Recurrence following Resection of Intraductal Papillary Mucinous Neoplasms: A Systematic Review to Guide Surveillance
Abstract
1. Background
2. Methods
2.1. Literature Search Strategy
2.2. Design and Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Literature Search and Study Selection
3.2. Baseline Characteristics
3.3. Type of Resection
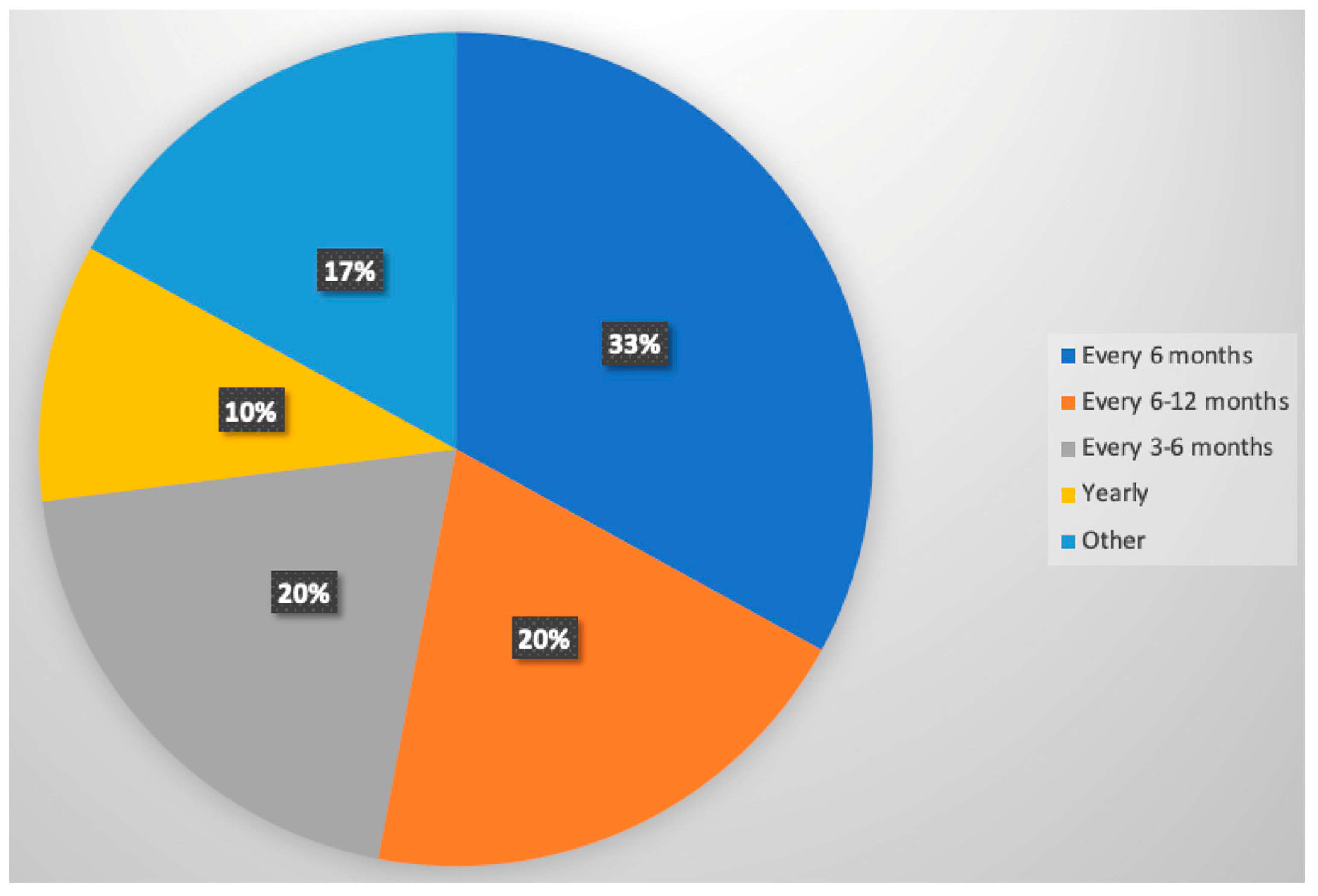
3.4. Patterns of Post-Resection Surveillance
3.5. Recurrence Rates and Patterns
3.6. Disease-Free and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aronsson, L.; Andersson, R.; Ansari, D. Intraductal papillary mucinous neoplasm of the pancreas–epidemiology, risk factors, diagnosis, and management. Scand. J. Gastroenterol. 2017, 52, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Malecka-Wojciesko, E. Pancreatic Incidentaloma. J. Clin. Med. 2022, 11, 4648. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas TESG. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cameron, J.L.; Ahuja, N.; Makary, M.A.; Hirose, K.; Choti, M.A.; Schulick, R.D.; Hruban, R.H.; Pawlik, T.M.; Wolfgang, C.L. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J. Am. Coll. Surg. 2013, 216, 657–667. [Google Scholar] [CrossRef]
- Nakamura, M.; Miyasaka, Y.; Sadakari, Y.; Date, K.; Ohtsuka, T. Comparison of guidelines for intraductal papillary mucinous neoplasm: What is the next step beyond the current guidelines? Ann. Gastroenterol. Surg. 2017, 1, 90–98. [Google Scholar] [CrossRef]
- Niedergethmann, M.; Grützmann, R.; Hildenbrand, R.; Dittert, D.; Aramin, N.; Franz, M.; Dobrowolski, F.; Post, S.; Saeger, H. Outcome of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas (IPMN): A 10-year experience. World J. Surg. 2008, 32, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Schnelldorfer, T.; Sarr, M.G.; Nagorney, D.M.; Zhang, L.; Smyrk, T.C.; Qin, R.; Chari, S.T.; Farnell, M.B. Experience With 208 Resections for Intraductal Papillary Mucinous Neoplasm of the Pancreas. Arch. Surg. 2008, 143, 639–646. [Google Scholar] [CrossRef]
- Nagai, K.; Doi, R.; Kida, A.; Kami, K.; Kawaguchi, Y.; Ito, T.; Sakurai, T.; Uemoto, S. Intraductal Papillary Mucinous Neoplasms of the Pancreas: Clinicopathologic Characteristics and Long-Term Follow-Up After Resection. World J. Surg. 2008, 32, 271–278. [Google Scholar] [CrossRef]
- Landa, J.; Allen, P.; D’Angelica, M.; Schwartz, L.H. Recurrence Patterns of Intraductal Papillary Mucinous Neoplasms of the Pancreas on Enhanced Computed Tomography. J. Comput. Assist. Tomogr. 2009, 33, 838–843. Available online: https://journals.lww.com/jcat/Fulltext/2009/11000/Recurrence_Patterns_of_Intraductal_Papillary.5.aspx (accessed on 10 October 2021). [CrossRef]
- Nakagohri, T.; Kinoshita, T.; Konishi, M.; Takahashi, S.; Gotohda, N.; Kobayashi, S.; Kojima, M.; Miyauchi, H.; Asano, T. Inferior head resection of the pancreas for intraductal papillary mucinous neoplasms. J. Hepatobiliary Pancreat. Sci. 2010, 17, 798–802. [Google Scholar] [CrossRef]
- Crippa, S.; Fernández-Del Castillo, C.; Salvia, R.; Finkelstein, D.; Bassi, C.; Domínguez, I.; Muzikansky, A.; Thayer, S.P.; Falconi, M.; Mino–Kenudson, M.; et al. Mucin-producing neoplasms of the pancreas: An analysis of distinguishing clinical and epidemiologic characteristics. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2010, 8, 213–219. [Google Scholar] [CrossRef]
- Lubezky, N.; Ben-Haim, M.; Nakache, R.; Lahat, G.; Blachar, A.; Brazowski, E.; Santo, E.; Klausner, J.M. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J. Surg. 2010, 34, 126–132. [Google Scholar] [CrossRef]
