Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression
Abstract
1. Introduction
2. Materials and Methods
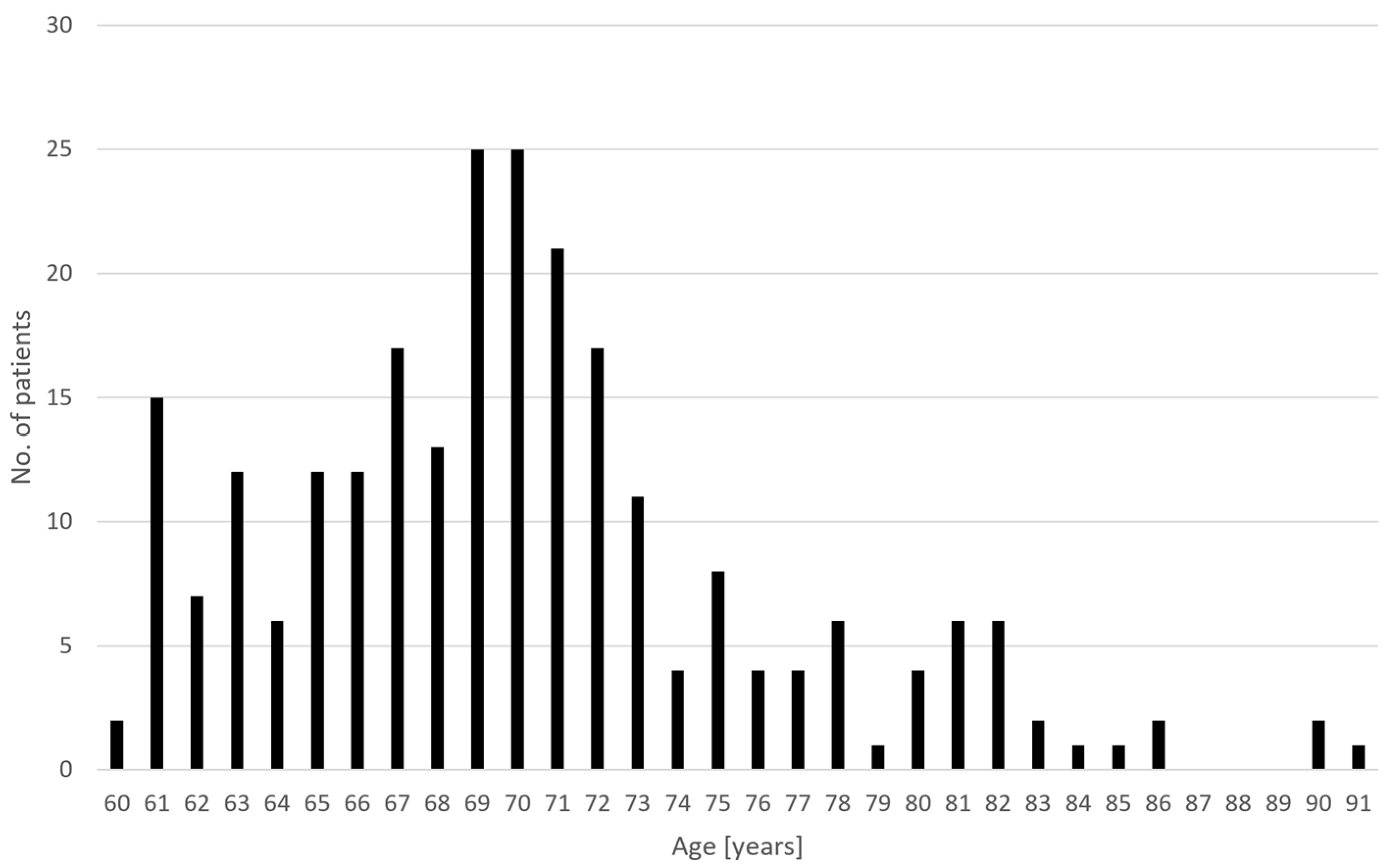
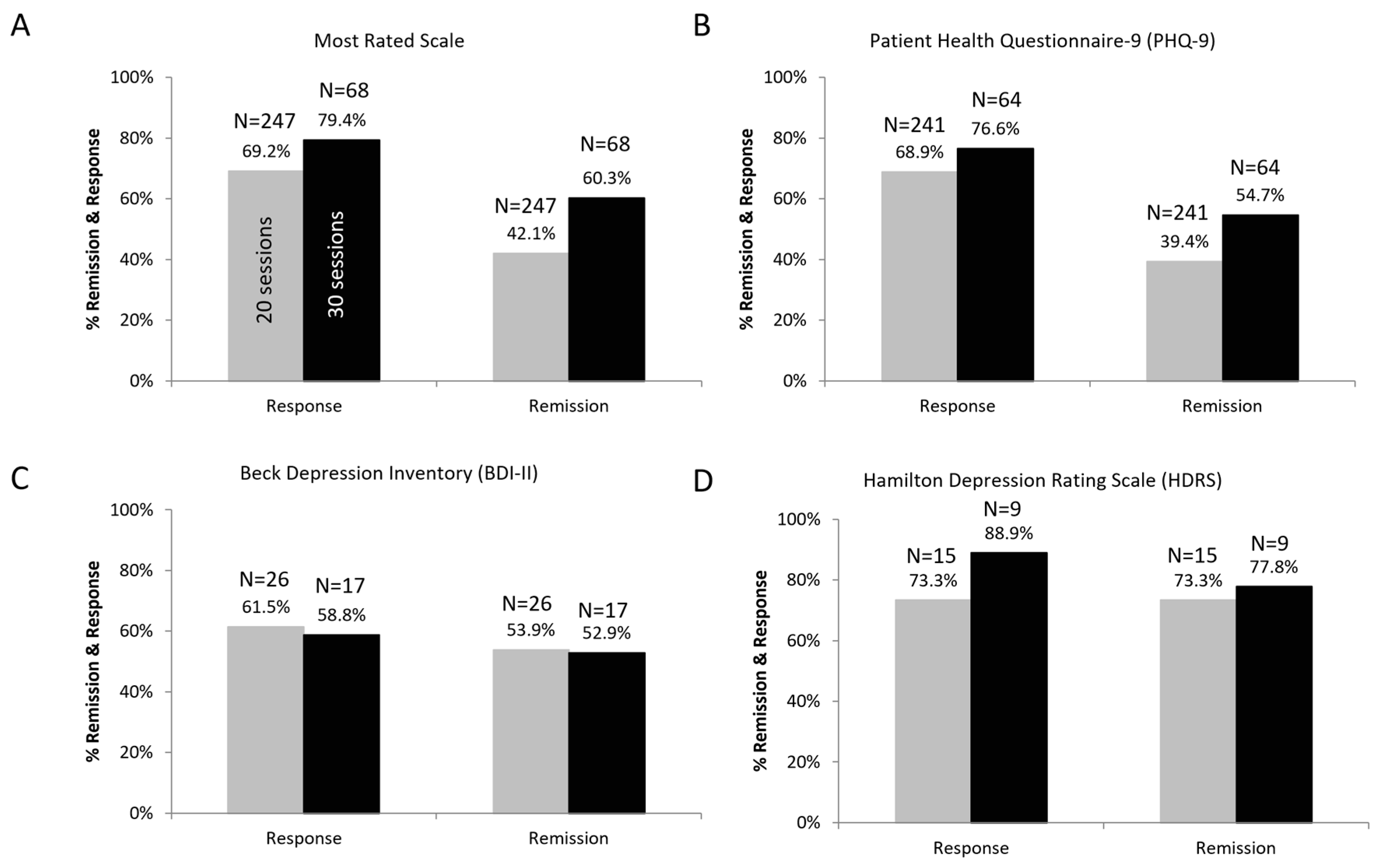
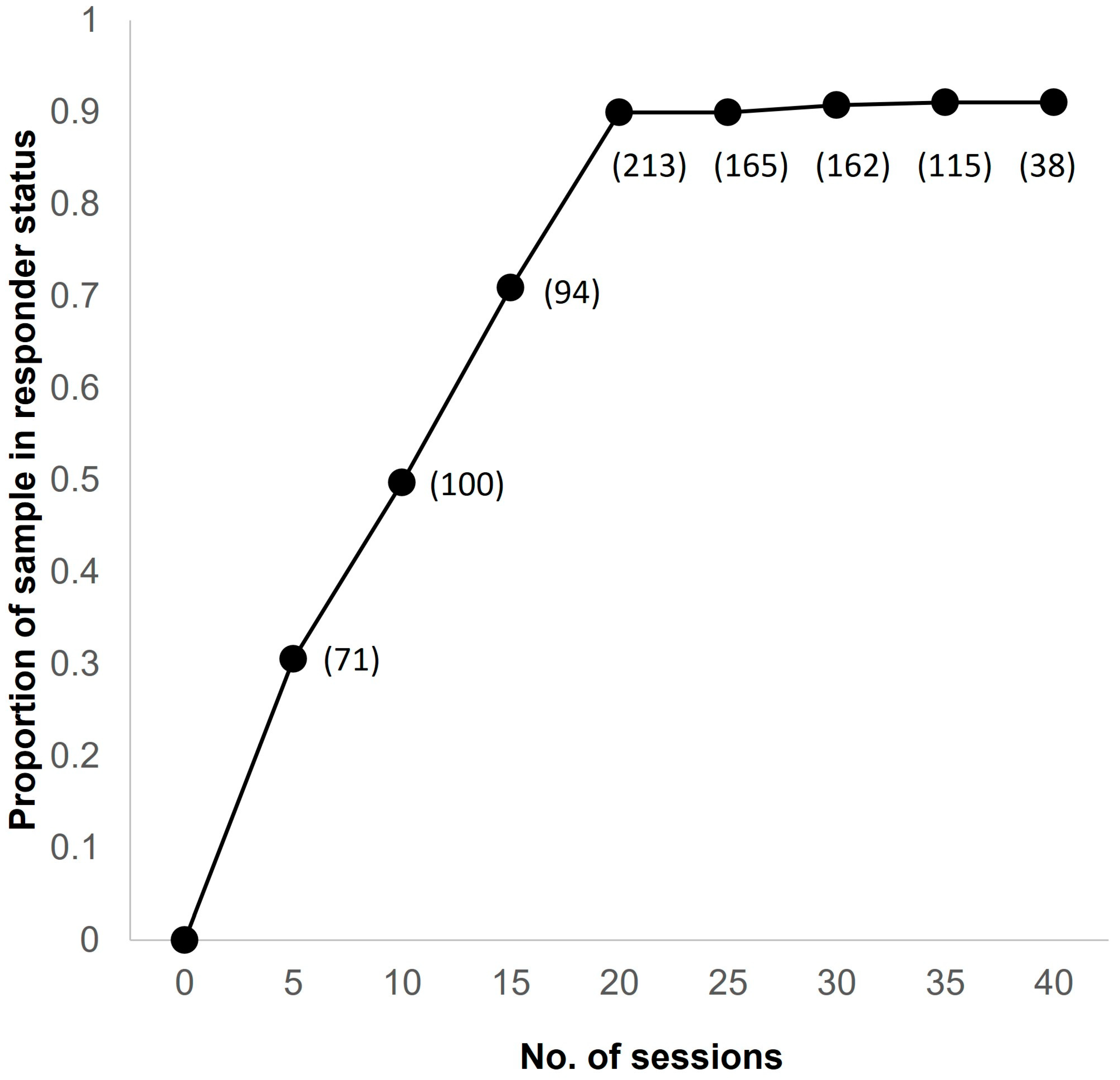
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing, 2019 Highlights; United Nations: New York, NY, USA, 2020. [Google Scholar]
- Friedrich, M.J. Depression is the leading cause of disability around the world. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef]
- Herbert, J.; Lucassen, P.J. Depression as a risk factor for Alzheimer’s disease: Genes, steroids, cytokines and neurogenesis—What do we need to know? Front. Neuroendocrinol. 2016, 41, 153–171. [Google Scholar] [CrossRef]
- Crown, W.H.; Finkelstein, S.; Berndt, E.R.; Ling, D.; Poret, A.W.; Rush, A.J.; Russell, J.M. The impact of treatment-resistant depression on health care utilization and costs. J. Clin. Psychiatry 2002, 63, 963–971. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR *D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Valiengo, L.; Pinto, B.S.; Marinho, K.A.; Santos, L.A.; Tort, L.C.; Benatti, R.G.; Teixeira, B.B.; Miranda, C.S.; Cardeal, H.B.; Suen, P.J.C.; et al. Treatment of depression in the elderly with repetitive transcranial magnetic stimulation using theta-burst stimulation: Study protocol or a randomized, double-blind, controlled trial. Front. Hum. Neurosci. 2022, 16, 941981. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.F., III; Lenze, E.; Mulsant, B.H. Assessment and treatment of major depression in older adults. Handb. Clin. Neurol. 2019, 167, 429–435. [Google Scholar]
- Hinrichsen, G.A. Recovery and relapse from major depressive disorder in the elderly. Am. J. Psychiatry 1992, 149, 1575–1579. [Google Scholar] [PubMed]
