The Association between Term Chorioamnionitis during Labor and Long-Term Infectious Morbidity of the Offspring
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higgins, R.D.; Saade, G.; Polin, R.A.; Grobman, W.A.; Buhimschi, I.A.; Watterberg, K.; Silver, R.M.; Raju, T.N.K.; Chorioamnionitis Workshop Participants. Evaluation and Management of Women and Newborns With a Maternal Diagnosis of Chorioamnionitis: Summary of a Workshop. Obstet. Gynecol. 2016, 127, 426–436. [Google Scholar] [CrossRef]
- Tita, A.T.N.; Andrews, W.W. Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 2010, 37, 339–354. [Google Scholar] [CrossRef]
- Malloy, M.H. Chorioamnionitis: Epidemiology of newborn management and outcome United States 2008. J. Perinatol. 2014, 34, 611–615. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. S4), S29–S52. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Moon, K.C.; Park, J.S.; Jun, J.K.; Romero, R.; Yoon, B.H. The Involvement of Human Amnion in Histologic Chorioamnionitis is an Indicator that a Fetal and an Intra-Amniotic Inflammatory Response is More Likely and Severe: Clinical Implications. Placenta 2009, 30, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Katz, B.; Schelonka, R.L. Mycoplasmas and Ureaplasmas as Neonatal Pathogens. Clin. Microbiol. Rev. 2005, 18, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Guzeloglu-Kayisli, O.; Kayisli, U.A.; Semerci, N.; Başar, M.; Buchwalder, L.F.; Buhimschi, C.S.; Buhimschi, I.A.; Arcuri, F.; Larsen, K.; Huang, J.S.; et al. Mechanisms of chorioamnionitis-associated preterm birth: Interleukin-1β inhibits progesterone receptor expression in decidual cells. J. Pathol. 2015, 237, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Rouse, D.J.; Landon, M.; Leveno, K.J.; Leindecker, S.; Varner, M.W.; Caritis, S.N.; O’Sullivan, M.J.; Wapner, R.J.; Meis, P.J.; Miodovnik, M.; et al. The maternal-fetal medicine units cesarean registry: Chorioamnionitis at term and its duration—Relationship to outcomes. Am. J. Obstet. Gynecol. 2004, 191, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Galinsky, R.; Polglase, G.R.; Hooper, S.B.; Black, M.J.; Moss, T.J.M. The consequences of chorioamnionitis: Preterm birth and effects on development. J. Pregnancy 2013, 2013, 412831. [Google Scholar] [CrossRef]
- Aziz, N.; Cheng, Y.W.; Caughey, A.B. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J. Matern. -Fetal Neonatal Med. 2009, 22, 780–784. [Google Scholar] [CrossRef]
- Yoon, B.H.; Jun, J.K.; Romero, R.; Park, K.H.; Gomez, R.; Kim, I.-O. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol. 1997, 177, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.; Wainstock, T.; Sheiner, E.; Beloosesky, R.; Fischer, L.; Landau, D.; Walfisch, A. Maternal chorioamnionitis & long term neurological morbidity in the offspring. Eur. J. Paediatr. Neurol. 2019, 23, 484–490. [Google Scholar] [PubMed]