- Fujii, T.; Kato, K.; Kodera, Y.; Kanda, M.; Nagai, S.; Yamada, S.; Kanzaki, A.; Sugimoto, H.; Nomoto, S.; Takeda, S.; et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery 2010, 148, 285–290. [Google Scholar] [CrossRef]
- Cheon, Y.K.; Cho, Y.D.; Jeon, S.R.; Moon, J.H.; Jeong, S.W.; Hur, K.Y.; So, Y.; Lee, J.S. Pancreatic Resection Guided by Preoperative Intraductal Ultrasonography for Intraductal Papillary Mucinous Neoplasm. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 1963–1969. Available online: https://journals.lww.com/ajg/Fulltext/2010/09000/Pancreatic_Resection_Guided_by_Preoperative.11.aspx (accessed on 10 October 2021). [CrossRef]
- Park, J.; Lee, K.T.; Jang, T.H.; Seo, Y.W.; Lee, K.H.; Lee, J.K.; Jang, K.-T.; Heo, J.S.; Choi, S.H.; Choi, D.W.; et al. Risk factors associated with the postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2011, 40, 46–51. [Google Scholar] [CrossRef]
- Cuillerier, E.; Cellier, C.; Palazzo, L.; Devière, J.; Wind, P.; Rickaert, F.; Cugnenc, P.-H.; Cremer, M.; Barbier, J.-P. Outcome after surgical resection of intraductal papillary and mucinous tumors of the pancreas. Am. J. Gastroenterol. 2000, 95, 441–445. [Google Scholar] [CrossRef]
- Fujii, T.; Kanda, M.; Kodera, Y.; Nagai, S.; Sahin, T.T.; Kanzaki, A.; Yamada, S.; Sugimoto, H.; Nomoto, S.; Morita, S.; et al. Comparison of pancreatic head resection with segmental duodenectomy and pylorus-preserving pancreatoduodenectomy for benign and low-grade malignant neoplasms of the pancreatic head. Pancreas 2011, 40, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Meyer, J.E.; Waters, J.A.; Al-Haddad, M.; DeWitt, J.; Sherman, S.; Lillemoe, K.D.; Schmidt, C.M. Outcome of the pancreatic remnant following segmental pancreatectomy for non-invasive intraductal papillary mucinous neoplasm. HPB 2011, 13, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Kono, H.; Tanabe, R.; Nagayoshi, Y.; Mori, Y.; Sadakari, Y.; Takahata, S.; Oda, Y.; Aishima, S.; Igarashi, H.; et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am. J. Surg. 2012, 204, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Passot, G.; Lebeau, R.; Hervieu, V.; Ponchon, T.; Pilleul, F.; Adham, M. Recurrences After Surgical Resection of Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Single-Center Study of Recurrence Predictive Factors. Pancreas 2012, 41, 137–141. Available online: https://journals.lww.com/pancreasjournal/Fulltext/2012/01000/Recurrences_After_Surgical_Resection_of.18.aspx (accessed on 10 October 2021). [CrossRef] [PubMed]
- Sahora, K.; Mino-Kenudson, M.; Brugge, W.; Thayer, S.P.; Ferrone, C.R.; Sahani, D.; Pitman, M.B.; Warshaw, A.L.; Lillemoe, K.D.; Fernandez-del Castillo, C.F. Branch duct intraductal papillary mucinous neoplasms: Does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann. Surg. 2013, 258, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Distler, M.; Kersting, S.; Niedergethmann, M.; Aust, D.E.; Franz, M.; Rückert, F.; Ehehalt, F.; Pilarsky, C.; Post, S.; Saeger, H.-D.; et al. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann. Surg. 2013, 258, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Frankel, T.L.; LaFemina, J.; Bamboat, Z.M.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Kingham, T.P.; Jarnagin, W.R.; Allen, P.J. Dysplasia at the surgical margin is associated with recurrence after resection of non-invasive intraductal papillary mucinous neoplasms. HPB 2013, 15, 814–821. [Google Scholar] [CrossRef]
