Long-Term Mortality Risk According to Cardiorespiratory Fitness in Patients Undergoing Coronary Artery Bypass Graft Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MET Extraction
2.3. CABG and Mortality
2.4. CRF Categories
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, K.D.X.J.; Arias, E. Mortality in the United States, 2019; NCHS Data Brief, no. 395; National Center for Health Statistics: Hyattsville, MD, USA, 2020. [Google Scholar]
- Odden, M.C.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Goldman, L.; Bibbins-Domingo, K. The impact of the aging population on coronary heart disease in the United States. Am. J. Med. 2011, 124, 827–833.e825. [Google Scholar] [CrossRef] [PubMed]
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; DiSesa, V.J.; Hiratzka, L.F.; Hutter, A.M.; et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 124, e652–e735. [Google Scholar]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Shahian, D.M.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; Vassileva, C.; von Ballmoos, M.C.W.; Thourani, V.H.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 1-Background, Design Considerations, and Model Development. Ann. Thorac. Surg. 2018, 105, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Feng, L.; He, X.; Xian, Y.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; et al. The Society of Thoracic Surgeons 2018 Adult Cardiac Surgery Risk Models: Part 2-Statistical Methods and Results. Ann. Thorac. Surg. 2018, 105, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Hamilton, C.; Grayburn, P.A.; Xu, H.; Hebeler, R.F., Jr.; Hamman, B. Established preoperative risk factors do not predict long-term survival in isolated coronary artery bypass grafting patients. Ann. Thorac. Surg. 2012, 93, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Shahian, D.M.; O’Brien, S.M.; Sheng, S.; DeLong, E.R.; Peterson, E.D.; Grau-Sepulveda, M.V.; Grover, F.L.; Mayer, J.E.; Jacobs, J.P.; Weiss, J.M.; et al. Predictors of long-term survival after coronary artery bypass grafting surgery: Results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study). Circulation 2012, 125, 1491–1500. [Google Scholar] [CrossRef]