- Singh, A.M.; Sherenian, M.G.; Kim, K.-Y.; Erickson, K.A.; Yang, A.; Mestan, K.; Ernst, L.M.; Kumar, R. Fetal cord blood and tissue immune responses to chronic placental inflammation and chorioamnionitis. Allergy Asthma Clin. Immunol. 2018, 14, 66. [Google Scholar] [CrossRef]
- The United Nations. Inter-Agency Group for Child Mortality Estimation. In Levels & Trends in Child Mortality; Report; UNICEF: Geneva, Switzerland, 2015. [Google Scholar]
- Davidesko, S.; Wainstock, T.; Sheiner, E.; Pariente, G. Long-Term Infectious Morbidity of Premature Infants: Is There a Critical Threshold? J. Clin. Med. 2020, 9, 3008. [Google Scholar] [CrossRef]
- Jain, V.G.; Willis, K.A.; Jobe, A.; Ambalavanan, N. Chorioamnionitis and neonatal outcomes. Pediatr. Res. 2022, 91, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Gallagher, K.; Taylor, L.A.; Goldstein, J.A.; Mithal, L.B.; Gernand, A.D. Chorioamnionitis and Risk for Maternal and Neonatal Sepsis: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2021, 137, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Stol, K.; Jans, J.; Ott de Bruin, L.; Unger, W.; van Rossum, A. Perinatal Infections with Ureaplasma. Pediatr. Infect. Dis. J. 2021, 40, S26–S30. [Google Scholar] [CrossRef]
- Ferreira, G.; Santander, A.; Savio, F.; Guirado, M.; Sobrevia, L.; Nicolson, G.L. SARS-CoV-2, Zika viruses and mycoplasma: Structure, pathogenesis and some treatment options in these emerging viral and bacterial infectious diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166264. [Google Scholar] [CrossRef]
- Zamstein, O.; Wainstock, T.; Sheiner, E. Intrapartum Maternal Fever and Long-Term Infectious Morbidity of the Offspring. J. Clin. Med. 2023, 12, 3329. [Google Scholar] [CrossRef]
- Chapman, E.; Reveiz, L.; Illanes, E.; Cosp, X.B. Antibiotic regimens for management of intra-amniotic infection. Cochrane Database Syst. Rev. 2014, 2014, CD010976. [Google Scholar] [PubMed]
- Niu, J.; Xu, L.; Qian, Y.; Sun, Z.; Yu, D.; Huang, J.; Zhou, X.; Wang, Y.; Zhang, T.; Ren, R.; et al. Evolution of the Gut Microbiome in Early Childhood: A Cross-Sectional Study of Chinese Children. Front. Microbiol. 2020, 11, 439. [Google Scholar] [CrossRef]
- Boursi, B.; Mamtani, R.; Haynes, K.; Yang, Y.X. The effect of past antibiotic exposure on diabetes risk. Eur. J. Endocrinol. 2015, 172, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Bridgman, S.L.; Becker, A.B.; Kozyrskyj, A.L. Pediatric original article infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes. 2014, 38, 1290–1298. [Google Scholar] [CrossRef]
- Örtqvist, A.K.; Lundholm, C.; Halfvarson, J.; Ludvigsson, J.F.; Almqvist, C. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: A population-based study. Gut 2019, 68, 218–225. [Google Scholar] [CrossRef]