- Winner, M.; Epelboym, I.; Remotti, H.; Lee, J.L.; Schrope, B.A.; Chabot, J.A.; Allendorf, J.D. Predictors of Recurrence in Intraductal Papillary Mucinous Neoplasm: Experience with 183 Pancreatic Resections. J. Gastrointest. Surg. 2013, 17, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Sahora, K.; Castillo, C.F.; Dong, F.; Marchegiani, G.; Thayer, S.P.; Ferrone, C.R.; Sahani, D.V.; Brugge, W.R.; Warshaw, A.L.; Lillemoe, K.D.; et al. Not all mixed-type intraductal papillary mucinous neoplasms behave like main-duct lesions: Implications of minimal involvement of the main pancreatic duct. Surgery 2014, 156, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Sauvanet, A.; Gaujoux, S.; Blanc, B.; Couvelard, A.; Dokmak, S.; Vullierme, M.-P.; Ruszniewski, P.; Belghiti, J.; Lévy, P. Parenchyma-sparing pancreatectomy for presumed noninvasive intraductal papillary mucinous neoplasms of the pancreas. Ann. Surg. 2014, 260, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Yadav, D.; Smyrk, T.C.; DiMagno, E.P.; Miller, L.J.; Raimondo, M.; Clain, J.E.; Norton, I.A.; Pearson, R.K.; Petersen, B.T.; et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology 2002, 123, 1500–1507. [Google Scholar] [CrossRef]
- Tamura, K.; Ohtsuka, T.; Ideno, N.; Aso, T.; Shindo, K.; Aishima, S.; Ohuchida, K.; Takahata, S.; Ushijima, Y.; Ito, T.; et al. Treatment strategy for main duct intraductal papillary mucinous neoplasms of the pancreas based on the assessment of recurrence in the remnant pancreas after resection: A retrospective review. Ann. Surg. 2014, 259, 360–368. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, S.C.; Song, K.-B.; Lee, J.H.; Hwang, D.W.; Park, K.-M.; Lee, Y.-J. Surgical outcomes of multifocal branch duct intraductal papillary mucinous neoplasms of pancreas. Korean J. Hepato-Biliary-Pancreatic Surg. 2014, 18, 152–158. [Google Scholar] [CrossRef][Green Version]
- Yuan, C.; Xiu, D.; Tao, M.; Ma, Z.; Jiang, B.; Li, Z.; Li, L.; Wang, L.; Wang, H.; Zhang, T. Data analysis of 36 cases with intraductal papillary mucinous neoplasm of the pancreas for their clinicopathological features, diagnosis, and treatment. Chin. Med. J. 2014, 127, 4087–4091. Available online: https://journals.lww.com/cmj/Fulltext/2014/12050/Data_analysis_of_36_cases_with_intraductal.16.aspx (accessed on 10 October 2021). [CrossRef] [PubMed]
- Kang, M.J.; Jang, J.-Y.; Lee, K.B.; Chang, Y.R.; Kwon, W.; Kim, S.-W. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: Implications for postoperative surveillance. Ann. Surg. 2014, 260, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Mino-Kenudson, M.; Ferrone, C.; Morales-Oyarvide, V.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F.-D. Patterns of Recurrence After Resection of IPMN. Ann. Surg. 2015, 262, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Mino-Kenudson, M.; Sahora, K.; Morales-Oyarvide, V.; Thayer, S.; Ferrone, C.; Warshaw, A.L.; Lillemoe, K.D.; Castillo, C.F.-D. IPMN involving the main pancreatic duct: Biology, epidemiology, and long-term outcomes following resection. Ann. Surg. 2015, 261, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Yogi, T.; Hijioka, S.; Imaoka, H.; Mizuno, N.; Hara, K.; Tajika, M.; Tanaka, T.; Ishihara, M.; Shimizu, Y.; Hosoda, W.; et al. Risk factors for postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas based on a long-term follow-up study: Proposals for follow-up strategies. J. Hepatobiliary Pancreat. Sci. 2015, 22, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Xourafas, D.; Tavakkoli, A.; Clancy, T.E.; Ashley, S.W. Noninvasive