; Yardley, D.; Campbell, F.; Randell, T.; Trevelyan, N.; Ghatak, A.; Hindmarsh, P.C. Real world use of hybrid-closed loop in children and young people with type 1 diabetes mellitus—A National Health Service pilot initiative in England. Diabet. Med. 2023, 40, e15015. [Google Scholar] [CrossRef]
- Rudnisky, C.J.; Hinz, B.J.; Tennant, M.T.; de Leon, A.R.; Greve, M.D. High-resolution stereoscopic digital fundus photography versus contact lens biomicroscopy for the detection of clinically significant macular edema. Ophthalmology 2002, 109, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Varma, R.; Bressler, N.M.; Doan, Q.V.; Gleeson, M.; Danese, M.; Bower, J.K.; Selvin, E.; Dolan, C.; Fine, J.; Colman, S.; et al. Prevalence of and Risk Factors for Diabetic Macular Edema in the United States. JAMA Ophthalmol. 2014, 132, 1334–1340. [Google Scholar] [CrossRef]
- Aiello, L.P. Diabetic Retinopathy and Other Ocular Findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 2014, 37, 17–23. [Google Scholar] [CrossRef]
- Kohner, E.M. Microvascular disease: What does the UKPDS tell us about diabetic retinopathy? Diabet. Med. 2008, 25, 20–24. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Bek, T. Diameter Changes of Retinal Vessels in Diabetic Retinopathy. Curr. Diabetes Rep. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Romeo, G.; Liu, W.-H.; Asnaghi, V.; Kern, T.S.; Lorenzi, M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002, 51, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.; Chekarova, I.; Ejaz, A.; Sohail, A.; Lim, C.W. Importance of pericytes and mechanisms of pericyte loss during diabetic retinopathy. Diabetes Obes. Metab. 2008, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Porta, M. Pericyte Loss in Diabetic Retinopathy: Mechanisms and Consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Huang, H.; He, J.; Johnson, D.; Wei, Y.; Liu, Y.; Wang, S.; Lutty, G.A.; Duh, E.J.; Carmeliet, P.; Semba, R.D. Deletion of Placental Growth Factor Prevents Diabetic Retinopathy and Is Associated with Akt Activation and HIF1α-VEGF Pathway Inhibition. Diabetes 2015, 64, 200–212. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of NADPH oxidase 4. Diabetes 2010, 59, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular Endothelial Growth Factor in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.; Zhang, J.; Muto, T.; Kim, D.; Sarthy, V.P.; Roy, S. High Glucose Induces Mitochondrial Dysfunction in Retinal Müller Cells: Implications for Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2017, 58, 2915–2921. [Google Scholar] [CrossRef]
- Guo, C.; Deshpande, M.; Niu, Y.; Kachwala, I.; Flores-Bellver, M.; Megarity, H.; Nuse, T.; Babapoor-Farrokhran, S.; Ramada, M.; Sanchez, J.; et al. HIF-1α accumulation in response to transient hypoglycemia may worsen diabetic eye disease. Cell Rep. 2023, 42, 111976. [Google Scholar] [CrossRef]
- Park, J.Y.; Hwang, J.H.; Kang, M.J.; Sim, H.E.; Kim, J.S.; Ko, K.S. Effects of glycemic variability on the progression of diabetic retinopathy among patients with type 2 diabetes. Retina 2021, 41, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-R.; Chen, Y.-T.; Sheu, W.H.-H. Glycemic variability and diabetes retinopathy: A missing link. J. Diabetes Its Complicat. 2015, 29, 302–306. [Google Scholar] [CrossRef]
- Bombaci, B.; Passanisi, S.; Alibrandi, A.; D’arrigo, G.; Patroniti, S.; Averna, S.; Salzano, G.; Lombardo, F. One-Year Real-World Study on Comparison among Different Continuous Subcutaneous Insulin Infusion Devices for the Management of Pediatric Patients with Type 1 Diabetes: The Supremacy of Hybrid Closed-Loop Systems. Int. J. Environ. Res. Public Health 2022, 19, 10293. [Google Scholar] [CrossRef]