- Weintraub, W.S.; Clements, S.D., Jr.; Crisco, L.V.T.; Guyton, R.A.; Craver, J.M.; Jones, E.L.; Hatcher, C.R., Jr. Twenty-year survival after coronary artery surgery: An institutional perspective from Emory University. Circulation 2003, 107, 1271–1277. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Kokkinos, P.; Faselis, C.; Myers, J.; Sui, X.; Zhang, J.; Blair, S.N. Age-specific exercise capacity threshold for mortality risk assessment in male veterans. Circulation 2014, 130, 653–658. [Google Scholar] [CrossRef]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef]
- Berry, J.D.; Pandey, A.; Gao, A.; Leonard, D.; Farzaneh-Far, R.; Ayers, C.; DeFina, L.; Willis, B. Physical fitness and risk for heart failure and coronary artery disease. Circ. Heart Fail. 2013, 6, 627–634. [Google Scholar] [CrossRef]
- Swift, D.L.; Lavie, C.J.; Johannsen, N.M.; Arena, R.; Earnest, C.P.; O’Keefe, J.H.; Milani, R.V.; Blair, S.N.; Church, T.S. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ. J. 2013, 77, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Chan, K.; Dandekar, E.; Yilmaz, B.; Nagare, A.; Faselis, C.; Soofi, M. Cardiorespiratory Fitness and Reclassification of Risk for Incidence of Heart Failure: The Veterans Exercise Testing Study. Circ. Heart Fail. 2017, 10, e003780. [Google Scholar] [CrossRef]
- Radford, N.B.; DeFina, L.F.; Leonard, D.; Barlow, C.E.; Willis, B.L.; Gibbons, L.W.; Gilchrist, S.C.; Khera, A.; Levine, B.D. Cardiorespiratory Fitness, Coronary Artery Calcium, and Cardiovascular Disease Events in a Cohort of Generally Healthy Middle-Age Men: Results from the Cooper Center Longitudinal Study. Circulation 2018, 137, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Verrill, T.A.; Boura, J.A.; Sakwa, M.P.; Shannon, F.L.; Franklin, B.A. Effect of cardiorespiratory fitness on short-term morbidity and mortality after coronary artery bypass grafting. Am. J. Cardiol. 2013, 112, 1104–1109. [Google Scholar] [CrossRef]
- Smenes, B.T.; Nes, B.M.; Letnes, J.M.; Slagsvold, K.H.; Wisløff, U.; Wahba, A. Cardiorespiratory fitness and the incidence of coronary surgery and postoperative mortality: The HUNT study. Eur. J. Cardiothorac. Surg. 2022, 62, ezac126. [Google Scholar] [CrossRef] [PubMed]