intraductal papillary mucinous neoplasms and mucinous cystic neoplasms: Recurrence rates and postoperative imaging follow-up. Surgery 2015, 157, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.K.; Ryu, J.K.; Chung, K.H.; Lee, B.S.; Park, J.K.; Lee, S.H.; Kim, Y.T. Risk Factors for Progression or Malignancy in Main-Duct and Mixed-Type Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2016, 45, 1027–1031. Available online: https://journals.lww.com/pancreasjournal/Fulltext/2016/08000/Risk_Factors_for_Progression_or_Malignancy_in.16.aspx (accessed on 10 October 2021). [CrossRef]
- Hirono, S.; Kawai, M.; Okada, K.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Ueno, M.; Yanagisawa, A.; Yamaue, H. Long-term surveillance is necessary after operative resection for intraductal papillary mucinous neoplasm of the pancreas. Surgery 2016, 160, 306–317. [Google Scholar] [CrossRef]
- D’Angelica, M.; Brennan, M.F.; Suriawinata, A.A.; Klimstra, D.; Conlon, K.C. Intraductal papillary mucinous neoplasms of the pancreas: An analysis of clinicopathologic features and outcome. Ann. Surg. 2004, 239, 400–408. [Google Scholar] [CrossRef]
- Miyasaka, Y.; Ohtsuka, T.; Tamura, K.; Mori, Y.; Shindo, K.; Yamada, D.; Takahata, S.; Ishigami, K.; Ito, T.; Tokunaga, S.; et al. Predictive Factors for the Metachronous Development of High-risk Lesions in the Remnant Pancreas After Partial Pancreatectomy for Intraductal Papillary Mucinous Neoplasm. Ann. Surg. 2016, 263, 1180–1187. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Kaneoka, Y.; Maeda, A.; Takayama, Y.; Onoe, S.; Isogai, M. Positive surgical margins in surgically treated unifocal and multifocal IPMN. Int. J. Surg. 2016, 28, 51–55. [Google Scholar] [CrossRef]
- Ridtitid, W.; DeWitt, J.M.; Schmidt, C.M.; Roch, A.; Stuart, J.S.; Sherman, S.; Al-Haddad, M.A. Management of branch-duct intraductal papillary mucinous neoplasms: A large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest. Endosc. 2016, 84, 436–445. [Google Scholar] [CrossRef]
- Marsoner, K.; Haybaeck, J.; Csengeri, D.; Waha, J.E.; Schagerl, J.; Langeder, R.; Mischinger, H.J.; Kornprat, P. Pancreatic resection for intraductal papillary mucinous neoplasm—A thirteen-year single center experience. BMC Cancer 2016, 16, 844. [Google Scholar] [CrossRef]
- Kimura, K.; Amano, R.; Ymazoe, S.; Ohira, G.; Nishio, K.; Hirakawa, K.; Ohira, M. The Clinical Indications for Limited Surgery of Intraductal Papillary Mucinous Neoplasms of the Pancreas. World J. Surg. 2017, 41, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Pea, A.; Yu, J.; Rezaee, N.; Luchini, C.; He, J.; Molin, M.D.; Griffin, J.F.; Fedor, H.; Fesharakizadeh, S.; Salvia, R.; et al. Targeted DNA Sequencing Reveals Patterns of Local Progression in the Pancreatic Remnant Following Resection of Intraductal Papillary Mucinous Neoplasm (IPMN) of the Pancreas. Ann. Surg. 2017, 266, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Dhar, V.K.; Merchant, N.B.; Patel, S.H.; Edwards, M.J.; Wima, K.; Imbus, J.; Abbott, D.E.; Weber, S.M.; Louie, R.; Kim, H.J.; et al. Does Surgical Margin Impact Recurrence in Noninvasive Intraductal Papillary Mucinous Neoplasms?: A Multi-institutional Study. Ann. Surg. 2018, 268, 469. Available online: https://journals.lww.com/annalsofsurgery/Fulltext/2018/09000/Does_Surgical_Margin_Impact_Recurrence_in.9.aspx (accessed on 10 October 2021). [CrossRef]