- Metzler, S.; Frei, R.; Schmaußer-Hechfellner, E.; von Mutius, E.; Pekkanen, J.; Karvonen, A.M.; Kirjavainen, P.V.; Dalphin, J.; Divaret-Chauveau, A.; Riedler, J.; et al. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatr. Allergy Immunol. 2019, 30, 423–433. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Simonsen, J.; Jensen, S.M.; Bønnelykke, K.; Bisgaard, H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J. Pediatr. 2013, 162, 832–838.e3. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Weitkamp, J.H.; Guthrie, S.O.; Wong, H.R.; Moldawer, L.L.; Baker, H.V.; Wynn, J.L. Histological chorioamnionitis shapes the neonatal transcriptomic immune response. Early Hum. Dev. 2016, 98, 1–6. [Google Scholar] [CrossRef]
- Bermick, J.; Gallagher, K.; DenDekker, A.; Kunkel, S.; Lukacs, N.; Schaller, M. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS J. 2018, 286, 82–109. [Google Scholar] [CrossRef] [PubMed]
- Yerkovich, S.T.; Wikström, M.E.; Suriyaarachchi, D.; Prescott, S.L.; Upham, J.W.; Holt, P.G. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr. Res. 2007, 62, 547–552. [Google Scholar] [CrossRef]
- Upham, J.W.; Zhang, G.; Rate, A.; Yerkovich, S.T.; Kusel, M.; Sly, P.D.; Holt, P.G. Plasmacytoid dendritic cells during infancy are inversely associated with childhood respiratory tract infections and wheezing. J. Allergy Clin. Immunol. 2009, 124, 707–713.e2. [Google Scholar] [CrossRef]
- Ygberg, S.; Nilsson, A. The developing immune system—From foetus to toddler. Acta Paediatr. Int. J. Paediatr. 2012, 101, 120–127. [Google Scholar] [CrossRef]
- Flaskerud, J.H.; Delilly, C.R. Social determinants of health status. Issues Ment. Health Nurs. 2012, 33, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; da Veiga, A.B.G.; Kaminski V de, L.; Valverde-Villegas, J.M.; de Freitas, A.W.Q.; Chies, J.A.B. Control and prevention of infectious diseases from a one health perspective. Genet Mol. Biol. 2021, 44, e20200256. [Google Scholar] [CrossRef]
- Nakitanda, A.O.; Kieler, H.; Odsbu, I.; Rhedin, S.; Almqvist, C.; Pasternak, B.; Pazzagli, L. In-utero antibiotic exposure and subsequent infections in infancy: A register-based cohort study with sibling analysis. Am. J. Obs. Gynecol. MFM 2023, 5, 100860. [Google Scholar] [CrossRef] [PubMed]
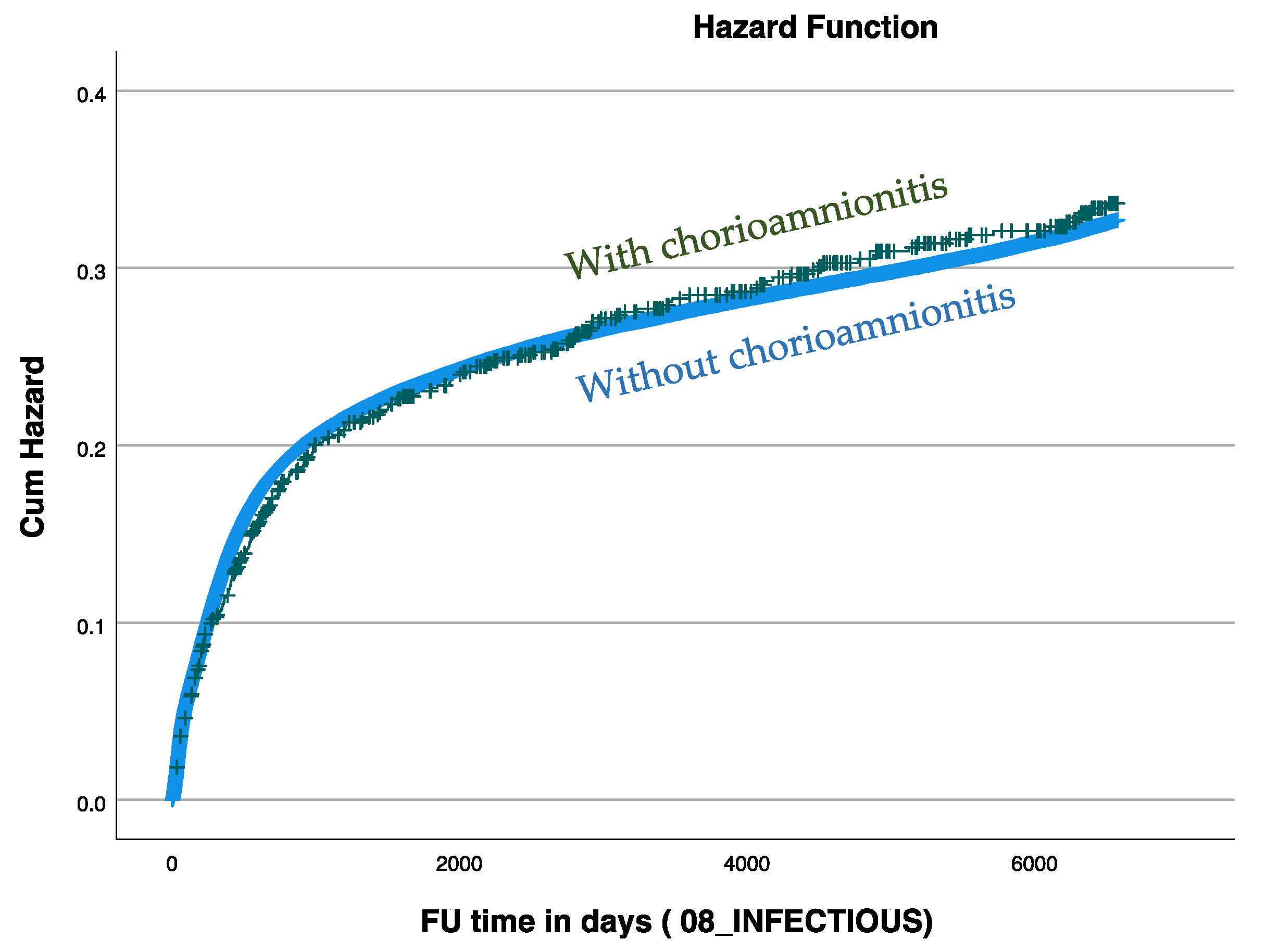
| Characteristics | With Chorioamnionitis [n = 988] | Without Chorioamnionitis [n = 330,610] | p-Value |
|---|---|---|---|
| Maternal age (mean, ±SD) | 29.0 ± 6.1 | 28.2 ± 5.7 | 0.003 |
| Nulliparity, n (%) | 423 (42.8%) | 72,478 (23.7%) | <0.001 |
| Obesity, n (%) | 19 (1.9%) | 3760 (1.1%) | 0.02 |
| Smoking, n (%) | 13 (1.3%) | 2274 (0.7%) | 0.017 |
| Fertility treatments, n (%) | 30 (3.0%) | 5195 (1.6%) | <0.001 |
| Hypertensive disorders, n (%) | 94 (9.5%) | 13,612 (4.1%) | <0.001 |
| Diabetes mellitus, n (%) | 93 (9.4%) | 15,270 (4.6%) | <0.001 |
| Pregnancy Outcome | With Chorioamnionitis [n = 988] | Without Chorioamnionitis [n = 330,610] | p-Value |
|---|---|---|---|