- McCarron, K.K.; Reinhard, M.J.; Bloeser, K.J.; Mahan, C.M.; Kang, H.K. PTSD diagnoses among Iraq and Afghanistan veterans: Comparison of administrative data to chart review. J. Trauma. Stress. 2014, 27, 626–629. [Google Scholar] [CrossRef]
- Floyd, J.S.; Blondon, M.; Moore, K.P.; Boyko, E.J.; Smith, N.L. Validation of methods for assessing cardiovascular disease using electronic health data in a cohort of Veterans with diabetes. Pharmacoepidemiol. Drug Saf. 2016, 25, 467–471. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Foster, C.; Jackson, A.S.; Pollock, M.L.; Taylor, M.M.; Hare, J.; Sennett, S.M.; Rod, J.L.; Sarwar, M.; Schmidt, D.H. Generalized equations for predicting functional capacity from treadmill performance. Am. Heart J. 1984, 107, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Myers, J.; Franklin, B.; Narayan, P.; Lavie, C.J.; Faselis, C. Cardiorespiratory Fitness and Health Outcomes: A Call to Standardize Fitness Categories. Mayo. Clin. Proc. 2018, 93, 333–336. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.-C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Thompson, P.D. Exercise Is Medicine: At Any Dose? JAMA 2015, 314, 1915–1916. [Google Scholar] [CrossRef]
- Myrstad, M.; Løchen, M.; Graff-Iversen, S.; Gulsvik, A.K.; Thelle, D.S.; Stigum, H.; Ranhoff, A.H. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand. J. Med. Sci. Sports 2014, 24, e238–e244. [Google Scholar] [CrossRef]
- Taylor, R.S.; Brown, A.; Ebrahim, S.; Jolliffe, J.; Noorani, H.; Rees, K.; Skidmore, B.; Stone, J.A.; Thompson, D.R.; Oldridge, N. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2004, 116, 682–692. [Google Scholar] [CrossRef]
- Kulik, A.; Ruel, M.; Jneid, H.; Ferguson, T.B.; Hiratzka, L.F.; Ikonomidis, J.S.; Lopez-Jimenez, F.; McNallan, S.M.; Patel, M.; Roger, V.L.; et al. Secondary prevention after coronary artery bypass graft surgery: A scientific statement from the American Heart Association. Circulation 2015, 131, 927–964. [Google Scholar] [CrossRef] [PubMed]
- Balady, G.J.; Williams, M.A.; Ades, P.A.; Bittner, V.; Comoss, P.; Foody, J.M.; Franklin, B.; Sanderson, B.; Southard, D.; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: A scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2007, 115, 2675–2682. [Google Scholar] [PubMed]