- Al Efishat, M.; Attiyeh, M.A.; Eaton, A.A.; Gönen, M.; Basturk, O.; Klimstra, D.; D’angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Balachandran, V.; et al. Progression Patterns in the Remnant Pancreas after Resection of Non-Invasive or Micro-Invasive Intraductal Papillary Mucinous Neoplasms (IPMN). Ann. Surg. Oncol. 2018, 25, 1752–1759. [Google Scholar] [CrossRef]
- Antoñanzas, J.; Cienfuegos, J.A.; Hurtado-Pardo, L.; Panadero, P.; Benito, A.; Pardo, F.; Rotellar, F.; Martí-Cruchaga, P.; Zozaya, G.; Valentí, V.; et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Clinicopathological features and long-term outcomes following a pancreatectomy. Rev. Española De Enfermedades Dig. 2018, 110, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Date, K.; Ohtsuka, T.; Nakamura, S.; Mochidome, N.; Mori, Y.; Miyasaka, Y.; Oda, Y.; Nakamura, M. Surveillance of patients with intraductal papillary mucinous neoplasm with and without pancreatectomy with special reference to the incidence of concomitant pancreatic ductal adenocarcinoma. Surgery 2018, 163, 291–299. [Google Scholar] [CrossRef]
- Salvia, R.; Fernández-del Castillo, C.; Bassi, C.; Thayer, S.P.; Falconi, M.; Mantovani, W.; Pederzoli, P.; Warshaw, A.L. Main-duct intraductal papillary mucinous neoplasms of the pancreas: Clinical predictors of malignancy and long-term survival following resection. Ann. Surg. 2004, 239, 677–678. [Google Scholar] [CrossRef]
- Majumder, S.; Philip, N.A.; Singh Nagpal, S.J.; Takahashi, N.; Mara, K.C.; Kendrick, M.L.; Smyrk, T.C.; Zhang, L.; Levy, M.J.; Gleeson, F.C.; et al. High-Grade Dysplasia in Resected Main-Duct Intraductal Papillary Mucinous Neoplasm (MD-IPMN) is Associated with an Increased Risk of Subsequent Pancreatic Cancer. Am. J. Gastroenterol. 2019, 114, 524–529. [Google Scholar] [CrossRef]
- Kwon, J.E.; Jang, K.-T.; Ryu, Y.; Kim, N.; Shin, S.H.; Heo, J.S.; Choi, D.W.; Han, I.W. Subtype of intraductal papillary mucinous neoplasm of the pancreas is important to the development of metachronous high-risk lesions after pancreatectomy. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Griffin, J.; Makary, M.A.; He, J.; Cameron, J.L.; Weiss, M.J.; Wood, L.D.; Goggins, M.; Wolfgang, C.L. Blood Type as a Predictor of High-Grade Dysplasia and Associated Malignancy in Patients with Intraductal Papillary Mucinous Neoplasms. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2019, 23, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Mizukami, Y.; Omori, Y.; Kin, T.; Yane, K.; Takahashi, K.; Ono, Y.; Sugitani, A.; Karasaki, H.; Shinohara, T.; et al. Metachronous intraductal papillary mucinous neoplasms disseminate via the pancreatic duct following resection. Mod. Pathol. 2020, 33, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Peng, L.; Jin, Z.; Sun, L.; Song, B. The pathological features and prognoses of intraductal papillary mucinous neoplasm and mucinous cystic neoplasm after surgical resection: A single institution series. World J. Surg. Oncol. 2020, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Habib, J.R.; Blair, A.; Rezaee, N.; Kinny-Köster, B.; Cameron, J.L.; Hruban, R.H.; Weiss, M.J.; Fishman, E.K.; Lafaro, K.J.; et al. Invasive and Non-Invasive Progression after Resection of Non-Invasive Intraductal Papillary Mucinous Neoplasms. Ann. Surg. 2020, 276, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Pflüger, M.J.; Griffin, J.F.; Hackeng, W.M.; Kawamoto, S.; Yu, J.; Chianchiano, P.; Shin, E.; Lionheart, G.; Tsai, H.L.; Wang, H.; et al. The Impact of Clinical and Pathological Features on Intraductal Papillary Mucinous Neoplasm Recurrence After Surgical Resection: Long-Term Follow-Up Analysis. Ann Surg. 2020, 275, 1165–1174. [Google Scholar] [CrossRef]