- Alexopoulos, G.S.; Meyers, B.S.; Young, R.C.; Kakuma, T.; Feder, M.; Einhorn, A.; Rosendahl, E. Recovery in geriatric depression. Arch. Gen. Psychiatry 1996, 53, 305–312. [Google Scholar] [CrossRef]
- Little, J.T.; Reynolds, C.F., III; Dew, M.A.; Frank, E.; Begley, A.E.; Miller, M.D.; Cornes, C.; Mazumdar, S.; Perel, J.M.; Kupfer, D.J. How common is resistance to treatment in recurrent, nonpsychotic geriatric depression? Am. J. Psychiatry 1998, 155, 1035–1038. [Google Scholar] [CrossRef]
- Kok, R.M.; Reynolds, C.F., 3rd. Management of depression in older adults: A review. JAMA 2017, 317, 2114–2122. [Google Scholar] [CrossRef]
- Tedeschini, E.; Levkovitz, Y.; Iovieno, N.; Ameral, V.E.; Nelson, J.C.; Papakostas, G.I. Efficacy of antidepressants for late-life depression: A meta-analysis and meta-regression of placebo-controlled randomized trials. J. Clin. Psychiatry 2011, 72, 1660–1668. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Guo, Q.; Li, C.; Wang, J. Updated Review on the Clinical Use of Repetitive Transcranial Magnetic Stimulation in Psychiatric Disorders. Neurosci. Bull. 2017, 33, 747–756. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; George, M.S.; Pridmore, S. The evidence is in: Repetitive transcranial magnetic stimulation is an effective, safe, and well-tolerated treatment for patients with major depressive disorder. Aust. N. Z. J. Psychiatry 2021, 56, 745–751. [Google Scholar] [CrossRef]
- Zhang, M.; Mo, J.; Zhang, H.; Tang, Y.; Guo, K.; Ouyang, X.; Huang, L.; Zhong, X.; Ning, Y. Efficacy and tolerability of repetitive transcranial magnetic stimulation for late-life depression: A systematic review and meta-analysis. J. Affect. Disord. 