- Hansen, D.; Dendale, P.; Leenders, M.; Berger, J.; Raskin, A.; Vaes, J.; Meeusen, R. Reduction of cardiovascular event rate: Different effects of cardiac rehabilitation in CABG and PCI patients. Acta Cardiol. 2009, 64, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease; StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
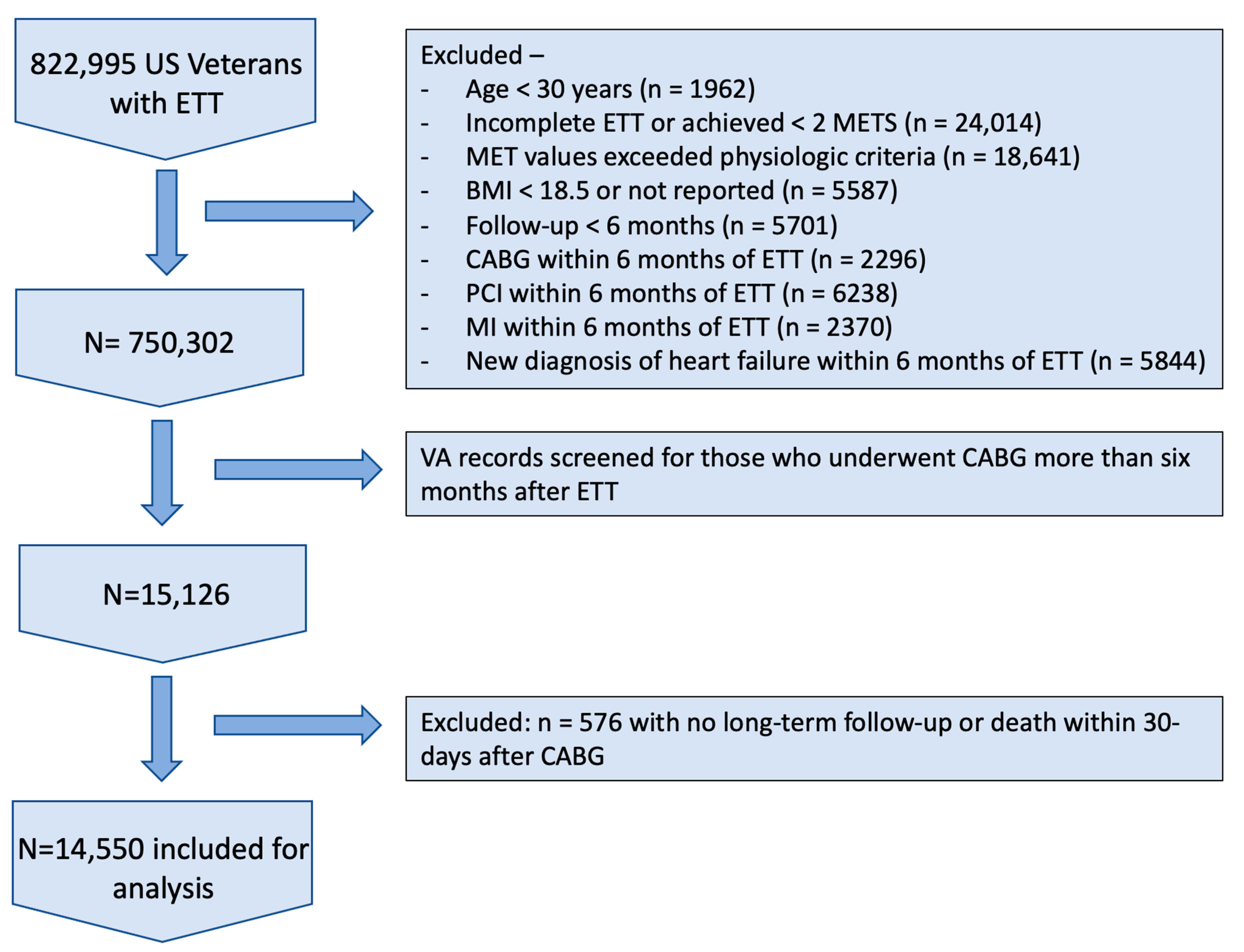
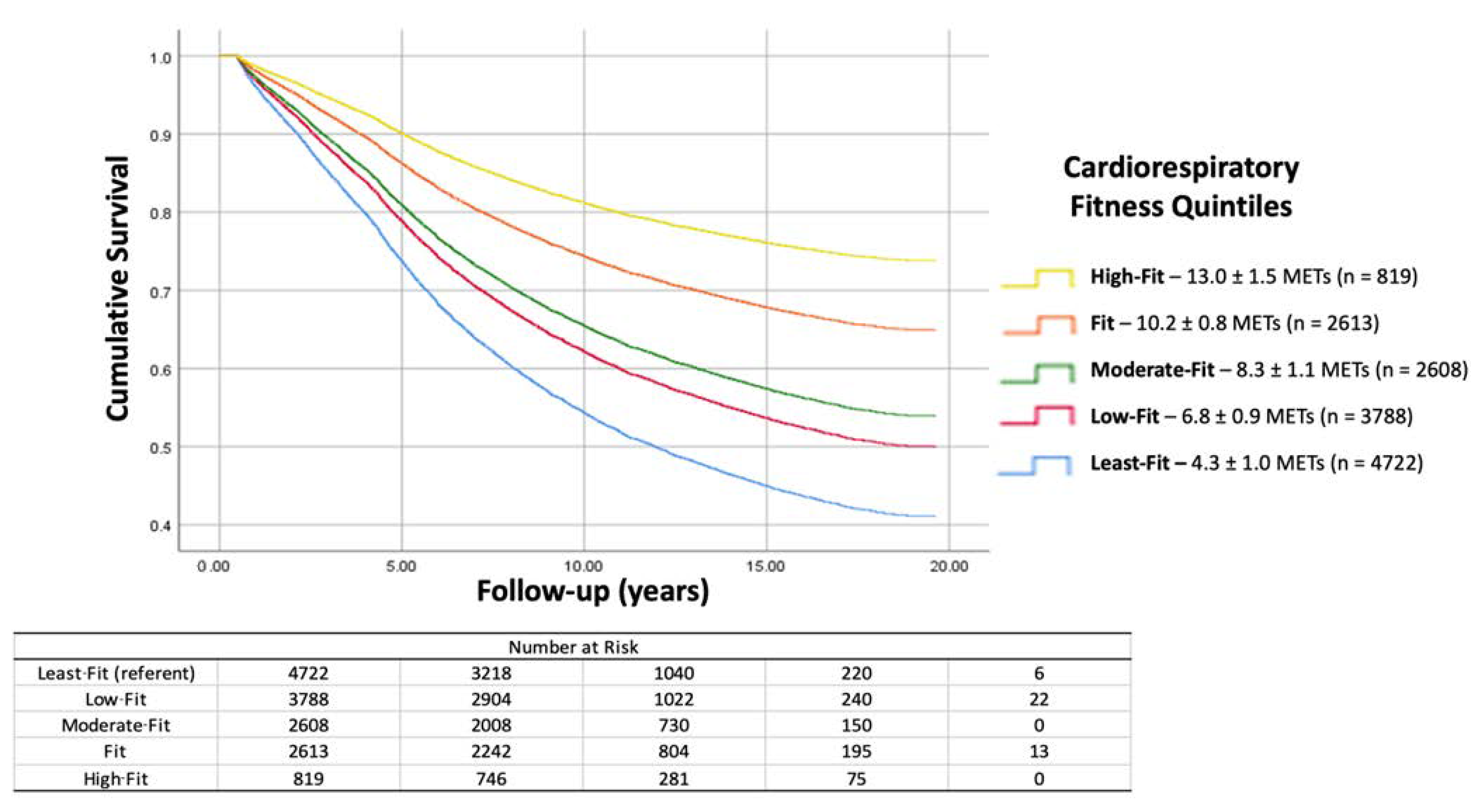
| Patient Characteristic | Least-Fit (n = 4722) | Low-Fit (n = 3788) | Moderate-Fit (n = 2608) | Fit (n = 2613) | High-Fit (n = 819) | Significance (p-Value) |
|---|---|---|---|---|---|---|
| Age at ETT (years) | 64.2 ± 8.2 | 63.3 ± 8.2 | 65.0 ± 9.0 | 62.0 ± 7.2 | 62.5 ± 8.5 | <0.001 |
| BMI (kg/m2) | 30.6 ± 5.7 | 30.2 ± 5.1 | 29.4 ± 4.5 | 29.0 ± 4.3 | 27.9 ± 4.0 | <0.001 |
| Body Weight (kg) | 95.7 ± 19.3 | 94.5 ± 17.9 | 91.7 ± 15.9 | 90.4 ± 15.2 | 86.4 ± 14.0 | <0.001 |
| Atrial Fibrillation or Flutter | 464 (9.8%) | 248 (6.5%) | 179 (6.9%) | 123 (4.7%) | 29 (3.5%) | <0.001 |
| Chronic Kidney Disease | 691 (14.6%) | 368 (9.7%) | 222 (8.5%) | 164 (6.3%) | 29 (3.5%) | <0.001 |
| Diabetes Mellitus | 1810 (38.3%) | 1158 (30.6%) | 663 (25.4%) | 477 (18.3%) | 100 (12.2%) | <0.001 |
| Cardiac/Hypertension Medications | 4171 (88.3%) | 3181 (84.0%) | 2161 (82.9%) | 1968 (75.3%) | 562 (68.6%) | <0.001 |
| CVD including CAD | 2878 (60.9%) | 2117 (55.9%) | 1401 (53.7%) | 1267 (48.5%) | 349 (42.6%) | <0.001 |
| Smoking | 1386 (29.4%) | 959 (25.3%) | 516 (19.8%) | 629 (24.1%) | 176 (21.5%) | <0.001 |
| History of Stroke | 69 (1.5%) | 28 (0.7%) | 28 (1.1%) | 10 (0.4%) | 5 (0.6%) | <0.001 |
| Hypertension | 3960 (83.9%) | 2989 (78.9%) | 2032 (77.9%) | 1866 (71.4%) | 514 (62.8%) | <0.001 |
| Statin Use | 2948 (36.6%) | 2125 (56.1%) | 1360 (52.1%) | 1253 (48.0%) | 361 (44.1%%) | <0.001 |
| Patient Characteristic | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Atrial Fibrillation or Flutter | 1.60 | 1.52–1.69 | <0.001 |
| Chronic Kidney Disease | 1.53 | 1.42–1.64 | <0.001 |
| Diabetes Mellitus | 1.44 | 1.37–1.52 | <0.001 |
| Cardiac/Hypertension Medications | 1.27 | 1.16–1.39 | <0.001 |
| CVD including CAD | 1.21 | 1.15–1.27 | <0.001 |
| Smoking | 1.14 | 1.08–1.21 | <0.001 |
| History of Stroke | 1.14 | 0.92–1.42 | 0.243 |
| Age | 1.04 | 1.04–1.05 | <0.001 |