- Hirono, S.; Shimizu, Y.; Ohtsuka, T.; Kin, T.; Hara, K.; Kanno, A.; Koshita, S.; Hanada, K.; Kitano, M.; Inoue, H.; et al. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J. Gastroenterol. 2020, 55, 86–99. [Google Scholar] [CrossRef]
- Asano, T.; Nakamura, T.; Noji, T.; Okamura, K.; Tsuchikawa, T.; Ebihara, Y.; Nakanishi, Y.; Tanaka, K.; Matsui, A.; Shichinohe, T.; et al. Outcomes of limited resection for patients with intraductal papillary mucinous neoplasm of the pancreas: A single-center experience. Pancreatology 2020, 20, 1399–1405. [Google Scholar] [CrossRef]
- Takigawa, Y.; Kitago, M.; Matsui, J. Independent predictors of secondary invasive pancreatic remnant tumors after initial resection of an intraductal papillary mucinous neoplasm: A nationwide large-scale survey in Japan. Surg. Today 2020, 50, 1672–1680. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakamori, S.; Nakahira, S.; Tsujie, M.; Takahshi, Y.; Marubashi, S.; Miyamoto, A.; Takeda, Y.; Nagano, H.; Dono, K.; et al. Surgical Outcomes of Noninvasive and Minimally Invasive Intraductal Papillary-Mucinous Neoplasms of the Pancreas. Ann. Surg. Oncol. 2006, 13, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.B.; Beckman, R.M.; Habib, J.R.; Griffin, J.F.; Lafaro, K.; Burkhart, R.A.; Burns, W.; Weiss, M.J.; Cameron, J.L.; Wolfgang, C.L.; et al. Should non-invasive diffuse main-duct intraductal papillary mucinous neoplasms be treated with total pancreatectomy? HPB 2021, 24, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Sugimachi, K.; Mano, Y.; Matsumoto, Y.; Nakanoko, T.; Uehara, H.; Nakashima, Y.; Sugiyama, M.; Ota, M.; Morita, M.; Toh, Y. Neutrophil-to-lymphocyte Ratio as a Predictor of Malignancy of Intraductal Papillary Mucinous Neoplasms. Anticancer. Res. 2021, 41, 1663–1669. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, Y.; Kang, J.S.; Choi, Y.J.; Byun, Y.; Kim, H.; Lee, K.B.; Kim, H.; Kwon, W.; Jang, J.-Y. Fate of Patients With Intraductal Papillary Mucinous Neoplasms of Pancreas After Resection According to the Pathology and Margin Status: Continuously Increasing Risk of Recurrence Even After Curative Resection Suggesting Necessity of Lifetime Surveillance. Ann. Surg. 2022, 276, e231–e238. Available online: https://journals.lww.com/annalsofsurgery/Fulltext/9000/Fate_of_Patients_With_Intraductal_Papillary.94199.aspx (accessed on 10 October 2021). [CrossRef]
- Rodriguez, J.R.; Salvia, R.; Crippa, S.; Warshaw, A.L.; Bassi, C.; Falconi, M.; Thayer, S.P.; Lauwers, G.Y.; Capelli, P.; Mino–Kenudson, M.; et al. Branch-Duct Intraductal Papillary Mucinous Neoplasms: Observations in 145 Patients Who Underwent Resection. Gastroenterology 2007, 133, 72–79. [Google Scholar] [CrossRef] [PubMed]
- White, R.; D’Angelica, M.; Katabi, N.; Tang, L.; Klimstra, D.; Fong, Y.; Brennan, M.; Allen, P. Fate of the Remnant Pancreas after Resection of Noninvasive Intraductal Papillary Mucinous Neoplasm. J. Am. Coll. Surg. 2007, 204, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Nagino, M.; Oda, K.; Nishio, H.; Ebata, T.; Abe, T.; Igami, T.; Nimura, Y. Clinicopathologic features of re-resected cases of intraductal papillary mucinous neoplasms (IPMNs). Surgery 2007, 142, 136–142. [Google Scholar] [CrossRef]
- Wada, K.; Kozarek, R.A.; Traverso, L.W. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am. J. Surg. 2005, 189, 632–637. [Google Scholar] [CrossRef]