- Guo, K.; Li, J.; Zhang, L.; Ye, J.; Fan, L.; Ding, Z.; Zhou, Q.; Li, X.; Yang, L.; Zhou, Z. Comparing the effectiveness of continuous subcutaneous insulin infusion with multiple daily insulin injection for patients with type 1 diabetes mellitus evaluated by retrospective continuous glucose monitoring: A real-world data analysis. Front. Public Health 2022, 10, 990281. [Google Scholar] [CrossRef]
- Lunati, M.E.; Morpurgo, P.S.; Rossi, A.; Gandolfi, A.; Cogliati, I.; Bolla, A.M.; Plebani, L.; Vallone, L.; Montefusco, L.; Pastore, I.; et al. Hybrid Close-Loop Systems Versus Predictive Low-Glucose Suspend and Sensor-Augmented Pump Therapy in Patients with Type 1 Diabetes: A Single-Center Cohort Study. Front. Endocrinol. 2022, 13, 816599. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Y.; Wang, F. The Protective Effect of HIF-1α in T Lymphocytes on Cardiac Damage in Diabetic Mice. Ann. Clin. Lab. Sci. 2016, 46, 32–43. [Google Scholar]
- Barber, A.J.; Lieth, E.; A Khin, S.; A Antonetti, D.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Agrawal, S.; Mishro, P.K.; Panda, R.; Dora, L.; Pachori, R.B. A Review on Retinal Blood Vessel Enhancement and Segmentation Techniques for Color Fundus Photography. Crit. Rev. Biomed. Eng. 2024, 52, 41–69. [Google Scholar] [CrossRef]
- Kylstra, J.A.; Brown, J.C.; Jaffe, G.J.; A Cox, T.; Gallemore, R.; Greven, C.M.; Hall, J.G.; E Eifrig, D. The importance of fluorescein angiography in planning laser treatment of diabetic macular edema. Ophthalmology 1999, 106, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Bini, S.; Midena, G.; Berton, M.; Pilotto, E.; Midena, E. Hyperreflective Intraretinal Spots in Diabetics without and with Nonproliferative Diabetic Retinopathy: An In Vivo Study Using Spectral Domain OCT. J. Diabetes Res. 2013, 2013, 491835. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-R.; Kim, Y.H.; Ha, S.J.; Byeon, H.-E.; Cho, C.-H.; Kim, J.H.; Lee, K. Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. J. Diabetes Res. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of diabetic macular edema with optical coherence tomography. Arch. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Shirasawa, M.; Otsuka, H.; Sonoda, Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina 2014, 34, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Torresin, T.; Berton, M.; Bini, S.; Convento, E.; Midena, E. Diabetic Macular Edema with and without Subfoveal Neuroretinal Detachment: Two Different Morphologic and Functional Entities. Arch. Ophthalmol. 2017, 181, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994, 125, 177–188. [Google Scholar] [CrossRef]
- Pratley, R.E.; Kanapka, L.G.; Rickels, M.R.; Ahmann, A.; Aleppo, G.; Beck, R.; Bhargava, A.; Bode, B.W.; Carlson, A.; Chaytor, N.S.; et al. Effect of Continuous Glucose Monitoring on Hypoglycemia in Older Adults with Type 1 Diabetes: A Randomized Clinical Trial. Jama 2020, 323, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, X.; Huang, J. Efficacy and Safety of Insulin Degludec versus Insulin Glargine: A Systematic Review and Meta-Analysis of Fifteen Clinical Trials. Int. J. Endocrinol. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Sherr, J.L.; Heinemann, L.; Fleming, G.A.; Bergenstal, R.M.; Bruttomesso, D.; Hanaire, H.; Holl, R.W.; Petrie, J.R.; Peters, A.L.; Evans, M. Automated insulin delivery: Benefits, challenges, and recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetologia 2023, 66, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, S.; Andersen, G.S.; Jørgensen, M.E.; Ridderstråle, M.; Vistisen, D.; Andersen, H.U. Treatment Modality–Dependent Risk of Diabetic Ketoacidosis in Patients with Type 1 Diabetes: Danish Adult Diabetes Database Study. Diabetes Technol. Ther. 2018, 20, 229–234. [Google Scholar] [CrossRef]