| Hypertension | 1.03 | 0.95–1.10 | 0.519 |
| BMI | 0.97 | 0.97–0.98 | <0.001 |
| Statin Use | 0.93 | 0.89–0.99 | 0.012 |
| History of PCI | 0.83 | 0.75–0.93 | 0.001 |
| Cardiorespiratory Fitness Quintile | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Entire Cohort (n = 14,550) | |||
| Least-Fit (referent) | 1.00 | -- | -- |
| Low-Fit | 0.76 | 0.72–0.81 | <0.001 |
| Moderate-Fit | 0.66 | 0.62–0.71 | <0.001 |
| Fit | 0.47 | 0.44–0.51 | <0.001 |
| High-Fit | 0.33 | 0.29–0.38 | <0.001 |
| No Known CVD (n = 6538) | |||
| Least-Fit (referent) | 1.00 | -- | -- |
| Low-Fit | 0.76 | 0.69–0.83 | <0.001 |
| Moderate-Fit | 0.63 | 0.56–0.7 | <0.001 |
| Fit | 0.45 | 0.40–0.51 | <0.001 |
| High-Fit | 0.33 | 0.27–0.41 | <0.001 |
| Known CVD (n = 8012) | |||
| Least-Fit (referent) | 1.00 | -- | -- |
| Low-Fit | 0.77 | 0.71–0.82 | <0.001 |
| Moderate-Fit | 0.69 | 0.63–0.75 | <0.001 |
| Fit | 0.49 | 0.45–0.54 | <0.001 |
| High-Fit | 0.34 | 0.28–0.41 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duggan, J.; Peters, A.; Antevil, J.; Faselis, C.; Samuel, I.; Kokkinos, P.; Trachiotis, G. Long-Term Mortality Risk According to Cardiorespiratory Fitness in Patients Undergoing Coronary Artery Bypass Graft Surgery. J. Clin. Med. 2024, 13, 813. https://doi.org/10.3390/jcm13030813
Duggan J, Peters A, Antevil J, Faselis C, Samuel I, Kokkinos P, Trachiotis G. Long-Term Mortality Risk According to Cardiorespiratory Fitness in Patients Undergoing Coronary Artery Bypass Graft Surgery. Journal of Clinical Medicine. 2024; 13(3):813. https://doi.org/10.3390/jcm13030813
Chicago/Turabian StyleDuggan, John, Alex Peters, Jared Antevil, Charles Faselis, Immanuel Samuel, Peter Kokkinos, and Gregory Trachiotis. 2024. "Long-Term Mortality Risk According to Cardiorespiratory Fitness in Patients Undergoing Coronary Artery Bypass Graft Surgery" Journal of Clinical Medicine 13, no. 3: 813. https://doi.org/10.3390/jcm13030813
APA StyleDuggan, J., Peters, A., Antevil, J., Faselis, C., Samuel, I., Kokkinos, P., & Trachiotis, G. (2024). Long-Term Mortality Risk According to Cardiorespiratory Fitness in Patients Undergoing Coronary Artery Bypass Graft Surgery. Journal of Clinical Medicine, 13(3), 813. https://doi.org/10.3390/jcm13030813