- McGinnis, T.; Bantis, L.E.; Madan, R.; Dandawate, P.; Kumer, S.; Schmitt, T.; Paluri, R.K.; Kasi, A. Survival Outcomes of Pancreatic Intraepithelial Neoplasm (PanIN) versus Intraductal Papillary Mucinous Neoplasm (IPMN) Associated Pancreatic Adenocarcinoma. J. Clin. Med. 2020, 9, 3102. [Google Scholar] [CrossRef]
- Elta, G.; Enestvedt, B.; Sauer, B.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Megibow, A.; Baker, M.; Morgan, D.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Vege, S.; Ziring, B.; Jain, R.; Moayyedi, P.; Adams, M.A.; Dorn, S.D.; Dudley-Brown, S.L.; Flamm, S.L.; Gellad, Z.F.; Gruss, C.B.; et al. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [PubMed]

| Author | Publication Year | Years Patient Data Collected | Non-Invasive IPMN Sample Size n |
|---|---|---|---|
| Marchegiani | 2015 | 1990–2013 | 299 |
| Al Efishat | 2018 | 1989–2015 | 319 |
| Marchegiani | 2015 | 1990–2013 | 106 |
| Pflüger | 2020 | 1995–2009 | 124 |
| Amini | 2020 | 1995–2018 | 449 |
| Li | 2020 | 2013–2019 | 125 |
| Kim | 2020 | 2000–2018 | 431 |
| Kwon | 2019 | 2005–2016 | 253 |
| Dhar | 2018 | 330 | |
| Jang | 2016 | 2004–2014 | 74 |
| Yogi | 2015 | 1988–2014 | 153 |
| Xourafas | 2015 | 2002–2012 | 87 |
| Kang | 2014 | 1995–2013 | 298 |
| Frankel | 2013 | 1990–2010 | 192 |
| Winner | 2013 | 1994–2011 | 183 |
| Passot | 2012 | 1994–2009 | 104 |
| Cheon | 2010 | 1998–2008 | 25 |
| Landa | 2009 | 1996–2006 | 67 |
| Nagai | 2007 | 1984–2006 | 42 |
| Yokoyama | 2007 | 1979–2005 | 100 |
| Takahashi | 2006 | 1992–2004 | 20 |
| Chari | 2002 | 1983–2002 | 60 |
| Hirono | 2020 | 1996–2014 | 827 |
| D’Angelica | 2004 | 1983–2000 | 32 |
| Fujii | 2010 | 1991–2009 | 81 |
| Salvia | 2004 | 1988–2002 | 140 |
| Schnelldorfer | 2008 | 1992–2005 | 143 |
| White | 2007 | 1983–2006 | 78 |
| Blair | 2021 | 2004–2016 | 127 |
| Sugimachi | 2021 | 2005–2020 | 25 |
| Majumder | 2019 | 1997–2014 | 138 |
| Asano | 2020 | 1990–2019 | 85 |
| Nagai | 2019 | 2004–2016 | 74 |
| Poruk | 2019 | 1997–2016 | 546 |
| Antoñanzas | 2018 | 1993–2016 | 18 |
| Date | 2018 | 1987–2015 | 135 |
| Pea | 2017 | 1996–2014 | 260 |
| Kimura | 2017 | 1994–2015 | 71 |
| Marsoner | 2016 | 24 | |
| Ridtitid | 2016 | 2001–2013 | 117 |
| Hirono | 2016 | 1999–2014 | 172 |
| Miyasaka | 2016 | 1987–2012 | 160 |
| Yamaguchi | 2016 | 2004–2013 | 40 |
| Kwon | 2014 | 1995–2013 | 19 |
| Sahora | 2014 | 1993–2012 | 43 |
| Sauvanet | 2014 | 1999–2011 | 75 |
| Tamura | 2014 | 1987–2012 | 36 |
| Yuan | 2014 | 2001–2011 | 24 |
| Sahora K | 2013 | 1995–2012 | 203 |
| Distler M | 2013 | 1995–2010 | 33 |
| He | 2013 | 130 | |
| Ohtsuka | 2012 | 136 | |
| Miller | 2011 | 191 | |
| Fujii | 2011 | 84 | |
| Park | 2011 | 1995–2009 | 68 |
| Nakagohri | 2010 | 1994–2007 | 13 |
| Crippa | 2010 | 1988–2006 | 389 |
| Lubezky | 2010 | 2002–2008 | 39 |
| Niedergethmann | 2008 | 1996–2006 | 29 |
| Rodriguez | 2007 | 1990–2005 | 113 |
| Cuillerier | 2000 | 1980–1996 | 20 |
| Author | Follow-Up Frequency | Imaging at Follow-Up |
|---|---|---|
| Al Efishat | 6–12 months | CT/MRI |
| Pflüger | 6 months | CT, MRI, & PET/CT |
| Amini | 6–12 months | CT, MRI, or EUS |
| Li | 6–12 months | CT, MRI, EUS; serum tumor markers |
| Kim | every 3 months for 1st year, 6 months for 2nd year, then yearly | CT, MRI |
| Kwon | 3–6 months for invasive, not given for noninvasive | CT or MRI |
| Jang | 6–12 months | Ultrasonography or CT |
| Yogi | 6 months | Contrast-enhanced CT |
| Kang | 3 months (first year), 6 months (second year), subsequent depended on tumor invasiveness | CT, MRI |
| Winner | 3–6 months | MRI, CT, or EUS |