| Gestational age at birth (weeks, ±SD) | 39.5 ± 1.3 | 39.3 ± 1.2 | <0.001 |
| Birthweight (g., ±SD) | 3185 ± 527 | 3263 ± 443 | <0.001 |
| Induced labor, n (%) | 436 (44.1%) | 70,788 (21.4) | <0.001 |
| Meconium-stained amniotic fluid, n (%) | 373 (37.8%) | 40,982 (12.4%) | <0.001 |
| Prolonged 2nd stage of labor, n (%) | 47 (4.8%) | 4169 (1.3%) | <0.001 |
| Cesarean delivery, n (%) | 617 (62.4%) | 41,746 (12.6%) | <0.001 |
| Low 5 min Apgar score a, n (%) | 31 (3.3%) | 1238 (0.4%) | <0.001 |
| SGA b, n (%) | 117 (11.8%) | 14,821 (4.5%) | <0.001 |
| Low birthweight c, n (%) | 98 (9.9%) | 11,434 (3.5%) | <0.001 |
| Postpartum hemorrhage, n (%) | 13 (1.3%) | 1937 (0.6%) | 0.003 |
| Mortality, n (%) | 53 (5.4%) | 860 (0.3%) | <0.001 |
| Infectious Morbidity | With Chorioamnionitis [n = 935] | Without Chorioamnionitis [n = 329,750] | p -Value |
|---|---|---|---|
| Bacterial, n (%) | 18 (1.9%) | 4589 (1.4%) | 0.165 |
| Viral, n (%) | 34 (3.6%) | 10,408 (3.2%) | 0.402 |
| Respiratory, n (%) | 135 (14.4%) | 44,582 (13.5%) | 0.412 |
| Central nervous system (CNS), n (%) | 7 (0.7%) | 1678 (0.5%) | 0.304 |
| Ear-nose-throat (ENT), n (%) | 63 (6.7%) | 20,947 (6.4%) | 0.629 |
| Gastrointestinal (GI), n (%) | 31 (3.3%) | 9650 (2.9%) | 0.481 |
| Ophthalmic, n (%) | 12 (1.3%) | 4039 (1.2%) | 0.871 |
| Dermatologic, n (%) | 23 (2.5%) | 7583 (2.3%) | 0.744 |
| Total infectious hospitalizations, n (%) | 242 (25.9%) | 78,409 (23.8%) | 0.131 |
| Variables | Adjusted Hazards Ratio (aHR) | 95%CI | p-Value | |
|---|---|---|---|---|
| Min | Max | |||
| Chorioamnionitis | 0.929 | 0.818 | 1.054 | 0.254 |
| Mother’s age at birth | 0.988 | 0.987 | 0.990 | <0.001 |
| Gestational age in weeks | 0.940 | 0.935 | 0.945 | <0.001 |
| Cesarean section | 1.091 | 1.068 | 1.114 | <0.001 |
| Hypertensive disorders a | 1.037 | 1.002 | 1.073 | 0.036 |
| Any diabetes b | 1.083 | 1.048 | 1.119 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidi, N.E.; Gutvirtz, G.; Sheiner, E. The Association between Term Chorioamnionitis during Labor and Long-Term Infectious Morbidity of the Offspring. J. Clin. Med. 2024, 13, 814. https://doi.org/10.3390/jcm13030814
Davidi NE, Gutvirtz G, Sheiner E. The Association between Term Chorioamnionitis during Labor and Long-Term Infectious Morbidity of the Offspring. Journal of Clinical Medicine. 2024; 13(3):814. https://doi.org/10.3390/jcm13030814
Chicago/Turabian StyleDavidi, Noa Efrat, Gil Gutvirtz, and Eyal Sheiner. 2024. "The Association between Term Chorioamnionitis during Labor and Long-Term Infectious Morbidity of the Offspring" Journal of Clinical Medicine 13, no. 3: 814. https://doi.org/10.3390/jcm13030814
APA StyleDavidi, N. E., Gutvirtz, G., & Sheiner, E. (2024). The Association between Term Chorioamnionitis during Labor and Long-Term Infectious Morbidity of the Offspring. Journal of Clinical Medicine, 13(3), 814. https://doi.org/10.3390/jcm13030814