2023, 323, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Almheiri, E.; Alhelali, A.; Abdelnaim, M.A.; Weber, F.C.; Langguth, B.; Schecklmann, M.; Hebel, T. Effectiveness of Repetitive Transcranial Magnetic Stimulation in the Treatment of Depression in the Elderly: A Retrospective Natural Analysis. J. Clin. Med. 2023, 12, 4748. [Google Scholar] [CrossRef] [PubMed]
- Roth, Y.; Zangen, A.; Hallett, M. A coil design for transcranial magnetic stimulation of deep brain regions. J. Clin. Neurophysiol. 2002, 19, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zibman, S.; Pell, G.S.; Barnea-Ygael, N.; Roth, Y.; Zangen, A. Application of transcranial magnetic stimulation for major depression: Coil design and neuroanatomical variability considerations. Eur. Neuropsychopharmacol. 2021, 45, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Isserles, M.; Padberg, F.; Lisanby, S.H.; Bystritsky, A.; Xia, G.; Tendler, A.; Daskalakis, Z.J.; Winston, J.L.; Dannon, P.; et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry 2015, 14, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Tendler, A.; Goerigk, S.; Zibman, S.; Ouaknine, S.; Harmelech, T.; Pell, G.S.; Zangen, A.; Harvey, S.A.; Grammer, G.; Stehberg, J.; et al. Deep TMS H1 Coil treatment for depression: Results from a large post marketing data analysis. Psychiatry Res. 2023, 324, 115179. [Google Scholar] [CrossRef] [PubMed]
- Kozel, F.A.; Nahas, Z.; deBrux, C.; Molloy, M.; Lorberbaum, J.P.; Bohning, D.; Risch, S.C.; George, M.S. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kaster, T.S.; Daskalakis, Z.J.; Noda, Y.; Knyahnytska, Y.; Downar, J.; Rajji, T.K.; Levkovitz, Y.; Zangen, A.; Butters, M.A.; Mulsant, B.H.; et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: A prospective randomized controlled trial. Neuropsychopharmacology 2018, 43, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Stultz, D.J.; Osburn, S.; Burns, T.; Gills, T.M.; Shafer, C.; Walton, R.; Roe, S.M. Treatment Tolerance and Depression Improvement in the Elderly > 70 Years Old with Transcranial Magnetic Stimulation (TMS). Brain Stimul. 