| Passot | At least yearly (dependent on invasiveness) | CT, MRI |
| Cheon | Seen at 6 and 12 months, then yearly | CT |
| Yokoyama | 3–6 months | US, CT, or MRI (ERCP, EUS, and IDUS used to confirm if recurrence was suspected) |
| Takahashi | 3–6 months | Abdominal US, CT, MRI |
| Hirono | 3–6 months | CT, MRI, EUS |
| Fujii | 6 months | CT/MDCT or EUS |
| Blair | every 6 months for 2 years, then yearly | CT, MRCP, EUS |
| Antoñanzas | 6–12 months | EUS/MRI/CT |
| Date | 6 months | CT/MRI alternating |
| Marsoner | 6 months | |
| Ridtitid | 3–12 months | CT, MRI, and/or EUS |
| Hirono | 6 months | CT/MRI, tumor markers |
| Miyasaka | 3–6 months | CT, MRI/MRCP, tumor markers |
| Yamaguchi | 3 months (for 2 years, 6 months thereafter) | Not specified |
| Kwon | At 1, 3, and 6 months, then yearly | CT |
| He | every 6 months for 2 years, then yearly | CT/MRCP/EUS |
| Ohtsuka | 6 months | CT/MRI alternating |
| Fujii | 6 months | CT or EUS |
| Niedergethmann | 1 year | CT/MRI |
| Rodriguez | 1 year | US/CT/MRI |
| Author | Median Follow-Up Time (months) | Overall Recurrence Rate (%) | Median (or Mean **) Time to Recurrence (months) |
|---|---|---|---|
| Marchegiani | 58 | 9 | 17 |
| Al Efishat | 42 | 22 | 28 |
| Marchegiani | 56 | 18.5 | 12 |
| Pflüger | 114 | 15 | 54 |
| Amini | 48.9 | 27.6 | 84 ** |
| Li | 38.5 | 9.6 | 8 |
| Dhar | 36 | 10.3 | 22 |
| Jang | 37.8 | 3.2 | 46.5 |
| Yogi | 46.4 | 17 | 20.4 |
| Xourafas | 16 | 59.4 | |
| Kang | 44.4 | 5.4 | 47.4 |
| Winner | 32 | 9.7 | 21.9 |
| Passot | 33.3 | 20.2 | 56.5 |
| Yokoyama | 60 | 5 | 41.6 |
| Chari | 36 | 8 | 40 |
| Hirono | 54.2 | 5.8 | 24 |
| D’Angelica | 32 | 9.375 | 20 |
| Fujii | 47 | 4.9 | 77.7 |
| White | 40 | 7.7 | 22 |
| Blair | 68 | 34 | |
| Antoñanzas | 92.4 | 5.5 | 46 |
| Pea | 19 | 27 | |
| Ridtitid | 53.9 | 6.8 | 21.5 ** |
| Hirono | 53.5 | 5.81 | 12.2 |
| Yamaguchi | 27.6 | 6.7 | 13.2 |
| Kwon | 25.3 | 4.55 | 17 |
| Sahora | 63 | 13 | |
| Sahora K | 60 | 8.5 | 34 |
| He | 38 | 17 | 46 |
| Ohtsuka | 64 | 15.4 | 23 |
| Park | 38.4 | 1.5 | 8 |
| Lubezky | 50 | 8 | 24 |
| Rodriguez | 46 | 7 | 34.7 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salahuddin, A.; Thayaparan, V.; Hamad, A.; Tarver, W.; Cloyd, J.M.; Kim, A.C.; Gebhard, R.; Pawlik, T.M.; Reames, B.N.; Ejaz, A. Recurrence following Resection of Intraductal Papillary Mucinous Neoplasms: A Systematic Review to Guide Surveillance. J. Clin. Med. 2024, 13, 830. https://doi.org/10.3390/jcm13030830
Salahuddin A, Thayaparan V, Hamad A, Tarver W, Cloyd JM, Kim AC, Gebhard R, Pawlik TM, Reames BN, Ejaz A. Recurrence following Resection of Intraductal Papillary Mucinous Neoplasms: A Systematic Review to Guide Surveillance. Journal of Clinical Medicine. 2024; 13(3):830. https://doi.org/10.3390/jcm13030830
Chicago/Turabian StyleSalahuddin, Aneesa, Varna Thayaparan, Ahmad Hamad, Willi Tarver, Jordan M. Cloyd, Alex C. Kim, Robyn Gebhard, Timothy M. Pawlik, Bradley N. Reames, and Aslam Ejaz. 2024. "Recurrence following Resection of Intraductal Papillary Mucinous Neoplasms: A Systematic Review to Guide Surveillance" Journal of Clinical Medicine 13, no. 3: 830. https://doi.org/10.3390/jcm13030830
APA StyleSalahuddin, A., Thayaparan, V., Hamad, A., Tarver, W., Cloyd, J. M., Kim, A. C., Gebhard, R., Pawlik, T. M., Reames, B. N., & Ejaz, A. (2024). Recurrence following Resection of Intraductal Papillary Mucinous Neoplasms: A Systematic Review to Guide Surveillance. Journal of Clinical Medicine, 13(3), 830. https://doi.org/10.3390/jcm13030830