- Cobelli, C.; Renard, E.; Kovatchev, B. Artificial Pancreas: Past, Present, Future. Diabetes 2011, 60, 2672–2682. [Google Scholar] [CrossRef]
- Infante, M.; Baidal, D.A.; Rickels, M.R.; Fabbri, A.; Skyler, J.S.; Alejandro, R.; Ricordi, C. Dual-hormone artificial pancreas for management of type 1 diabetes: Recent progress and future directions. Artif. Organs 2021, 45, 968–986. [Google Scholar] [CrossRef]
- Champakanath, A.; Akturk, H.K.; Alonso, G.T.; Snell-Bergeon, J.K.; Shah, V.N. Continuous Glucose Monitoring Initiation within First Year of Type 1 Diabetes Diagnosis Is Associated with Improved Glycemic Outcomes: 7-Year Follow-Up Study. Diabetes Care 2022, 45, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, X.; Zhou, J.; Zhang, L.; Mo, Y.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Vigersky, R.A.; et al. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, with Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care 2018, 41, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; Kanapka, L.G.; Akturk, H.K.; Polsky, S.; Forlenza, G.P.; Kollman, C.; Beck, R.W.; Snell-Bergeon, J. Time in Range is Associated with Incident Diabetic Retinopathy in Adults with Type 1 Diabetes: A Longitudinal Study. Diabetes Technol. Ther. 2023. [Google Scholar] [CrossRef]
- Sartore, G.; Chilelli, N.C.; Burlina, S.; Lapolla, A. Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol. 2013, 50, 437–442. [Google Scholar] [CrossRef]
- Zhu, D.-D.; Cheng, X.-X.; Ding, N. Time in range as a useful marker for evaluating retinal functional changes in diabetic retinopathy patients. Int. J. Ophthalmol. 2023, 16, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Arunachalum, S.; Velado, K.; Vigersky, R.A.; Cordero, T.L. Glycemic Outcomes During Real-World Hybrid Closed-Loop System Use by Individuals with Type 1 Diabetes in the United States. J. Diabetes Sci. Technol. 2023, 17, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Beato-Víbora, P.I.; Gallego-Gamero, F.; Ambrojo-López, A. Real-world outcomes with different technology modalities in type 1 diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1845–1850. [Google Scholar] [CrossRef]
- Guo, Y.M.; Zheng, X.M.; He, H.M.; Zheng, S.M. Retinal Microvasculopathy with Different Insulin Infusion Therapies in Children with Type 1 Diabetes Mellitus without Clinical Diabetic Retinopathy. Retina 2023. [Google Scholar] [CrossRef]
- Kesavadev, J.; Srinivasan, S.; Saboo, B.; Krishna, M.B.; Krishnan, G. The Do-It-Yourself Artificial Pancreas: A Comprehensive Review. Diabetes Ther. 2020, 11, 1217–1235. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tecce, N.; Cennamo, G.; Rinaldi, M.; Costagliola, C.; Colao, A. Exploring the Impact of Glycemic Control on Diabetic Retinopathy: Emerging Models and Prognostic Implications. J. Clin. Med. 2024, 13, 831. https://doi.org/10.3390/jcm13030831
Tecce N, Cennamo G, Rinaldi M, Costagliola C, Colao A. Exploring the Impact of Glycemic Control on Diabetic Retinopathy: Emerging Models and Prognostic Implications. Journal of Clinical Medicine. 2024; 13(3):831. https://doi.org/10.3390/jcm13030831
Chicago/Turabian StyleTecce, Nicola, Gilda Cennamo, Michele Rinaldi, Ciro Costagliola, and Annamaria Colao. 2024. "Exploring the Impact of Glycemic Control on Diabetic Retinopathy: Emerging Models and Prognostic Implications" Journal of Clinical Medicine 13, no. 3: 831. https://doi.org/10.3390/jcm13030831
APA StyleTecce, N., Cennamo, G., Rinaldi, M., Costagliola, C., & Colao, A. (2024). Exploring the Impact of Glycemic Control on Diabetic Retinopathy: Emerging Models and Prognostic Implications. Journal of Clinical Medicine, 13(3), 831. https://doi.org/10.3390/jcm13030831