2023, 16, 10. [Google Scholar] [CrossRef]
- Cusin, C.; Yang, H.; Yeung, A.; Fava, M. Chapter 2. Rating scales for depression. In Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health; Baer, L., Blais, M.A., Eds.; Current Clin Psychology; Humana Press: New York, NY, USA, 2010; pp. 7–36. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Friedman, M.; Boerescu, D.A.; Attiullah, N.; Toba, C. How can we use depression severity to guide treatment selection when measures of depression categorize patients differently? J. Clin. Psychiatry 2012, 73, 1287–1291. [Google Scholar] [CrossRef]
- Cole, E.J.; Stimpson, K.H.; Bentzley, B.S.; Gulser, M.; Cherian, K.; Tischler, C.; Nejad, R.; Pankow, H.; Choi, E.; Aaron, H.; et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant de-pression. Am. J. Psychiatry 2020, 177, 716–726. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Cappon, D.; den Boer, T.; Jordan, C.; Yu, W.; Metzger, E.; Pascual-Leone, A. Transcranial magnetic stimulation (TMS) for geriatric depression. Ageing Res. Rev. 2022, 74, 101531. [Google Scholar] [CrossRef] [PubMed]
- Roth, Y.; Amir, A.; Levkovitz, Y.; Zangen, A. Three-Dimensional Distribution of the Electric Field Induced in the Brain by Transcranial Magnetic Stimulation Using Figure-8 and Deep H-Coils. J. Clin. Neurophysiol. 2007, 24, 31–38. [Google Scholar] [CrossRef]
- Trevizol, A.P.; Goldberger, K.W.; Mulsant, B.H.; Rajji, T.K.; Downar, J.; Daskalakis, Z.J.; Blumberger, D.M. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int. J. Geriatr. Psychiatry 2019, 34, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, D.M.; Mulsant, B.H.; Thorpe, K.E.; McClintock, S.M.; Konstantinou, G.N.; Lee, H.H.; Nestor, S.M.; Noda, Y.; Rajji, T.K.; Trevizol, A.P.; et al. Effectiveness of Standard Sequential Bilateral Repetitive Transcranial Magnetic Stimulation vs. Bilateral Theta Burst Stimulation in Older Adults with Depression: The FOUR-D Randomized Noninferiority Clinical Trial. JAMA Psychiatry 2022, 79, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Sabesan, P.; Lankappa, S.; Khalifa, N.; Krishnan, V.; Gandhi, R.; Palaniyappan, L. Transcranial magnetic stimulation for geriatric depression: Promises and pitfalls. World J. Psychiatry 2015, 5, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Bauer, M.; Carvalho, A.F.; Eyre, H.; Fava, M.; Kasper, S.; Kennedy, S.H.; Khoo, J.P.; Lopez Jaramillo, C.; Malhi, G.S.; et al. A clinical approach to treatment resistance in depressed patients: What to do when the usual treatments don’t work well enough? World J. Biol. Psychiatry 2021, 22, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Khawam, E.A.; Laurencic, G.; Malone, D.A., Jr. Side effects of antidepressants: An overview. Clevel. Clin. J. Med. 2006, 73, 351–353, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Tendler, A.; Harmelech, T.; Gersner, R.; Roth, Y. Seizures provoked by H-coils from 2010 to 2020. Brain Stimul. 2021, 14, 66–68. [Google Scholar] [CrossRef]
- Tendler, A.; Gersner, R.; Roth, Y.; Stein, A.; Harmelech, T.; Hanlon, C.A. Deep TMS: A comprehensive summary of adverse events from five multicenter trials. Brain Stimul. 2023, 16, 1123–1125. [Google Scholar] [CrossRef]
- Isserles, M.; Rosenberg, O.; Dannon, P.; Lerer, B.; Zangen, A. Cognitive emotional reactivation during deep transcranial magnetic stimulation over the prefrontal cortex of depressive patients affects antidepressant outcomes. J. Affect. Disord. 2011, 128, 235–242. [Google Scholar] [CrossRef]
- Cole, E.J.; Phillips, A.L.; Bentzley, B.S.; Stimpson, K.H.; Nejad, R.; Barmak, F.; Veerapal, C.; Khan, N.; Cherian, K.; Felber, E.; et al. Stanford neuromodulation therapy (SNT): A double-blind randomized controlled trial. Am. J. Psychiatry 2022, 179, 132–141. [Google Scholar] [CrossRef]
- Roth, Y.; Hanlon, C.A.; Pell, G.; Zibman, S.; Harmelech, T.; Muir, O.S.; MacMillan, C.; Prestley, T.; Purselle, D.C.; Knightly, T.; et al. Real world efficacy and safety of various accelerated deep TMS protocols for major depression. Psychiatry Res. 2023, 328, 115482. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, Y.; Munasifi, F.; Harvey, S.A.; Grammer, G.; Hanlon, C.A.; Tendler, A. Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression. J. Clin. Med. 2024, 13, 816. https://doi.org/10.3390/jcm13030816
Roth Y, Munasifi F, Harvey SA, Grammer G, Hanlon CA, Tendler A. Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression. Journal of Clinical Medicine. 2024; 13(3):816. https://doi.org/10.3390/jcm13030816
Chicago/Turabian StyleRoth, Yiftach, Faisal Munasifi, Steven A. Harvey, Geoffrey Grammer, Colleen A. Hanlon, and Aron Tendler. 2024. "Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression" Journal of Clinical Medicine 13, no. 3: 816. https://doi.org/10.3390/jcm13030816
APA StyleRoth, Y., Munasifi, F., Harvey, S. A., Grammer, G., Hanlon, C. A., & Tendler, A. (2024). Never Too Late: Safety and Efficacy of Deep TMS for Late-Life Depression. Journal of Clinical Medicine, 13(3), 816. https://doi.org/10.3390/jcm13030816






